Combination of Ethyl Acetate Extract of Trichoderma hamatum Fermentation Broth and Fungicide Carbendazim enhances Inhibition against Scleromitrula shiraiana under Laboratory Conditions
Combination of Ethyl Acetate Extract of Trichoderma hamatum Fermentation Broth and Fungicide Carbendazim enhances Inhibition against Scleromitrula shiraiana under Laboratory Conditions
Xiaqing Chen, Yü Huang and Zhen Huang*
Department of Entomology, College of Agriculture, South China Agricultural University, Guangzhou 510642, China
Xiaqing Chen and Yü Huang are joint first authors.
ABSTRACT
Scleromitrula shiraiana is a fungal pathogen that attacks mulberry fruits and causes disease named as “popcorn disease”. Many Trichoderma spp. produce a series of antibiotic metabolites that can inhibit plant pathogens. Trichoderma hamatum isolated from infected mulberry fruits was evaluated for its efficiency to suppress plant pathogen S. shiraiana alone or in combination with fungicide carbendazim under laboratory conditions. The results showed that S. shiraiana was not detected on mulberry leaves and branches, whereas it was only detected in mulberry fruits. T. hamatum fermentation broth and its ethyl acetate extracts caused high mycelial growth inhibition and germination inhibition of S. shiraiana, with the inhibition value up to 80% and 90%, respectively. An apparent increase in mycelial growth inhibition in a dose-dependent manner was observed when ethyl acetate extract and carbendazim were applied individually. The EC50 of T. hamatum ethylacetate extract or carbendazim in mycelial growth inhibition of S. shiraiana was 6.6 μg/ml or 39.5 μg/ml under laboratory condition, respectiviely. The level of synergism between ethylacetate extract and carbendazim was affected by the concentration of each component in the mixtures. The synergistic effects were observed in treatments T4 (3.5+40.0), T5 (7.0+20.0) and T6 (7.0+40.0), respectively. The treatment T3 (3.5+20.0) was determined as additive effects according to their cumulative adjusted mortalities, Me and Chi-square values. The current research on the joint action of ethylacetate extracts from T. hamatum with carbendazim offered a promising, safe and effective alternative to fungicides in treatment against S. shiraiana, popcorn disease of mulberry.
Article Information
Received 19 April 2017
Revised 04 June 2017
Accepted 25 July 2017
Available online 05 October 2018
Authors’ Contribution
ZH conceived and designed the study. XC and YH performed all the experiments and analyzed the data. ZH wrote the article.
Key words
Trichoderma hamatum, Joint action, Mycelial growth inhibition, Germination inhibition, Scleromitrula shiraiana, Carbendazim.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.6.2239.2247
* Corresponding author: [email protected]
0030-9923/2018/0006-2239 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Introduction
Mulberry tree is cultivated worldwide in the temperate, subtropical, or tropical regions, and has been grown to rear silkworms (Ercisli and Orhan, 2007; Özgen et al., 2009), recently growing area of this tree has increased sharply (Kishi, 1998) for mulberry fruits taste, nutritive value and health benefits (Kim et al., 2013), with fruits containing polyphenol pigment antioxidants, minerals, and vitamins. However, mulberry fruits are susceptible to plant pathogens such as Scleromitrula shiraiana (Henn.) Imai, Ciboria carunculoides (Siegler and Jenkins) Whetzel, and Ciboria shiraiana (Henn.) Whetzel (Whetzel and Wolf, 1945; Kohn and Nagasawa, 1984; Kishi, 1998; Lǚ et al., 2017; Ye et al., 2014b). These pathogens release ascospores spreading by wind to infect mulberry blossom, causing disease named as “popcorn disease”, leading to significant annual yield loss (Siegler and Jenkins, 1923; Blain, 1931). S. shiraiana is one of the major pathogens attacking mulberry fruits, producing large amounts of black polymer called “sclerotia” and the sclerotia formed in the diseased fruit is coated with a thick black substance (Li and Rollins, 2009). S. shiraiana is also a worldwide distribution phytopathogen and can infect more than 408 species of plants, including many crops and vegetables such as oilseed rape, soybean, peanut, sunflower, cauliflower and carrot (Boland and Hall, 1994; Bolton et al., 2006). With the chemical fungicide widely used in plant disease management and development of fungicide resistance of disease, it is more and more difficult to control “popcorn disease” with chemical fungicides, including iprodione, carbendazium, procymidone, glyphosate, vinclozolin and dicloraz and so on (Ben-Yephet et al., 1986; Hubbard et al., 1997; Ye et al., 2014a).
Carbendazim is a widely used, broad-spectrum benzimidazole fungicide and a metabolite of benomyl, has been extensively used to control S. sclerotiorum for more than three decades in China in cereals and fruits, including citrus, bananas, strawberries, pineapples, mulberry tree, oilseed rape and pomes (Wright, 2009). Repetitive and extensive applications of carbendazim have resulted in emergence and prevalence of carbendazim resistance strains of pathogen in oilseed rape fields in eastern China (Zhou et al., 2012; Wang et al., 2014). Therefore, many physical and biological measures are being used as a safer alternative to chemical control. These alternate tools include the use of biological control agents, plant bioactive compounds and physico-chemical methods. Many application-specific fungal antagonists, such as, nonspecific Trichoderma and Gliocladium species, were used to control plant disease (Mishike, 1998; Mishra, 1996; Harman et al., 2004; Verma et al., 2007). Trichoderma species are well-reported as biocontrol agents against several fungal pathogens through mechanisms such as mycoparasitism (mycelial coiling), antibiosis, cell wall degrading enzymes and induced resistance in host plant against diseases by altering plant gene expression (Pandya and Saraf, 2010; Alfano et al., 2007). Studies showed that T. hamatum can reduce the occurrence of foliar diseases of several vegetable crops by altering genes involved in stress and protein metabolism (Khan et al., 2004; Al-Dahmani et al., 2005; Horst et al., 2005).
