Effect of Fenugreek as a Feed Additive on the Growth, Body Composition and Apparent Nutrients Digestibility of Striped Catfish Pangasius hypophthalmus Fry
Effect of Fenugreek as a Feed Additive on the Growth, Body Composition and Apparent Nutrients Digestibility of Striped Catfish Pangasius hypophthalmus Fry
Aqsa Mehboob1, Noor Khan1,*, Usman Atiq1, Khalid Javed Iqbal2, Rafia Tayyab1, Syeda Suhaira Batool1, Hafiza Saleha Batool1, Sana Amjad1 and Mehwish Tanveer1
1Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore, Pakistan
2Department of Life Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
ABSTRACT
The study was conducted to evaluate the effect of fenugreek as a feed additive on the survival, growth and body composition of striped catfish Pangasius hypophthalmus. The experiment was conducted in fiberglass tank having capacity of 18 L water with a stocking density of 25 fish fry per aquaria. There were two treatment groups; T1, T2 and a control (T0) each was replicated twice. Fenugreek as an additive was added in experimental feed at the rate of 0.5% in T1 and 1.0% in T2 while control T0 was without fenugreek supplementation. Fish was fed for six days a week at the rate of 4% of its body weight twice a day. Results showed significant (P≤0.05) higher growth performance among the three treatment groups. Highest weight gain (5.07±0.72g), increase in total length (24.25±4.31 mm) was observed in T2. Better feed conversion ratio (FCR) 1.8 and specific growth rate 1.4 were observed in T1. Proximate analysis revealed significant differences (P≤0.05) among treatments and control for crude protein while rest of the parameters remained same. Apparent digestibility of crude protein and fat was found non-significantly different among treatments. It is concluded that addition of fenugreek in fish feed can improve growth performance and also protein content of the fish meat as well.
Article Information
Received 25 June 2016
Revised 04 March 2017
Accepted 23 September 2017
Available online 25 October 2017
Authors’ Contribution
AM and NK designed and executed the experiment. UA helped in sampling. RT, SSB and HSB studied the growth, proximate and physico-chemical parameters. SA and MT studied the apparent nutrient digestibility. NK and KJI analyzed the data statistically and wrote the article.
Key words
Fish, Pangasius hypophthalmus, Feed additive, Growth, Body composition, Digestibility.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.6.2037.2042
* Corresponding author: noorkhan@uvas.edu.pk
0030-9923/2017/0006-2037 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Fenugreek (Trigonellafoenum graecum L.) is a leguminous plant grown in Northern Africa, the Mediterranean, Western Asia, Northern India, and currently cultivated in North America. The seeds of fenugreek are used medicinally as well as food ingredient for several years. It has enormous beneficial effects to digestive system such as a laxative, intestinal lubricant, carminative, vomiting, colitis swell, febrifuge, digestive and tonic. It also helps to dissolve fat and cholesterol deposits, prevents fat accumulation and water retention, and lowering blood glucose levels (Ahmad et al., 2016; Moradi Kor and Moradi, 2013; Madar and Stark, 2002; Platel and Srinivasan, 2000). It has been traditionally used to treat wounds, inflammation, abscesses, arthritis, coughs and bronchitis as well as to reduce mucus production and good for asthma and lung disorder (Castleman, 1991; Ody, 1993; Duke, 2002; Sahalian, 2004). It contains an amino acid called 4- hydroxideisolucine which appears to increase the production of insulin when blood sugar level is high. It may reduce the amount of calcium oxalate in the kidneys and conditions affecting the male reproductive traits (Hannana et al., 2003; Sauvaire et al., 1998). Recent studies suggested that fenugreek has anti-carcinogenic potential and potential allergens (Pandian et al., 2002).
