An Overview of Bacterial Lipases and their Enormous Applications
An Overview of Bacterial Lipases and their Enormous Applications
Ghalia Nawal1, Muhammad Irfan2*, Saira Ashfaq3, Hafiz Abdullah Shakir1
1Department of Zoology, University of the Punjab, New Campus, Lahore, Pakistan.
2Department of Biotechnology, University of Sargodha, Sargodha, Pakistan.
3Department of Biotechnology, Lahore College for Women University, Lahore, Pakistan.
Abstract | Lipases are reputably honored as cleavers of fatty compounds that have been recognized from innumerable organisms. Among microbial lipases, the bacterial lipases holds very prominent stance. The specificity of lipases is far-fetched for their point selection and kinds of reactions they undergo on their discernible substrates. Most suitable production plan involves Sub-merged liquid fermentation in fed batch manner using agricultural wastes. Bacterial lipases are preferred due to their extra cellularity, easy step down approach and practicability of increased productions via technological modifications. They have wide- ranging applications in diverse sectors of the food, medical, cosmetic, detergent sectors and in paper industry which establishes their irrefutable noteworthiness.
Article History
Received: August 05, 2018
Revised: April 20, 2019
Accepted: May 08, 2019
Published: June 21, 2019
Authors’ Contributions
GN reviewed the literature and wrote the manuscript. MI and HAS revised the draft critically. SA helped in literature review.
Keywords
Bacteria, Lipases, Production, Commercialized applications
Corresponding author
Muhammad Irfan, [email protected]
To cite this article: Nawal, G., Irfan, M., Ashfaq, S. and Shakir, H.A., 2019. An overview of bacterial lipases and their enormous applications Punjab Univ. J. Zool., 34(1): 61-71. http://dx.doi.org/10.17582/journal.pujz/2019.34.1.61.71
Introduction
Enzymes – The tiny proteinaceous biomachines that come in various orders of forms, shapes, sizes and names; each endeavoring to boost up a certain chemical reaction of our metabolism, work continuously but specifically until a cascade of desired product is acquired without being ended and still available for the next series of reactions.
Word lipases stands for an assembly of catabolic enzymes that principally acts on lipids (a broad category of compounds that are made up of fatty acids possessing anester that is hydrolyzed by lipases) to rip them apart setting off lipolysis. They were first recognized from bacterial origin in start of 19th century and with advancement in scientific knowledge they were given an enzyme commission number 3.1.1.3. The digit 3 in beginning shows they belong to serine hydrolases family (Aravindan et al., 2007).
The substrate of lipases is the hydrocarbon back bone of organic fatty compounds and upon catalysis by means of hydrolyzing ester part they engender minor free building blocks of fatty acids in addition to Triglycerides and glycerol.
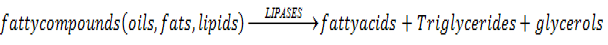
The catalytic site of lipases has special arrangement of amino acids; it begins and ends with Glycine with respective histidine and glutamic acid on sides of middle serine as G-X-S-X-G as described in Figure 1. Analysis of its 3D configuration reveals that it has an oxyanion hole lying about seventy to hundred amino acids in advance of this site and transuding sequence consisting of thirty five amino acids is sited ten to forty spaces forward to this hole. The common molecular weight is 40-50 kilo Daltons (Kwoun Kim et al., 2004).
Concerning their chemical properties lipasesare reported to catalyze transesterification, interesterification, esterification, acidolysis, aminolysis, alcoholysis types of chemical reactions, working on wide-ranging substrates with high specificity (Houde et al., 2004). Lipases illustrate immense point and location selectivity for bond fissure and formulation during catalysis.
Sources
Lipases are universal in origin. They exist in the majority of living organisms, from largest visible human beings to smallest invisible microbial organisms like bacteria. Since the inception of biotechnology, gradually the noteworthiness of enzymes and their production has become irrefutable. These enzymes producing organism can be either of fungal or bacterial origin. They are reported as intracellular enzymes in spit and Gastrointestinal tract whereas extracellular liposome within humans. There are grouped according to their Physiological locality. Other than animals, some researchers have also reported presence of lipases in vegetation of bean family (Gupta et al., 2004).
They are ideally recovered from microbial means through a tone down approach that withdraw lipases after culturing them on a larger scale. Among microbial lipases, lipases gained from fungal sources are favored but the bacterial lipases are also employed up to a greater extent in commercialization because of their extracellular secretion. Pandey et al. (1999), Jaeger et al. (1999) has reported following bacterial species which are significant producers of lipases are :ten species of Achromobacter, Acinobacter, Alcaligenes, Arthrobactor, Eleven species of Bacillus, Burkholderia, chromobacterierium, Proteus, five species of staphylococcus, nine species of Pseudomonas. Out of all, the later is much more reputable for lipases production than anyone else.