Although many studies have been conducted to investigate the effects of Trichoderma species on the growth and development of a number of fungal pathogens, few studies have directly examined their ability of T. hamatum fermentation broth ethyl acetate extracts or together with carbendazim to control S. shiraiana. Therefore, in the present study, we examined the inhibition of T. hamatum fermentation broth ethyl acetate extracts alone or together with carbendazim on mycelial growth and germination of S. shiraiana.
Materials and methods
Fungal strains
The Trichoderma hamatum strain Th-N5 (CCTCC accession # M 2016251) was isolates from soil in Guangzhou city, China. Scleromitrula shiraiana isolate (Ss-01), originally isolated from infected mulberry fruit in Guangzhou city, deposited at Laboratory of Entomopathogenic Fungus, College of Agriculture, South China Agricultural University, identified as the method described by White et al. (1990), was used in the present study. The fungus was identified by sequencing of ITS region as amplified from genomic DNA using the primer pairs ITS1/ITS4 (5’-TCCGTAGGGTGAACCTGCGG-3’ / 5’-TCCTCCGCTTATTGATATGC-3’). To produce the inoculum used in this study, S. shiraiana and T. hamatum were cultured on potato dextrose agar (PDA) medium (Potato infusion 200 g/L, Dextrose 20 g/L and Agar 20 g/L dissolved in water 1000 ml and sterilized at 121°C at 15 psi for 20 min) and incubated at 22 ± 1°C for 23 days and 26 ± 1°C for 10 days, respectively. Spore of S. shiraiana and T. hamatum were harvested from PDA plates into sterile distilled H2O + 0.1% Tween-80. Spores were counted using a Fuchs-Rosenthal hemocytometer and a compound microscope. The suspensions were adjusted to indicate concentrations as needed.
Detection of the dynamics of S. shiraiana in mulberry leaf, fruit and soil
Samples, including mulberry small branch with leaves or fruits and soil near mulberry trees, were collected one time every 15 days interval from February to September in mulberry orchard. Mulberry leaves, small branch and fruits used for extracting DNA were detached from the mulberry samples, and then washed with deionized water three times after sterilized with 75% alcohol 10 min, respectively. S. shiraiana was identified with morphological characters and by PCR with primers ITS1/ITS4 using the methods as White et al. (1990) and Lǚ et al. (2017). The specific fragment clone (about 600 bp) were amplified in a 50-ul reaction system (1 ul DNA solution as template, 0.2 uM each primer, 0.2 mM each dNTP, 2.5mM MgCl2, 1×Taq Polymerase Buffer, and 2.5 U Taq Polymerase) by denaturation at 94°C for 5 min, followed by 30 cycles of 30 s at 94°C, 1 min at 55°C, 1 min at 72°C and terminating with a final extension at 72°C for 10 min. The amplified fragments were sequenced at Invitrogen Com. (China) to verify the sequence. The soil sample contained the surface and 5 cm depth of the soil near mulberry tree. The drupelets were obtained from the soil sample (300g soil) and then rinsed with water, disinfected with 70% alcohol for 30 seconds followed by 0.1% mercuric chloride surface sterilized for five min (Zhang et al., 2009). The drupelets were then rinsed five times with sterile distilled water to ensure complete cleansing of alcohol and mercuric chloride. A sterile scalpel was used to slice the drupelets, then inoculated into PDB broth (Potato infusion 200 g/L, Dextrose 20 g/L dissolved in water 1000 ml and sterilized at 121°C at 15 psi for 20 min) and incubated at 22 ± 1°C in darkness for germination examination after 48 h.
Preparation of ethyl acetate extract of T. hamatum fermentation broth
Fungal conidia (10 ml, 1×106 conidia ml-1) of T. hamatum was inoculated into shake cultures in a 1 liter flask containing 300 ml of Czapek-Dox broth + 1% peptone (CZP, composed of Peptone 0.5%, NaNO3 0.2%, K2HPO4 0.1%, MgSO4·7H2O 0.05%, KCl 0.05%, FeSO4·7H2O 0.001%, sucrose 3% (w/v)) and incubated with aeration (180 rpm) at 26 ± 1°C for 3 days for the production of the seed inoculum. The seed inoculum were added to fresh CZP at 1: 9 ratios (v/v, 3000 ml total volume) and the mixture was incubated with aeration (240 rpm) at 26 ± 1°C for 6 days. After 6 days, fungal cells were removed by centrifugation (12000 x g, 15 min) and the cell-free culture supernatant stored at 4oC for use.
Metabolites were extracted from the cell-free culture supernatant using ethyl acetate (EthOAc). Aliquots (6000 ml) of the cell-free supernatant were mixed with an equal volume of ethyl acetate (1:1) and mixed vigorously for 30 min. The organic phase from above extraction mixture was collected and concentrated (rotary evaporator RE -52A, Shanghai Ya Rong Biochemical Instrument Factory, Shanghai, China) under reduced pressure to obtain EthOAc-fraction and stored at -20oC for use. All chemicals used in this experiment were purchased at Qianghui Bio-chemical Company.