Aquaculture is growing worldwide with significant higher rate compared to other agro industries. The intensification of aquaculture increased fish production manifold but it also created many disease problems especially stress and pathogenic disorders among aquatic species. An outbreak of disease jeopardizes sustainable aquaculture and threatens fish yields. The controlling of disease is one of the most vital tasks in aquaculture. Most diseases can be treated, but treatment is often of doubtful value. Due to indiscriminate use of antibiotics, various pathogenic microbes are gaining resistance to different antibiotics. For these reasons herbal treatments would be an alternate choice for prevention and control of fish diseases (Das et al., 2017; Pandiyan et al., 2013; Bondad-Reantaso et al., 2005). Medicinal plants are important elements of traditional medicine in virtually all cultures and promise a cheaper source for therapeutics, greater accuracy than chemotherapeutic agents and a viable solution for several problems (Ahmed et al., 2009). Many kinds of herbs possess antibacterial and antifungal activity that can be used to control diseases but very little attempts have been undertaken for the treatment of fish diseases (Gültepe, 2014). Nutritionists are trying their best to use various ingredients and additives in formulated aqua feed as growth promoters, feed acceptability and enhanced resistance against various infectious diseases in finfish and shellfish. At present Indian major carps and Chinese carps are the dominant culturable species in the present aquaculture system of Pakistan. Introduction of new species like stripped catfish (Pangasius hypophthalmus) in the country’s prevailing culture system is utmost important to increase per acre fish production and quality protein to cater the protein demand of growing population of the country. It is generally believed that culture of Pangasius would be successful and profitable in Pakistan due to its fast growth, wider tolerance to changes in water quality, disease resistance and comparatively high market price. Another important fact is that this fish can easily be acclimatized to the artificial feed due to its omnivorous nature. The aim of this study was to evaluate the effect of various levels of fenugreek seeds (0, 0.5 and 1 %) as feed additives on the survival, growth performance, feed utilization and body composition of striped catfish (Pangasius hypophthalmus) fry.
Materials and methods
Experimental site and design
The experimental trial was conducted in fiberglass aquaria having 18L water holding capacity with proper supply of oxygen through aerators at Research and Training Facilities of the Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Ravi Campus, Pattoki. The experimental fish P. hypophthalmus was imported from Thailand and kept for one month under quarantine conditions to avoid any disease and fed with commercial fish diet containing 40% crude protein 5 times a day at 2% fish wet body weight daily. After one month six fiberglass aquariums were prepared and stocked 25 fish per aquaria. There were three treatments and each treatment was replicated twice designated as control (T0), T1 and T2.
Feed formulation and preparation
The experimental feed containing 39.60% crude protein was prepared with locally available ingredients (Table I). The ingredients were grinded and mixed well in dough form before preparation of moist pellets which were then passed in local pelleting machine. The fenugreek was added at 0.5% and 1.0% in diet T1 and T2 while control T0 was without supplementation of fenugreek. The moist pellets were sun dried and crumbles were again grinded to form mash which were packed in plastic jars and stored at room temperature and fed to experimental fish for study period as per 4% body weight daily.
Table I.- Fish feed formulation supplemented with different levels of fenugreek seed supplementation.
| Ingredient used |
Inclusion level (%) |
Contributed CP % |
| Fish meal (54%) |
30 |
16.2 |
| Soybean meal (44%) |
10 |
4.4 |
| Corn glutton (60%) |
22 |
13.2 |
| Rice polish (12%) |
7 |
0.85 |
| Wheat bran (16.5%) |
29 |
4.95 |
| Minerals, vitamins and oil |
1 |
- |
| Chromic oxide |
1 |
- |
| Fenugreek |
0%, 0.5%, 1% |
- |
| Total |
100 |
39.6% |
Growth parameters
Fish having average weight of 0.98 g were stocked at 25 fish per aquaria in each treatment groups. The morphometric parameters viz., wet body weight and total body length of each fish was measured and recorded at the time of stocking. Fish was regularly fed at the rate of 4% of its body weight twice a day up to six days a week. All the fish were caught on fortnightly basis for morphometric records (total body length and weight) and were released back to their respective aquaria. The mortality rate was also recorded if found. Growth parameters net weight gain, specific growth rate (SGR%) and feed conversion ratio (FCR) were calculated as per following formulas:
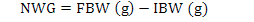
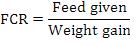
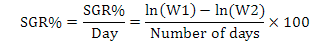
Where, NWG is net weight gain, FBW is final body weight (g), IBW is initial body weight (g), W1, W2, and T are the initial weight, final weight, and number of days in the feeding trial, respectively.