Production
For producing lipase on larger scale, starter culture of bacteria are grown in fermentation reactors with addition of growth supporting sterilized medium and monitoring of physical and chemical factors like agitation for appropriate amalgamation, aeration for suitable oxygen supply, pH harmonization via acids and bases, apt temperature and moisture echelon. Medium includes a variety of nutritional elements like carbon, nitrogen, electron donors, hydrogen ions some vitamins and metals ions as coenzyme to accelerate bacterial growth and primary metabolites so that more enzyme production takes place during the growth phase by as secondary metabolites (Vargas et al., 2008).
According to the amount of water used in fermentor; there are two manners of carrying out fermentation i.e. culturing of microbes in controlled and monitored conditions, first one is called solid state fermentation SSF that is mostly suited for fungal growth or filamentous organisms that necessitate very minute moisture level (water), this process is reasonably cost-effective and trouble-free to control whereas the second is submerged liquid fermentation (SLF) for mushrooming bacterial cells necessitating more moisture level, even though it is pricey but step down approach is unproblematic (Pandey, 2003).
Many researchers have reported enhanced lipase production employing (SSF) by using agricultural waste as nutritional delivery which reduces the cost effect as well (Imandi et al., 2010; Vargas et al., 2008; Kempka et al., 2008; Alkan et al., 2007; Mala et al., 2007). Whereas Submerged Liquid Fermentation has been also employed for production (Thakur et al., 2014; Pinheiro et al., 2008; de Azeredo et al., 2007; Falony et al., 2006). These fermentations manners are drive in batch (taking out the entire product upon completion of session), Fed-Batch (taking out product and inoculating at intermissions) and continuous, in chorus addition of new media and taking out of formed products (Thakur, 2012). It is common observation that the later approach significantly time saving, less prone to contamination and cheaper. It has proved a 5.67 IU/ml, fold raised yield of lipases (Li et al., 2005).
Improved production
Since breakthrough of lipases on industrial scale researchers are incessantly working on improving the lipase yield, their distinctiveness of competence through amendments in their properties and existing and novel strains and this all is to be indebted to contemporary biotechnologies. One of most frequent method in this regard is site-directed mutagenesis (monitored mutation induction at specific sites of genome) by means of radiations and chemicals (Bapiraju et al., 2004). More than an one hundred and tenfold enhancement in catalytic performance of lipases from mutant sources as compared to the ordinary source has been achieved using NTG mutation at random locations on DNA have comprehensively converse about better production of lipases via technological manipulation (Shu et al., 2010). They have explained the employment of homologous expression for obtaining twenty three percent additional yields of pseudomonas lipases by increasing the number of lipase coding regions introduced in expression vector for replication. Ahn et al. (2001) reported manufacturing lipases together with their respective group of proteins in designed recombination gives seventy percent more production. Another policy of getting more lipases is to produce them in absence of lipase slicing entities (proteases) so that more intact enzymes can be recovered (Takahashi et al., 1999). Similarly the insertion of potent transcription maker sequences and coalescing chaperons synthesis has encouraging influences on lipase production (Liu et al., 2006). Additionally ,monitoring triplet gene codes for enhanced productions has resulted in much greater yield of lipases (Chang et al., 2006). Adding acacia gum in cell growth medium also improves the expression of bacterial lipases (Martinez and Nudel, 2002).
Factors affecting lipase production
Amplified yield of lipase depends on optimization of fermentation conditions. Therefore, factors like carbon and nitrogen sources, temperature, incubation period, pH and agitation speed matter the most (Ugo et al., 2017).
Carbon and nitrogen sources
Most excellent substrates for lipases are various classes of organic compounds like oils, lipids and fats of all sorts. Mostly carbon acts as a limiting factor for the synthesis of lipases. Highest yield of lipases for B.subtilis KPL13 was observed with olive oil, tributyrin and glucose respectively when used as carbon source (Veerapagu et al., 2013).Organic nitrogen sources are well suited for increased lipase production. For P.stutzeri cotton seeds provide best supply of Nitrogen and carbon respectively (Thakur et al., 2014). Bacillus spp. and Pseudomonas spp. reveal enhanced growth with use of peptone or alternatively yeast as nitrogen source (Lanser et al., 2002). Peptone was used as nitrogen sources for lipase production (3.7U/ml) from B.subtilis KPL13 using peptone (Veerapagu et al., 2013).