Inhibitory effect of the ethyl acetate extract on ascospore germination and mycelial growth of S. shiraiana
Freshly prepared fungal suspension of S. shiraiana ascospore (100 µl of 1 × 106 spore ml-1) was inoculated in the centre of plates with PDA medium using a micro applicator and was spread to cover the whole plate. The plates were incubated for mycelial growth at 22 ± 1 °C, 80 ± 5% R.H., and L14:D10 h for 2-3 days. Mycelial discs from the actively growing margins together with medium (Ø1 cm) were removed and cultured on PDA medium having the ethyl acetate extract of T. hamatum and incubated at 22 ± 1oC. The same mediums without the ethyl acetate extract served as a control. There were 5 Petri dishes for different concentrations of ethyl acetate extract treatments. Colony diameters were measured after 7d. 5 ml of the ascospore suspension was added to 45 ml PDB broth (250-ml flask) contain different concentration of ethyl acetate extract. The flasks were incubated in rotary shaker (200 rpm) at 22 ± 1°C. The culture was examined under microscope after 48 h incubated, with ascospore considered germinated when the length of the germ tube was equal or greater than the diameter of the conidia. At least 800 ascospores were examined per sample and the percentage of germination was calculated. All experiments were performed in triplicate, and experiments were repeated with at least one independent batch of ascospore as inoculum.
Inhibitory effect of carbendazim on ascospore germination and mycelial growth of S. shiraiana
Mycelial discs of S. shiraiana was prepared as described above, mycelial discs together with medium (Ø1 cm) were removed and cultured on PDA medium having fungicide carbendazim and incubated at 22 ± 1 oC. The same mediums without carbendazim served as a control. There were 5 Petri dishes for different concentrations of carbendazim treatments. Colony diameters were measured after 7 d. All the treatments were replicated three times, and experiments were repeated with at least one independent batch of carbendazim and ascospore as inoculum. Inhibition of carbendazim on ascospore germination at 48 h was performed as described above.
Joint action of the ethyl acetate extract and carbendazim on ascospore germination and mycelial growth of S. shiraiana under laboratory conditions
The activities of different ethyl acetate extract and carbendazim mixtures were tested against ascospore germination and mycelial growth of S. shiraiana. Basis on the resulted of experiment above, three concentrations of carbendazim (0, 20.0, 40.0 ug/mL) and ethyl acetate extract (0, 3.5, 7 ug/mL) were used, respectively. The different mixtures of ethyl acetate extract and carbendazim were prepared by serial dilutions with 0.05% Tween-80, and bioassays were carried out using the methods previously described. The different mixtures of ethyl acetate extract (ug/mL) and carbendazim (µg/mL) were as follows: T1=3.5+0.0, T2=7.0+0.0, T3=3.5+20.0, T4=3.5+40.0, T5=7.0+20.0, T6=7.0+40.0, T7=0.0+20.0, T8=0.0+40.0 and T9=0.0+0.0.
Statistical analysis
The average mycelial growth diameter of colony was calculated as (long diameter + short diameter) / 2. Inhibitory percentage on germination or mycelial growth was calculated as: % inhibition = [(Gc-Gt)/Gc]*100, where Gc = conidial germination or mycelial growth diameter in control and Gt = conidial germination or mycelial growth diameter in treatment. Inhibitory percentage on mycelial growth and germination data were analyzed by Analysis of variance (ANOVA) and treatment means were compared by using Tukey’s HSD test for mean comparisons at 5% level of significance. The curves of (log concentration – probit line (LC-p)) were calculated and tested by chi-square test, median effective concentrations (EC50) and their confidence intervals were calculated by probit analysis using SPSS (Statistical Package for Social Science) 8.0 for windows (SPSS, 2008). The data were control-corrected (Abbott, 1925) first, and converted into proportion (i.e., 0-100% → 0-1). The inhibitory percentage expected for no interaction (additive effect) was calculated as follows (Huang et al., 2016):
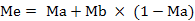
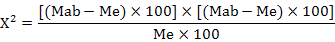
Where, Me is the expected inhibitory percentage for additive mortality, Ma, Mb and Mab are the observed inhibitory percentage for agents ethyl acetate extract, carbendazim and their combination, respectively. Then P-value were looked up in a chi-square table for df=1. If Mab significantly <Me, it meant antagonism; if Mab significantly >me, it meant synergism. Otherwise the inhibition rate was additive.
Results
Dynamics of S. shiraiana in mulberry leaf/branch, fruit and soil
Total 270 leaves and branches were tested by PCR with the primers (Ss-F/Ss-R) to identify infection of S. shiraiana, the result showed that the specific DNA fragment was detected from infected samples and was identified with 100% similarity to the sequence of KP229357.1 in NCBI (Fig. 1).
The data regarding infection rate of mulberry leaf and fruit infected by S. shiraiana from February to May has been shown in Table I. S. shiraiana was not detected by PCR method on mulberry leaves throughout the sample collection period, however it was detected in all the infected mulberry fruits from February 13th to May 13th until fruit harvested from May 10th to 18th. The infection rate of S. shiraiana on appearance no-infected mulberry fruits showed very different during the sample collection periods. The maximum infection rate on appearance no infected fruit was 69.3% (Table I).
Table I.- Percentage infection of S. shiraiana on mulberry leaf/branch and fruit (%).
|
Collection time |
Leaf/ branch |
Infected fruit |
Appearance no infection fruit |
|
February 13 |
0 |
0.0 |
0.0 |
|
February 28 |
0 |
0.0 |
0.0 |
|
March 13 |
0 |
100.0 |
30.6±2.5 c |
|
March 28 |
0 |
100.0 |
39.8 ± 3.3 bc |
|
April 13 |
0 |
100.0 |
51.7 ± 4.3 b |
|
April 28 |
0 |
100.0 |
69.3 ± 4.5 a |
|
May 13 |
0 |
100.0 |
32.6 ± 3.5 c |
|
F, df, P |
89.54, 4, <0.0001 |
Means ± SE in the same column followed by different letters are significantly different (Tukey’s HSD Test, α=0.05).