Table II.- Fortnightly average growth of Pangasius hypophthalmus using various levels of Fenugreek in artificial feed (n=50).
| Parameters |
Control (0.0%) |
T1 (0.5%) |
T2 (1.0%) |
|||
|
Initial |
Final |
Initial |
Final |
Initial |
Final |
|
| Weight (g) |
0.46± 0.00b |
3.40 ±0.00c |
0.47 ±0.01b |
4.87±0.02b |
0.85 ± 0.03a |
5.92± 0.00a |
| Length (mm) |
53.20±0.99a |
74.85±0.92a |
33.60±40.59a |
45.30±0.42b |
52.85±0.21a |
77.10± 4.52a |
| Biomass (g) |
11.62±0.07b |
74.86±0.03c |
11.87±0.28b |
78.03±0.34b |
21.39±0.83a |
130.20± 0.19a |
| Net weight gain (g) |
2.94 ±0.10c |
4.40±0.05b |
5.07±0.72a |
|||
| Weight gain (%) |
639.13±4.18b |
936.17±19.99b |
596.47±26.32a |
|||
| Length gain (mm) |
21.65±0.07a |
11.70±1.63a |
24.25±4.31a |
|||
| FCR |
2.32±0.00a |
1.84±0.01c |
2.08±0.02b |
|||
| SGR% |
1.15±0.00b |
1.35±0.01a |
1.12 ±0.02b |
|||
Proximate analysis
At the end of the experimental trial five to seven fish from each treatment were collected and processed for proximate analysis (dry matter, crude protein, crude lipids and ash contents) were determined while using Near Infra-Red (NIR) Spectrophotometry Technology (Martinez et al., 2003; Iqbal et al., 2014).
Digestibility test
For digestibility tests fish faeces were collected on daily basis through siphoning method. The faeces were put in petri dishes and freeze at -4°C in refrigerator on daily basis. Finely faeces were grounded and weighed up to 50 to 500 mg placed in a 100 ml kjeldahl flask for nutrient digestibility tests. Then added 10ml HNO3 in the flask and digested at a gentle boil for at least 30 min or until yellowish vapours stop rising. The sample was cooled to room temperature and added 5ml per chloric acid. The flask was placed in the digester again and continued boiling until the solution turn from green to lemon yellow, and then cooled again. There was a reddish ring around the edge of the liquid. Then cooled liquid was transferred to 25 ml volumetric flask and diluted with distilled water. The spectrophotometer was adjusted to ‘0’ with a blank reagent and read at 350 nm and was calculated by following Furukawa and Tsukahara (1966).
Physico-chemical parameters
Physico-chemical parameters such as, dissolved oxygen (DO), pH, water temperature (°C), total dissolved solids (TDS) and salinity were monitored and recorded on daily basis by using DO meter (YSI 55 Incorporated, Yellow Springs, Ohio, 4387, USA), pH meter (LT-Lutron pH-207 Taiwan) and TDS meter, respectively.
Statistical analysis
The data obtained was analyzed using SAS 9.1 version statistical software. The data on different variables was statistically analyzed by using Analysis of Variance (ANOVA) technique and means were compared by Duncan’s Multiple Range Test.