Temperature
Temperature significantly affects flourishing of bacteria in fermentation medium. An optimum temperature of 36oC is reported for Bacillus and Pseudomonas species (Priya and Reddy, 2015). Thakur et al. (2014) have reported 86 Fahrenheit as optimal for maximum lipase output from P.stutzeri. While optimum temperature for P.gessardii has been reported as 37oC (Veerapagu et al., 2013). Even though lipase producing bacteria belong to mesophilic temperature range they show appropriate growth between twenty to forty five degrees centigrade but with increasing warmth an increase in synthesis is reported, higher temperature at which first-rated growth of bacterial lipase takes place is reported fifty degrees centigrade by Sharma et al. (2002).
Incubation period
From smaller laboratory scale fermentation to larger reactor scale production requires incubation; this time varies from various hours to days depending upon the fermentation type and microbe used. Incubation periods are slightly longer in SSF. Imandi et al. (2010) has reported 14 days of incubation in SSF reactor with 86F temperature, pH of 6.8 and sixty percent of humidity for optimized production. Whereas incubation period of 3 days was reported for Bacillus strains (Priya and Reddy, 2015; Kumar and Valsa, 2007). Mahler et al. (2000) has reported an incubation duration of twelve hours works finest for Bacillus spp. while an incubation duration of three to four days in case of Pseudomonas sp.
Agitation speed
Speed at which reactor media is stirred increase the rate of lipase production as it results in uniformity of substrate distribution, providing more surface area and greater rate of oxygen transfer (Gulati et al., 2000). A range of 110-160 rpm is usually employed by different researchers (Veerapagu et al., 2013). The optimum speed for maximum production by Pseudomonas and Staphylococcus species has been reported as 160 rpm (Priya and Reddy, 2015; Sirisha et al., 2010).
pH
Bacterial growth is significantly affected by the pH of growth medium, which ultimately influences the products being harbored. Although, bacteria can produce lipases in a pH range from 4-10 but studies have reported that initial pH is much more significant (Ugo et al., 2017). pH 6± 0.5 has been reported for higher yields of lipases from Bacillus, Pseudomonas, Klebsiella and Corynebacterium strains (Bharathi et al., 2018). Optimized pH for lipase produced by P.gessardi was 7.0 (Veerapagu et al., 2013). In a similar study, pH 7.0 has been stated as optimized for Bacillus species (Priya and Reddy, 2015).
Table 1 Summarizing the yields and fermentation modes with respect to bacteria
Table 1: Summarizing the yields and fermentation modes with respect to bacteria.
|
Bacterial Genus |
Mode of Production |
Substrate |
Enzyme Units (units/ml) |
Reference |
|
Pseudomonas luteola |
SMF |
Olive oil |
2059 |
(Litthauer et al., 2002) |
|
Pseudomonas Pseudomalleli 12Sm |
SMF |
Hexadecane |
69 |
(Kanwar and Goswami, 2002) |
|
Pseudomonas |
SMF |
Coconut oil |
960 |
(Rathi et al., 2001) |
|
Bacillus alcalophilus |
SMF |
Soyabean Starch Yeast Extract |
59.4 |
(Ghanem et al., 2000) |
|
BacillusLBN2 |
SMF |
Ground nut oil |
2394 |
(Bora and Bora, 2012) |
|
BacillusSpp.(locally collected) |
SSF |
Mustard Oil cake and wheat Bran |
20 |
(Bokhari et al.,2013) |
|
Serratia mercescens |
SMF |
Tributyrin emulsion |
380.6 |
(Abdou, 2003) |
|
Serratia mercescens |
SSF |
Olive Oil |
11.94 |
(Gupta et al., 2013) |
|
Acinetobactorradioresistens |
SMF |
Synthetic (Tween 80) |
84.5 |
(Neelambari et al., 2011) |
Purification
During cultural growth the lipases are secreted extracellularly otherwise intracellularly; either in very last of log or at beginning of the equilibrium phase (Shah and Bhatt, 2011). Depending upon location type of product synthesized. The first step is to take apart the cell biomass (CBM) in case of intracellular product or the supernatant in case of extracellular product from fermentation medium by sedimentation (through settling down the CBM) or flotation, for supernatants or cell pellet ultracentrifuges are used, afterwards mechanical or non-mechanical ways are employed to break cells leading to clarification using filtration and concentrated products are obtained by several techniques like: precipitation via Ammonium Sulphate or solvent and Gel Chromatography. At last final product is preserved through drying and crystallization (Waites et al., 2009).