The numbers of collected drupelets in soil samples from March 13th to September 28th and the ascospore germination rates have been shown in Table II. The numbers of collected drupelets increased from March 13th to May 28th and then decrease gradually afteraward. The highest number of S. shiraiana drupelets (518) was detected on 28th May whereas the lowest number of drupelets (51) was observed on September 28th. The germination of S. shiraiana ascospore in soil drupelets remained constant during March 13th to April 28th with the highest value 100% and decreased afterwards with the lowest germination 43.5% on September 28th (Table II).
Inhibitory effect of ethyl acetate extract on ascospore germination and mycelial growth
Fermentation broth and its ethyl acetate extracts of T. hamatum inhibited significantly the mycelial growth and ascospore germination of S. shiraiana among different treatments (Table III). For T. hamatum fermentation broth the inhibition rate was 83.7%. In case of ethyl acetate extract, the lowest inhibition rate was 20.7% at 7 d in 2.5 µg/ml concentration treatment and the highest inhibition rate was 97.8% at 40 µg/ml concentration treatment. The EC50 of T. hamatum ethyl acetate extract in mycelial growth inhibition of S. shiraiana was 6.6µg/ml, with the regression equation of Y=3.1469+2.2612X (R2=0.9816, X2=16.845, P0.05 (5.71, 7.63), LC95=35.24 ug/ml). The lowest ascospore germination inhibition rate was 32.8% at 48 h in 2.5 µg/ml concentration treatment and the highest germination rate was 96.7% in 40 µg/ml concentration treatment.
Table II.- Examination the number of drupelets in soil and ascospore germination rate.
|
Sample collection time |
Number of drupelets |
Germination rate (%) |
|
March 13 |
0 |
0.0± 0.00 |
|
March 28 |
87 |
100.0± 0.00 a |
|
April 13 |
169 |
100.0± 0.00 a |
|
April 28 |
351 |
100.0± 0.00 a |
|
May 13 |
427 |
95.0± 0.00 ab |
|
May 28 |
518 |
92.0 ± 0.00 b |
|
June 13 |
426 |
90.0 ± 0.00 b |
|
June 28 |
353 |
89.5 ± 3.1 b |
|
July 13 |
288 |
76.3 ± 2.6 c |
|
July 28 |
182 |
69.6 ± 3.2 c |
|
August 13 |
173 |
64.2 ± 4.3 cd |
|
August 28 |
80 |
53.9 ± 4.6 d |
|
September 13 |
65 |
49.7 ± 3.9 d |
|
September 28 |
51 |
43.5 ± 2.5 d |
|
F, df, P |
187.4, 13, <0.0001 |
Means ± SE in the same column followed by different letters are significantly different (Tukey’s HSD Test, α=0.05).
Table III.- Inhibitory effect of fermentation broth and metabolite extract on ascospore germination and mycelial growth of S. shiraiana.
|
Treatment |
Concent. |
Inhibition of germination (%) (48h) |
Inhibition of growth (%) (7d) |
|
Fermentation broth |
Crude broth |
86.5 ± 4.3 b |
83.7 ± 4.8 b |
|
Ethyl acetate extract |
2.5µg/ml |
32.8 ± 2.5 e |
20.7 ± 2.3 e |
|
5µg/ml |
47.3 ± 3.6 d |
36.1 ± 4.2 d |
|
|
10µg/ml |
73.7 ± 4.3 c |
65.9 ± 4.9 c |
|
|
20µg/ml |
87.3 ± 5.2 b |
78.5 ± 5.5 b |
|
|
40µg/ml |
96.7 ± 6.1 a |
97.8 ± 5.8 a |
|
|
F, df, P |
145.7, 5, 0.0001 |
121.5, 5, 0.0001 |
Means (M ± SE) in the same column followed by different letters are significantly different (Tukey’s HSD Test, α=0.05). Data on mean (±SE) inhibitory percentage were subjected to arcsine transformation prior to computation.
Inhibitory effect of carbendazim on ascospore germination and mycelial growth
The inhibition of carbendazim on the ascospore germination and mycelial growth of S. shiraiana differed significantly among different treatments (Table IV). The lowest mycelial growth inhibition rate was 19.6 % observed at 5 µg/ml concentration treatment and the highest inhibition rate was 63.8 % at 80 µg/ml concentration treatment. In case of inhibition radial growth, The EC50 of carbendazim in mycelial growth inhibition of S. shiraiana was 39.5 µg/ml, with the regression equation of Y=3.3870+1.0101X (R2=0.9839, X2=7.2474, P0.05(28.6, 54.5), EC95=1679.80 µg/ml). The lowest inhibition germination rate was 24.1% at 48 h in 5 µg/ml concentration treatment and the highest one was 89.4% in 80 µg/ml concentration treatment.
Table IV.- Inhibitory effect of carbendazim on ascospore germination and mycelial growth of S. shiraiana.
|
Concentration (μg/ml) |
Inhibition of germination (%) (48h) |
Inhibition of growth (%) (7d) |
|
5 |
24.1 ± 1.3 d |
19.6 ± 4.1 d |
|
10 |
31.8 ± 3.2 d |
28.1 ± 4.3 c |
|
20 |
48.5 ± 3.9 c |
32.5 ± 2.5 c |
|
40 |
67.3 ± 4.1 b |
51.7 ± 3.9 b |
|
80 |
89.4 ± 5.4 a |
63.8 ± 4.7 a |
|
F, df, P |
92.7, 4, <0.0001 |
53.7, 4, <0.0001 |
Means (M ± SE) in the same column followed by different letters are significantly different (Tukey’s HSD Test, α=0.05). Data on mean (±SE) inhibitory percentage were subjected to arcsine transformation prior to computation.