RESULTS
Growth data
The growth parameters of fish under all the treatments are presented in Table II. The initial weight of fish at the time of stocking was 0.73±0.002 g, 0.97±0.01g and 0.85± 0.03g in control, T1 and T2, respectively. Statistical analysis revealed significant difference among all the three treatment groups. The final weight of fish recorded after three month trial was 3.40 ±0.001g, 4.87±0.02g and 5.91±0.004g in Control, T1 and T2, respectively. Statistical analysis using one-way ANOVA reveals a significant difference among three groups where T2 showed significantly (P≤0.05) higher followed by T1 and control (Table II). The net weight gain was found significantly higher in T2 (5.07±0.72g) followed by T1 (4.40±0.52g) and control (2.94±0.10g), respectively (Table II). The percentage weight gain also indicated significantly higher values in T1 (936.17±19.99%) followed by control (639.13±4.18%) and in T2 (596.47±26.32%). The initial body length was found non-significant in all the three groups. Final body length indicated highly significant differences where T2 showed 77.10±4.525mm increase followed by 74.85±0.91mm in control group and 45.30±0.42 mm in T1. Increase in length revealed significant differences (P≤0.05) in T2 (24.250±4.313mm) followed by control (21.63±0.07mm) and T1 (11.70±1.63mm). The initial biomass was 11.62±0.07g, 11.87±0.28g and 21.38±0.830g in control, T1 and T2, respectively with significant differences among treatments. The final biomass also revealed that values were 74.86±0.03g, 78.03±0.33g and 130.20±0.10g in control, T1 and T2 with significant differences. The FCR values in control were 2.31±0.004, T1 (1.84±0.01) and in T2 (2.07±0.02) with significant differences (P≤0.05) among treatment groups. The SGR% of three groups revealed 1.15±0.003, 1.349±0.011 and 1.12±0.02% indicating significant differences among treatments (Table II).
Proximate composition
The fish was analyzed for its proximate composition at the start and at the end of the experiment. The detail of proximate composition of P. hypophthalmus fingerlings are given in Table III. Dry matter values revealed non-significant differences among pre and post-trial treatment groups. Crude protein was found significantly higher in T1 (63.50±0.42%) followed by T2 and control than pre-trial with significantly lower value (58.10±0.49%). Crude fat, fiber and ash contents were found non-significantly different among pre and post-trial treatments (Table III).
Apparent nutrient digestibility
Crude protein (CP) and fat digestibility remained non-significant (P≤0.05) but highest CP (17.40±4.38) and fat (2.52±0.00) digestibility were found in treatment T2 while these parameters were lowest in control, CP (13.05±1.62) and fat (2.31±0.14) (Table IV).
Table III.- Pre and post-trial proximate analysis of Pangasius hypophthalmus (n=5).
| Para- meters |
Pre-trial |
Post-trial |
||
|
Control (0.0%) |
T1 (0.5%) |
T2 (1.0%) |
||
|
Dry matter% |
7.69±0.36a |
7.9±0.14a |
8.5±0.28a |
8.35±0.35a |
| Crude protein% |
58.10±0.5c |
59.35 ±0.21b |
63.50±0.42a |
59.2 ±0.14b |
| Crude fat% |
8.20±0.14a |
8.40±0.28a |
7.50±0.42a |
8.25 ±0.35a |
| Ash % |
17.4± 0.14a |
18.4±0.14a |
17.55±0.49a |
17.55 ±0.35a |
| Crude fiber% |
0.78±0.09a |
0.75±0.07a |
0.82±0.03a |
0.85±0.07a |
*Figures with different superscripts are significantly different (P≤0.05).
Table IV.- Statistical analysis of apparent nutrient digestibility.
| Parameters |
Control (0.0%) |
T1 (0.5%) |
T2 (1.0%) |
|
Crude protein(CP) |
13.05a±1.62a |
14.15±3.74a |
17.40±4.38a |
| Fat |
2.31±0.14a |
2.35±0.01a |
2.52±0.00a |
Physico-chemical parameters
The statistical analysis of water quality parameters during the experimental trial showed non-significant (P≤0.05) difference. The temperature of water in glass aquaria at different feeds i.e. control, T1 and T2 were 26.82±0.013°C, 26.35±0.068°C and 26.68±0.054°C, respectively. The dissolved oxygen in the water was measured as 4.634±0.178, 4.585±0.341 and 4.750± 0.257 mgL-1 in control, T1 and T2, respectively. The total dissolved solids (TDS) in each aquarium were noted as 1243.03±0.479 mgL-1 in control, 1230.56±11.54 mgL-1 in T1 and 1208.15±2.719 mgL-1 in T2. The salinity of water in aquaria with different feeds showed statistically significant difference (P≤0.05) in control (1.051±0.001) and T2 (1.025±0.012). The pH was measured as control 7.67±0.013, T1 (7.65±0.005), T2 (7.56±0.034) with non-significant differences among treatments (Table V).