Shah and Bhatt (2011) has reported the following steps of purification of lipases from Bacillus spp. as: cell separation achieved by a centrifugation at 10,000 rpm, 15 min to acquire supernatant and icy acetone precipitation using sixty percent of it for the night and recuperating lipase by subsequent centrifuge as well as drying. Impurities and contaminations were removed by dialysis using forty percent solution of sucrose. For higher resolution cellulose column with pore size of G-100 was used for purifying analyte in an ion-exchange way of chromatography. Use of sepharose column has also been reported by (Nawani and Kaur, 2000)
Characterization
All enzymes are naturally designed to function at some set parameters; which severely influence the effectiveness of enzyme activity. All the factors that affect production of lipase actually characterize the lipases as well. Other factor that contributes to chemical properties and characterization of lipases are:
Metal ions
Lipolytic action of bacterial lipases is observed to be increased as well as decreased by certain bivalent metal ions. Since, certain lipases are metalloenzymes therefore effect of these should be considered (Ugo et al., 2017). Metal ions which cause an augmented performance are calcium and Magnesium while the one which reduce this activity are Zinc and copper (Ran et al., 2015). In the middle of a variety of metal ions tested as inducer of lipases, five Molar concentration of CaCl₂ was found to give highest expression with exposure to air at 0.4 liters per minute at an incubation of 2 days (Thakur et al., 2014). The lipolytic activity of lipases obtained from P.aeruginosa was reported to be reduced with high concentrations of metal ions like Hg+, Pb+2, Al+3, Zn+2 and Fe+3 (Bakir and Metin, 2016) while it was reported to be increased with Ca+2 (Borkar et al., 2009).
pH, Metal chelators and Surfactants: The pH of fermentor in which culturing is taking place is very important for regulating the growth of any enzyme, same is true for lipases. Bacterial lipases is most excellently formed at neutral pH i.e. 7.0, although at slightly higher pH upper limit of production is reported but this is critical (Barbaro et al., 2001). Ran et al., (2015) has reported lipase from Acinetobacter show excellent catalysis at slight basic pH 8.0 and trivial higher temperature 40oC.
Shah and Bhatt, (2011) have reported an investigation of pH range from strong acidic 4 to moderately basic 10 but the maximum lipolytic activity of lipases was marked at nearly neutral 7.4 at 98.6 Fahrenheit temperature for bacterial lipases from Bacillus subtilius. while Staphylococcus spp. are reported to work best at weak acidic pH 6. Among inhibiters of their lipolysis capabilities significant was the effect of EDTA and among the agents that arouse this capability were Magnesium and calcium metals. Other substances that affect are some surfactants like Tween and existence of fatty compounds of lipid category (Rathi et al., 2001).
Table 2: Physical and chemical optima of bacterial lipases.
|
Bacteria |
Opti-mum pH |
Opti-mum temp |
Mole-cular weight |
Metal ion effect |
KineticsKm=mM, Vmax= µM/min, Kcat=S-1 |
Reference |
|
Bacillus spp. (lipase gene expressed in E.coli) |
7 |
19.85 |
Not menti-oned |
Ca+ activate |
Km=66.68 Vmax=400 Kcat=228 Kcat/km= 3.4×106 |
(Masuch et al., 2015) |
|
Bacillus sp.J33 |
8 |
70 |
45KDa |
Inhibited by Hg+ |
Km=2.5 Vmax=0.4 |
(Nawani and Kaur,2000) |
|
Bascillusthe-rmolevorans (BDIT A) |
9 |
60-65 |
18 KDa |
Inhibited by cu+, co+, Hg |
Km=1.82 Vm=12.8 |
(Lee et al., 2001) |
|
Bascillusthe-rmolevorans (BDIT B) |
9 |
60-65 |
43 KDa |
Ca+, Co+, Mn+ activate |
Km=6.24 Vmax=63.3 |
(Lee et al., 2001) |
|
Pseudom-onas spp. |
7 |
40 |
Not Mentioned |
Ca+, Mg+ activate |
Km=0.50±0.05 Vmax=128.16 |
(kermanshahi et al. 1998) |
|
Pseudom-onas Pseuom-allei12 Sm |
10 |
35 |
143KDa |
Ca+ has no effect |
Km=178±12 Vmax=172±8 Kcat=409±10 Kcat/Km=2.3 |
(Kanwar and Goswami,20002) |
|
Pseudomonas spp. |
11 |
90 |
Not Mentioned |
Not Mentioned |
Km=40mg/ml |
(Rathi et al., 2000) |
Even though colossal research has been done on lipases but only a few articles have discussed the Enzyme kinetics of bacterial lipases, the published data mainly of fungal lipases has been more generous in this regard. Each bacterial lipase catalyzes the specific reaction of its own kind at a specific rate of Km, Vmax and Kcat numbers as following:
Table 2 abridging the Physical & chemical optima of Bacterial lipases
Applications
Lipases stand amongst the most significant enzymes from biotechnological point of view because of their all-encompassing utilization in most of sectors concerning human beings and quality of their life by sharing five percent of the total enzyme demand (Vakhlu, 2006). Immobilized as well as silcoat enzymes are more ready to lend a hand for large scale applications (Mohamad et al., 2015).