Table V.- Joint action of metabolite extract and carbendazim on ascospore germination and mycelial growth of S. shiraiana.
|
Treatment |
Inhibition of germination (%) (48h) |
Inhibition of growth (%) (7d) |
|
T1 |
40.1 ± 1.3 d |
29.6 ± 4.1 c |
|
T2 |
57.3 ± 3.2 c |
46.7 ± 4.3 bc |
|
T3 |
71.4 ± 3.6 b (68.2, 0.144) |
57.3 ± 3.8 b (51.2, 0.647) |
|
T4 |
89.5* ± 4.5 ab (78.9, 1.266 ) |
81.8* ± 5.7 a (65.8, 3.135) |
|
T5 |
93.8* ± 4.2 a (77.3, 2.893) |
85.6* ± 4.9 a (63.1, 5.934) |
|
T6 |
97.9* ± 5.3 a (84.9, 1.719) |
89.5* ± 6.2 a (74.1, 2.651) |
|
T7 |
46.9 ± 3.1 d |
30.7 ± 2.2 c |
|
T8 |
64.7 ± 3.4 bc |
51.4 ± 2.8 b |
|
F, df, P |
129.2, 7, 0.0001 |
103.7, 7, 0.0001 |
Means (M ± SE) in the same column followed by different letters are significantly different (Tukey’s HSD Test, α=0.05). Data on mean (±SE) inhibitory percentage were subjected to arcsine transformation prior to computation. Data in bracket shows Me (the expected inhibitory percentage for additive inhibitory percentage) subjected to arcsine transformation and the chisquare value, respectively. * represent the combined treatment having synergistic interaction through the data analysis. T1=3.5 (metabolite extract) + 0.0 (carbendazim), T2 = 7.0 + 0.0, T3 = 3.5 + 20.0, T4 = 3.5 + 40.0, T5 = 7.0 + 20.0, T6 = 7.0 + 40.0, T7 = 0.0 + 20.0, and T8 = 0.0 + 40.0.
Joint action of the ethyl acetate extract and carbendazim on ascospore germination and mycelial growth
The mean inhibitory percentage of T. hamatum, together with Me (expected inhibitory percentage for additive inhibitory percentage) and Chi-square values are presented in Table V. Most ethyl acetate extracts / carbendazim combinations tested caused high inhibitory percentage on germination and mycelial growth of S. shiraiana and the combination (marked with *) showed a substantial level of synergism. The ascospore germination and mycelial growth in control were 97.3% and 4.3 cm under laboratory conditions, respectively. The level of synergism between ethyl acetate extracts and carbendazim was affected by the concentration of each component in the mixtures, i.e., the inhibitory percentage of germination and mycelial growth increased with an increase in the concentration of ethyl acetate extracts and carbendazim. The inhibitory percentage in the treatments containing ethyl acetate extracts or carbendazim alone differed significantly from the relevant mixtures of ethyl acetate extracts and carbendazim. The best synergistic effect was observed in treatment T5 (7.0+20.0) against S. shiraiana having inhibitory percentage values of 93.8 and 85.6 on germination and mycelial growth after 7 d treatment, respectively. Similar synergistic actions were also observed for treatments T4 (3.5+40.0) and T6 (7.0+40.0). The treatment T3 (3.5+20.0) was determined as additive effects according to their inhibitory percentage, Me and Chi-square values (Table V).
Discussion
Scleromitrula shiraiana ascospores are the only source of primary infection and infect mulberry inflorescences causing the popcorn disease in mulberry trees (Lǚ et al., 2016). The appearance of the disease manifested with the growing mulberry fruits and then formed sclerotia in drupelet, finally, the mulberry fruits turned to mummified white fruits. Paraffin sections of diseased fruits showed that the fungal mycelium infected mulberry drupelet and then disintegrated into small black particles, which formed the outer wall of the sclerotia. Sclerotia have two structures with a hard, dense black outer layer called the pseudoparenchyma and a loose inner layer called the prosenchyma (Lǚ et al., 2016). The hard black layer can withstand adverse environmental impacts, has the chemical properties of melanin, such as, black color, insolubility in water and organic solvents, resistance to degradation by concentrated acids, bleaching by oxidizing agents, and solubilization and degradation by hot alkali solutions (Butler and Day, 1998; Wu et al., 2008). Once the mycelium of S. shiraiana infected and entered into mulberry inflorescences, it is difficult to control mycelium growing in inflorescences and fruit with chemical fungicide for the reason of systemic fungicide residue. So this is the reason why we focused on the S. shiraiana ascospores and mycelium growth to inhibit the primary infection in this study.
T. hamatum can be an efficient endophytic and saprophytic colonizer of the above ground parts of plant (El-Hassan et al., 2013), is one of the promising biological control agents against popcorn disease in mulberry trees (Metcalf and Wilson, 2001; Hohmann et al., 2011; Ye et al., 2014). In order to survive and compete, Trichoderma produces a series of toxic and antibiotic metabolites against plant pathogens and secretes extracellular hydrolytic enzymes to inhibit, compete with phytopathogenic fungi. (Thrane et al., 2000; Eziashi et al., 2006; Vinale et al., 2008; Andrabi et al., 2011). Our results showed that T. hamatum fermentation broth and ethyl acetate extracts inhibited mycelium growth and ascospore germination of S. shiraiana on agar plates under laboratory condition. The appearance of mycelium growth and ascospore germination inhibition on agar plates is considered as antibiosis and competition for nutrients and / or space for growth (Cook and Baker, 1983). The antibiotic metabolites produced by Trichoderma species diffused in the medium and occupied the whole area, which may inhibit the pathogen activities or direct effect on the target pathogen growth. On the other hand, the toxicity of antibiotic compounds released in the culture filtrate by T. hamatum which inhibited the growth of S. shiraiana mycelium may be similar to the metabolites produced by other Trichoderma species. Our results also showed that different concentrations of T. hamatum ethyl acetate extracts and crude fermentation broth caused ascospore germination inhibition of S. shiraiana and this inhibition increased with increase in extract concentrations. These results encourage us to find alternative tools to inhibit ascospores, the only primary infection source and infection on mulberry inflorescences.