Table V.- Physico-chemical parameters of aquariums water of Pangasius hypophthalmus under various treatments.
| Parameters |
Control (0.0%) |
T1 (0.5%) |
T2 (1.0%) |
|
Temperature (◦C) |
26.82±0.01a |
26.35±0.07a |
26.68±0.05a |
|
Dissolved oxygen (mgL-1 ) |
4.63±0.178a |
4.585±0.34a |
4.750 ±0.26a |
|
Total dissolved solids (TDS)(mgL-1) |
1243.03 ± 0.48a |
1230.56± 11.54a |
1208.15 ± 2.72a |
| Salinity (ppt) |
1.051±0.00a |
1.098 ±0.01a |
1.025 ±0.01b |
| pH |
7.67±0.01a |
7.65±0.00a |
7.56±0.034a |
DISCUSSION
The survival rate in present study was observed 80% in control and 90% in T1 and T2. Results of our study are supported by the findings of Abdel-Zaher et al. (2009) who fed different levels of fenugreek seeds meal (FKSM) to Nile tilapia and reported survival rate 93.33% to 100% in treated groups. Statistically significant (P≤0.05) difference in growth parameters were observed among the three groups. Highest weight gain (108.98±0.722), length increase (24.250±4.313) and %weight gain (926.97±26.316) were observed in T2 which indicated that 1.0% addition of fenugreek in fish feed improves the growth performance. Better feed conversion ratio (FCR) 1.8 and specific growth rate 1.4 were observed in T1 (0.5%). The results of our study confirmed the findings of Abdel-Zaher et al. (2009) who reported that fish fed diets containing 1% fenugreek seed meal (FKSM) significantly improve (P<0.05) growth performance (body weight: weight gain, % weight gain, SGR%) in mono-sex fingerlings of Nile tilapia Oreochromis niloticus. Similarly, Awad et al. (2015) observed the effect of the addition of fenugreek 0% (control), 1%, 5% and 10% for four weeks on gilthead seabream (Sparus aurata L.) and reported significant effect on the growth parameters (final weight, weight gain, specific growth rate and length) on the experimental fish. Shaikhlar et al. (2011) replaced fish meal (FM) with fenugreek seed meal (FSM) in the feeds for P. hypophthalmus and observed optimum growth performance and feed consumption with the replacement of FSM (30%) in the diet of African catfish. Roohi et al. (2015) studied fenugreek effect on common carp (Cyprinus carpio) and reported significant higher weight gain, specific growth rate and better FCR in treated than control.
Proximate analysis of current study revealed significant differences among treated and control groups for crude protein contents while non-significant differences observed in crude fat, moisture, ash and fiber contents. Abdel-Zaher et al. (2009) fed different levels of fenugreek seeds meal (FKSM) to Nile tilapia and reported non-significant difference in dry matter, protein, lipid and ash content. Abdelhamid and Soliman (2012) studied the effect of dietary levels (0, 1, 2%) of fenugreek seed meal (FSM) or cresson seeds meal (CSM) on Nile tilapia fry growth performance and stated that FSM significantly improved the feed utilization in the form of protein productive value and energy retention. It also significantly improves fish carcass protein percent with the addition of 2%. Similarly, some researchers reported that use of medicinal plants (garlic, onion and garlic, Allium sativum and Thymus vulgaris) in aquaculture diet often improves fish nutrient composition (El-Saidy and Gaber, 1997; Zaki and El-Ebiary, 2003; Attalla, 2009).
The results of apparent nutrient digestibility revealed that protein digestibility (20.5±5.06%) and fat digestibility (2.5229±0.756) were highest in T2 as compared to T1 and control. These results are similar to the results of digestibility study conducted by Sotolu and Sule (2011) who found that apparent digestibility coefficient (ADC) of protein and energy were the highest in traditional diet followed by leaf meal diet.
The physico-chemical parameters play significant role in the growth of poikilothermic animals such as fish. All the parameters were found within acceptable range other than temperature which showed fluctuations that might be due to addition of freshwater. Other water quality parameters were recorded throughout the study period and were within the acceptable ranges.