Much information regarding to the secondary metabolites from T. harzianum against pathogen growth by affecting the physiology of competitors organisms were available (Sivasithamparam and Ghisalberti, 1998; Keszler, 2000), but a few reports are available on compatibility of the secondary metabolites with synthetic fungicides against S. shiraiana. Therefore, current studies were conducted to observe the compatibility of ethyl acetate extracts from T. harzianum with carbendazim against S. shiraiana. The results showed that even the low dose of ethyl acetate extracts could promote the inhibition of carbendazim against S. shiraiana ascospores germination and mycelium growth. Joint application of ethyl acetate extracts and carbendazim resulted in different joint actions on S. shiraiana. There were additive and synergistic effects when different concentrations of ethyl acetate extracts and carbendazim were mixed. The level of synergism was most evident when 7.0 ug/mL ethyl acetate extracts was mixed with 20 or 40 ug/mL carbendazim.
The possible reason behind the additive and synergistic action of metabolite ethyl acetate extracts and carbendazim can be related to their different mode and mechanism of target systems. The possible outcome of interaction in mixture is influenced by different factors like target pathogen species, differences in biochemical properties of different fungicides and target of the chemicals. Although, the mechanism of metabolite from T. harzianum was not very clearly illustrated yet to date, the theory of metabolite as an inhibitor of pathogen growth has been supporting by many researchers (Dennis and Webster, 1971a, b; Okuda et al., 1982; Claydon et al., 1987; Ghisalberti et al., 1990), the inhibitor include simple aromatic compounds, some polyketides as pyrones and butenolides, volatile terpenes, isocyano metabolites, and relatively non-polar substances. The molecular mechanism of carbendazim is to inhibit β-tubulin assembly in mitosis of cell in growing pathogen, which implies an inherent high risk for resistance development (Brent and Hollomon, 2007). Studies on molecular mechanisms demonstrated that a single mutation in the β-tubulin gene resulting in the replacement of glutamic acid by alanine at codon 198 (E198A) confers high-level resistance to carbendazim and the replacement of phenylalanine by tyrosine at codon 200 (F200Y) confers moderate-level resistance (Li et al., 2003; Chen et al., 2009). Carbendazim has strong power in controlling plant disease with both protective and curative activity up to 10 days and was used as a systemic fungicide. In our pre-experiment, the mycelia growth of pathogen was out of control with metabolite after 5 day or so. So, in the joint action a distinct advantage of carbendazim was its systemic activity, which not only protected plants from infection but also provided disease control when applied after the early stages of infection in plant tissue. The results indicated that the metabolite was an alternative agent used alone, or at low dose promotion the inhibition efficiency of carbendazim, against S. shiraiana for reduction the dose of fungicide applied in field.
Our studies strongly recommend the joint application of metabolite ethyl acetate extracts from T. harzianum and carbendazim because of their synergistic action against S. shiraiana. Such combination can improve the inhibition efficacy on pathogen by using low dose of ethyl acetate extracts, providing an opportunity to reduce the likelihood of development of fungicide resistant pathogens and reduce the dose of fungicide used.
Acknowledgements
The research was funded by grants from Science and Technology Planning Project of Guangzhou city (2014Y2-00514) and Science and Technology Planning Project of Guangdong province (2016A050502049).
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abbott, W.S., 1925. A method for computing the effectiveness of an insecticide. J. econ. Ent., 18: 265–267.
Aldahmani, J.H., Abbasi, P.A., Sahin, F., Hoitink, H.A.J. and miller, S.A., 2005. Reduction of bacterial leaf spot severity on radish, lettuce, and tomato plants grown in compost-amended potting mixes. Canadian J. Pl. Pathol., 27: 186-193. https://doi.org/10.1080/07060660509507215
Alfano, G., Lewis ivey, M.L., Cakir, C., Bos, J.I.B., Miller, S.A., Madden, L.V., Kamoun, S. and Hoitink, H.A.J., 2007. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology, 97: 429-437. https://doi.org/10.1094/PHYTO-97-4-0429
Andrabi, M., Vaid, A. and Razdan, V.K., 2011. Evaluation of different measures to control wilt causing pathogens in chickpea. J. Pl. Prot. Res., 51: 55–59. https://doi.org/10.2478/v10045-011-0010-3
Ben-yephet, Y., Bitton, S. and Greenberger, A., 1986. Control of lettuce drop disease, caused by Sclerotinia sclerotiorum, with metham-sodium soil treatment and foliar application of benomyl. Pl. Pathol., 35: 46-51. https://doi.org/10.1111/j.1365-3059.1986.tb01997.x
Blain, W.L., 1931. A list of diseases of economic plants in Alabama. Mycologia, 23: 300-304. https://doi.org/10.2307/3753830
Boland, G.J. and Hall, R., 1994. Index of plant hosts of Sclerotinia sclerotiorum. Canadian J. Pl. Pathol., 16: 93-108.