Conclusion
In conclusion, it has been observed that fenugreek is an important feed additive that can enhance fish growth, survival and digestibility of protein coefficient index without any significant effects on fish nutrient profile. It has been recommended that P. hypophthalmus can be fed 1.0% supplemented diets of fenugreek can significantly enhance growth and digestibility of nutrients.
Acknowledgements
The authors are thankful to the Department of Fisheries & Aquaculture, UVAS for providing facilities for conducting experiment and analysis and Higher Education Pakistan (HEC) for providing financial support for this study conducted under research project “Prospects of culturing and breeding of Catfish (Pangasius pangasius) in Pakistan”, project #20-2219/NRPU/R&D/HEC/12 4231 Rs. 3.954 million.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abdelhamid, A.M. and Soliman, A.A.A., 2012. Possibility of using Fenugreek seeds or Cresson seeds in tilapia diets. J. Arab. Aquacul. Soc., 7: 75-90.
Abdel-Zaher, A., Mostafa, Z. M., Ahmad, M. H., Mousallamy, A. and Samir, A., 2009. Effect of using dried Fenugreek seeds as natural feed additives on growth performance, feed utilization, whole-body composition and entropathogenic Aeromonas hydrophila-challinge of monsex Nile tilapia O. niloticus (L) fingerlings. Aust. J. Basic appl. Sci., 3: 234-1245.
Ahmad, A., Alghamdi, S.S., Mahmood, K. and Afzal, M., 2016. Fenugreek a multipurpose crop: Potentialities and Improvements. Saudi J. biol. Sci., 23: 300-310. https://doi.org/10.1016/j.sjbs.2015.09.015
Ahmed, I.E., Noor, E.I.D. and Mahmud, R.A., 2009. Application of some medicinal Plants to eliminate Trichodina sp. in tilapia (Oreochromis niloticus). Rep. Opin., 1: 1-5.
Attalla, R.F., 2009. Influence of some feed additives on growth rates and physiological measurements of blue tilapia (Oreochromis aureus). Egypt. J. aquat. Res., 35: 231-241.
Awad, E., Cerezuela, R. and Esteban, M.A., 2015. Effects of fenugreek (Trigonella foenum graecum) on gilthead seabream (Sparus aurata L.) immune status and growth performance. Fish Shellf. Immunol., 45: 454-464.
Bondad-Reantaso, M.G., Subasinghe, R.P. and Arthur, J.R., 2005. Disease and health management in Asian aquaculture. Vet. Parasitol., 132: 249-272. https://doi.org/10.1016/j.vetpar.2005.07.005
Castleman, M., 1991. The healing herbs: The ultimate guide to the curative power of nature’s medicines. Bantam Books, NY, USA.
Das, S., Mondal, K. and Haque, S., 2017. A review on application of probiotic, prebiotic and synbiotic for sustainable development of aquaculture. J. Ent. Zool. Stud., 5: 422-429.
Duke, J.A., 2002. Handbook of medicinal herbs, 2nd Ed. CRC Press, USA. https://doi.org/10.1201/9781420040463
El-Saidy, D.M.S.D. and Gaber, M.M.A., 1997. Effect of different levels of dry garlic meal supplemented to the diets on growth performances, survival and body composition of Nile tilapia (Oreochromis niloticus) fingerlings. Annls. Agric. Sci., Moshtohor, 35: 1197-1209.
Furukawa, A. and Tsukahara, H., 1966. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull. Jpn. Soc. Sci. Fish., 32: 502-506. https://doi.org/10.2331/suisan.32.502
Gültepe, N., Bilen, S., Yılmaz, S., Güroy, D. and Aydın, S., 2014. Effects of herbs and spice on health status of tilapia (Oreochromis mossambicus) challenged with Streptococcus iniae. Acta Vet. Brno, 83: 125-131. https://doi.org/10.2754/avb201483020125
Hannana, J.M.A., Rokeya, B., Faruque, O., Nahar, N., Mosihuzzaman, M., Khana, A.K. and Alia, L., 2003. Effect of soluble dietary fiber fraction of Trigonella foenum-graecum on glycemic, insulinemic, lipidemic and platelet aggregation status of Type 2 diabetic model rats. J. Ethnopharmacol., 88: 73-77. https://doi.org/10.1016/S0378-8741(03)00190-9
Iqbal, K.J., Ashraf, M., Abbas, F., Javid, A., Hafeez-Ur-Rehman, M., Abbas, S., Rasool, F., Khan, N., Khan, S.A. and Altaf, M., 2014. Effect of plant-fishmeal and plant by-product based feed on growth, body composition and organoleptic flesh qualities of Labeo rohita. Pakistan J. Zool., 46: 253-260.