Bolton, D.M., Thomma, B.P. and Nelson, B.D., 2006. Sclerotinia sclerotiorum (Lib.) de Bray: Biology and molecular traits of a cosmopolitan pathogen. Mol. Pl. Pathol., 7: 1-16. https://doi.org/10.1111/j.1364-3703.2005.00316.x
Brent, K.J. and Hollomon, D.W., 2007. Fungicide resistance: The assessment of risk. FRAC Monograph No. 2, second ed. Belgium, Brussels. Available at: htpp://www.frac.info/publication/anhang/FRAC_Mono2_2007.pdf
Butler, M.J. and Day, A.W., 1998. Fungal melanins: A review. Canadian J. Microbiol., 44: 1115-1136. https://doi.org/10.1139/w98-119
Chen, C.J., Zhao, W., Lu, Y.J., Wang, J.X., Chen, Y., Li, H.X. and Zhou, M.G., 2009. Highthroughput detection of highly benzimidazole-resistant allele E198A with mismatch primers in allele-specific real-time polymerase chain reaction. Pest Manage. Sci., 65: 413-419. https://doi.org/10.1002/ps.1691
Claydon, N., Allan, M., Hanson, J.R. and Avent, A.G., 1987. Antifungal alkyl pyrones of Trichoderma harzianum. Trans. Br. Mycol. Soc., 88: 503-513. https://doi.org/10.1016/S0007-1536(87)80034-7
Cook, R.J. and Baker, K.F., 1983. The nature and practice of biological control of plant pathogens. APS Press, St. Paul, Minnesota, USA, pp. 539.
Dennis, C. and Webster, J., 1971a. Antagonistic properties of species groups of Trichoderma. I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc., 57: 25-39. https://doi.org/10.1016/S0007-1536(71)80078-5
Dennis, C. and Webster, J., 1971b. Antagonistic properties of species groups of Trichoderma. II. Production of volatile antibiotics. Trans. Br. Mycol. Soc., 57: 41-48. https://doi.org/10.1016/S0007-1536(71)80077-3
El-Hassan, S.A., Gowen, S.R. and Pembroke, B., 2013. Use of Trichoderma hamatum for biocontrol of lentil vascular wilt disease: efficacy, mechanisms of interaction and future prospects. J. Pl. Protect. Res., 53: 12-26. https://doi.org/10.2478/jppr-2013-0002
Ercisli, S. and Orhan, E., 2007. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Fd. Chem., 103: 1380-1384. https://doi.org/10.1016/j.foodchem.2006.10.054
Eziashi, E.I., Uma, N.U., Adekunle, A.A. and Airede, C.E., 2006. Effect of metabolites produced by Trichoderma species against Ceratocystis paradoxa in culture medium. Afri. J. Biotechnol., 5: 703–706.
Ghisalberti, E.L., Narbey, M.J., Dewan, M.M. and Siva sinthamparam, K., 1990. Variability among strains of Trichoderma harzianum in their ability to reduce take-all and to produce pyrones. Pl. Soil, 121: 287-291. https://doi.org/10.1007/BF00012323
Harman, G., Howell, C.R., Viterbo, A., Chet, I. and Lorito, M., 2004. Trichoderma species opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol., 2: 43-56. https://doi.org/10.1038/nrmicro797
Hohmann, P., Jones, E.E., Hilla, R.A. and Stewart, A., 2011. Understanding Trichoderma in the root system of Pinus radiate: associations between rhizosphere colonisation and growth promotion for commercially grown seedlings. Fungal Biol., 115: 759–767. https://doi.org/10.1016/j.funbio.2011.05.010
Horst, L.E., Locke, J., Krause, C.R., Mcmahon, R.W., Madden, L.V. and Hoitink, H.A.J., 2005. Suppression of Botrytis blight of begonia by Trichoderma hamatum 382 in peat and compostamended potting mixes. Pl. Dis., 89:1195-1200. https://doi.org/10.1094/PD-89-1195
Huang, Z., Ali, S. and Ren, S., 2016. The combination of destruxin A with insecticide chlorantraniliprole increases virulence against Plutella xylostella L. Pakistan J. Zool., 48: 1849-1855.
Hubbard, J.C., Subbarao, K.V. and Koike, S.T., 1997. Development and significance of dicarboximide resistance in Sclerotinia minor isolates from commercial lettuce fields in California. Pl. Dis., 81: 148-153. https://doi.org/10.1094/PDIS.1997.81.2.148
Keszler, A., Forgacs, E., Kotai, L., Vizcaíno, J.A., Monte, E. and García-Acha, I., 2000, Separation and identification of volatile components in the fermentation broth of Trichoderma atroviride by solid-phase extraction and gas chromatography–mass spectrometry. J. Chromat. Sci., 38: 421. https://doi.org/10.1093/chromsci/38.10.421
Khan, J., Ooka, J.J., Miller, S.A., Madden, L.V. and Koitink, H.A.J., 2004. Systemic resistance induced by Trichoderma hamatum 382 in cucumber against Phytophthora crown rot and leaf blight. Pl. Dis., 88: 280-286. https://doi.org/10.1094/PDIS.2004.88.3.280
Kim, S.B., Chang, B.Y., Jo, Y.H., Lee, S.H., Han, S.-B., Hwang, B.Y., Kim, S.Y. and Lee, M.K., 2013. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol., 145: 393-396. https://doi.org/10.1016/j.jep.2012.11.007
Kishi, K., 1998. Plant disease in Japan. Zenkaku Noson Kyoiku Kyokai Co., Ltd., Tokyo, Japan.
Kohn, L.M. and Nagasawa, E., 1984. The genus Scleromitrula (Sclerotiniaceae), Episclerotium gen. nov. (Leotiacwae) and allied stipitate-capitate species with reduced ectal excipula. Trans. Mycol .Soc. Japan, 25: 23-38.
Li, H.X., Lu, Y.J., Zhou, M.G. and Wang, X.F., 2003. Mutation in b-tubulin of Sclerotinia sclerotiorum conferring resistance to carbendazim in rapeseed field isolate. Chin. J. Oil Crop Sci., 25: 56-60.