Madar, Z. and Stark, A.H., 2002. New legume sources as therapeutic agents. Br. J. Nutr., 88: S287-S292. https://doi.org/10.1079/BJN2002719
Martinez, S., Navalon, J.L., Ortiz, A., Montalvo, G., Ferrero, J.L. and Gonzalez, A., 2003. The use of NIRS to predict chemical composition of ungrounded poultry feeds. 14th Eur. Symp. Poult. Nutr., Aug. 2003, Lillehammer, Norway, pp. 91-92.
Moradi kor, N. and Moradi, K., 2013. Physiological and pharmaceutical effects of fenugreek (Trigonella foenum-graecum L.) as a multipurpose and valuable medicinal plant. Glob. J. med. Pl. Res., 1: 199-206.
Ody, P., 1993. The complete medicinal herbal. Dorling Kindersley, New York.
Pandian, R.S., Anuradha, C.V. and Viswanathan, P., 2002. Gastroprotective effect of fenugreek seeds (Trigonella foenum graecum) on experimental gastric ulcer in rats. J. Ethnopharmacol., 81: 393-397. https://doi.org/10.1016/S0378-8741(02)00117-4
Pandiyan, P., Balaraman, D., Thirunavukkarasu, R., George, E.G.J., Subaramaniyam, K. and Manikkam, S., 2013. Probiotics in aquaculture. Drug Invent. Today, 5: 55-59. https://doi.org/10.1016/j.dit.2013.03.003
Platel, K. and Srinivasan, K., 2000. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung, 44: 42-46. https://doi.org/10.1002/(SICI)1521-3803(20000101)44:1<42::AID-FOOD42>3.0.CO;2-D
Roohi, Z., Imanpoor, M. R., Jafari, V. and Taghizadeh, V., 2015. The use of fenugreek seed meal in fish diets: growth performance, haematological and biochemical parameters, survival and stress resistance of common carp (Cyprinus carpio L.). Aquacul. Res., 48: 1209-1215. https://doi.org/10.1111/are.12962
Sahalian, M.D., 2004. Diosgenin, asteroid saponins of Trigonella foenum graecum (fenugreek), inhibits azoxymethane- induced aberrant crypt foic formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidermiol. Biomark. Prev. Aug., 13: 1392-1398.
Sauvaire, Y., Petit, P., Broca, C., Manteghetti, M. and Ribes, G., 1998. 4-hydroxy leucine: a novel amino acid potentiator of insulin secretion. Diabetes, 47: 206-210. https://doi.org/10.2337/diabetes.47.2.206
Shaikhlar, A., Alimon, A.R. and Daud, M.H., 2011. Effect of replacing fish meal by fenugreek seed meal on growth performance of African cat fish. In: 4th International conference on Animal nutrition (4th ICAN 2010): Sustainable feeds – Maximizing productivity and profitability, 21-23 September 2010, Persada Johor International Convention Centre, Johor Bahru, pp. 277.
Sotolu, A.O. and Sule, S.O., 2011. Digestibility and performance of water hyacinth meal in the diets of african catfish (Clarias gariepinus; BURCHELL, 1822). Trop. Subtrop. Agroecosyst., 14:245 –250.
Zaki, M.A. and El-Ebiary, E.H., 2003. Effect of incorporation of onion and garlic into diets on growth performance and body composition of mono-sex Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish., 7: 113-126.
To share on other social networks, click on any share button. What are these?