Li, M. and Rollins, J.A., 2009. The development-specific protein (Ssp1) from Sclerotinia sclerotiorum is encoded by a novel gene expressed exclusively in sclerotium tissues. Mycologia, 101: 34-43. https://doi.org/10.3852/08-114
Lǚ, Z., Kang, X., Xiang, Z. and He, N., 2016. A laccase gene Sh-lac is involved in the growth and melanin biosynthesis of Scleromitrula shiraiana. Phytopathology, 107: 353-361. https://doi.org/10.1094/PHYTO-04-16-0180-R
Metcalf, D.A. and Wilson, C.R., 2001. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Pl. Pathol., 50: 249–257. https://doi.org/10.1046/j.1365-3059.2001.00549.x
Mischke, S., 1998. Mycoparasitism of selected sclerotia forming fungi by Sporidesmium sclerotivorum. Canadian J. Bot., 76: 460-466. https://doi.org/10.1139/b98-019
Mishra, R.R., 1996. Microorganisms associated with plant roots. In: Soil microbiology. CBS Publishers and Distributors, New Dehli, pp. 52-82.
Okuda, T., Fujiwara, A. and Fujiwara, M., 1982. Correlation between species of Trichoderma and production patterns of isonitril antibiotics. Agric. Biol. Chem., 46: 1811-1822. https://doi.org/10.1271/bbb1961.46.1811
Özgen, M., Serçe, S. and Kaya, C., 2009. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Horticul., 119: 275-279. https://doi.org/10.1016/j.scienta.2008.08.007
Pandya, U. and Saraf, M., 2010. Application of fungi as a biocontrol agentand their biofertilizer potential in agriculture. J. Adv. Develop. Res., 1: 90-99.
Siegler, E.A. and Jenkins, A.E., 1923. Sclerotinia carunculouloides, the cause of a carious disease of the Mulberry (Morus alba). J. agric. Res., 23: 833-836.
Sivasithamparam, K. and Ghisalberti, E.L., 1998. Trichoderma and Gliocladium (eds. G.E., Harman and C.P. Kubicek). Taylor and Francis, London, pp. 139.
SPSS, 2008. SPSS Statistics for Windows, Version 17.0. SPSS Inc., Chicago IL.
Thrane, C., Jensen, D.F. and Tronsmo, A., 2000. Substrate colonization, strain competition, enzyme production in vitro, and biocontrol of Pythium ultimum by Trichoderma spp. isolates P1 and T3. Eur. J. Pl. Pathol., 10: 215–225. https://doi.org/10.1023/A:1008798825014
Verma, M., Brar, S.K., Tyagi, R.D., Sahai, V., Prévost, D., Valéro, J.R. and Surampalli, R.Y., 2007. Bench-scale fermentation of Trichoderma viride on wastewater sludge: rheology, lytic enzymes and biocontrol activity. Enzyme Microb. Technol., 41: 764-771. https://doi.org/10.1016/j.enzmictec.2007.06.013
Vinale, F., Sivasithamparam, K., Ghisalberti, E.L., Marra, R., Woo, S.L. and Lorito, M., 2008. Trichoderma plant pathogen interactions. Soil Biol. Biochem., 40: 1-10. https://doi.org/10.1016/j.soilbio.2007.07.002
Wang, Y., Hou, Y.P., Chen, C.J. and Zhou, M.G., 2014. Detection of resistance in Sclerotinia sclerotiorum to carbendazim and dimethachlon in Jiangsu Province of China. Australas. Pl. Pathol., 43: 307-312. https://doi.org/10.1007/s13313-014-0271-1
Whetzel, H.H. and Wolf, F.A., 1945. The cup fungus, ciboria-carunculoides, pathogenic on mulberry fruits. Mycologia, 37: 476-491. https://doi.org/10.2307/3754633
White, T.J., Bruns, T., Lee, S. and Taylor, J., 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: A guide to methods and applications. Academic Press, San Diego, CA, USA, pp. 315-322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Wight, A., (14 January 2009). Two-headed fish mystery deepens. Stock and Land. Archived from the original on 19 October 2009.
Wu, Y., Shan, L., Yang, S. and Ma, A., 2008. Identification and antioxidant activity of melanin isolated from Hypoxylon archeri, a companion fungus of Tremella fuciformis. J. Basic Microbiol., 48: 217-221. https://doi.org/10.1002/jobm.200700366
Ye, M., Kuang, Z., Zhao, X., Yang, Q., Li, Q., Xiao, Y., Wang, Z., Dai, F. and Luo, G., 2014a. Screening of fungicide to control Ciboria carunculoides under laboratory conditions. Pakistan J. Zool., 46: 53-59.
Ye, M., Yue, H., Luo, G.Q., Yang, Q. and Kuang, Z.S., 2014b. Effect of a fungal pathogen, Trichoderma hamatum, on growth and germination of Ciboria carunculoides under laboratory conditions. Pakistan J. Zool., 46: 1377-1384.
Zhang, G., Ma, L., Beuchat, L.R., Erickson, M.C., Phelan, V.H. and Doyle, M.P., 2009. Evaluation of treatments for elimination of foodborne pathogens on the surface of leaves and roots of lettuce (Lactuca sativa L.). J. Fd. Protect., 72: 228-234. https://doi.org/10.4315/0362-028X-72.2.228
Zhou, F., Wang, Y.F., Zhang, X.L. and Zhu, F.X., 2012. Advances in resistance of Sclerotinia sclerotiorum to carbendazim. Hunan agric. Sci., 17: 82-84.
To share on other social networks, click on any share button. What are these?