Food sector
Fat monitored quality products: Lipases are capable of boosting interesterification reaction that promises dawdling rancidity, altered melting points, and nutritionally healthier oils with less saturated fat contents that can be adopted more easily for cooking. This type of hydrolysis reaction occurs optimally at 0.9 water to lipase ratio, products diffuse from bulk phase by a water saturated oil phase (Kuo and Gardner, 2002). Sales of such products throughout the globe annually crosses billion.
Flavor enrichment: In common terms flavor enrichment can be described as making edible items more superior in taste and nutrition by the means of definite flavor enhancers in food industry that is why the gross sale of these agents crosses billions. Lipases are one of these flavor enriching enzymes that can generate the esters by removing water molecules and squeezing the atoms of alcohol and acid together in compact form. Esters are prevalently known for increasing the zest of milk made items and processing of other foodstuffs (Rajendran et al., 2009). For this purpose, out of many esters created are a Hexyl acetate that confer distinctive fruit heady scent, this compound is tasteless and green in color (Shieh and Chang, 2001). Another ester of these category is Hexyl butyrate widely known as Natural flavor enrichment compound made through gentle transesterification of a six carbon alcohol along with tributrin, using lipozyme IM-77 obtained from fungal sources for treatment of edible food and drinking items (Chang et al., 2003).
Baking: In all sorts of bakery items like from cakes, biscuits, cookies to puddings and pie, Eggs are extensively used for their fat content that imparts aromatic flavor and act as a binding, moisture giving and leavening ingredient. Phospholipases (PLs) are used in baking sector for their hydrolytic action whose primary purpose is to improve the quantitative and qualitative aspects of dough, employed in baking. These PLs interact with protein components of eggs like lecithin and act as biocatalyst in their hydrolysis which results in greater emulsifying role and more thermo-resistance to increased temperature (Reimerdes et al., 2004). Increased stability and improved texture of the dough is made with dried egg yolks which were pretreated with Phospolipases A2 as compared to non-treated with these PLs (Zhao et al., 2010).
Dairy trading: Their key intervention in dairy industry is as hydrolytic agents for dropping the level of fat part of milk as well as to amend the length of fatty acids. Lipases also confer cheesy aroma to manifold cheeses with hastened time of cheese ripening. Customary devices for this purpose are pancreatic glands of animal origin that are mostly bovine tissues. They also possess the competence to substitute the lipases of pre-gastric origin A large number of dairy products (like butter, curd, cream and fats) are treated by their hydrolytic reactions to attain the desired properties through the process of lipolysis, this generates free TAGs, esters and fatty acids that yield diverse dairy products further. A class of such lipases is reported from a number of bacteria (Rajendran et al., 2009).
Fatty acids which are cleaved and released during this catalysis turn out flavor enriching products most likely beta keto acids and lactones. Addition of lipases in experimental cases is the reason of speeding up the release free fatty acids in contrast to control groups under observation (Rooney and Weatherley, 2001). Enzyme Modified Cheese (EMC) is the revolution that lipases together with pack of other enzymes have brought in dairy industry. According to the preferred aroma needed, cheese without any prior treatment is incubated with particular lipases at amplified temperature ranges to perform catalysis to ensure intense aroma and fat percentages.EMC has tenfold greater fat content than normal cheese (Rajendran et al., 2009).
Commercial tea leaves preparation: The inborn flavor of tea usually drank in Asian countries, comes from the type of plant and mode of preparation employed in processing tea leaves. Starting from picking leaves it involves drying and breakdown of leaves with help of lipases for oxidation and exclusion of water, which is made through the help of lipases due to their hydrolytic capacity (Ferreira-Dias et al., 2013).
Medical sector
Various enzymes are deliberately operated to function preferably in organic media rather than using water this is because extraction of products which are further to be used as drugs become quite laborious and is a lengthy procedure whereas organic reaction media like lipases are handy to use for this purpose since years, in manufacturing drugs because of ease in product recovery and purification. Lipases are employed for multipurpose functions: as non aqueous media and as biocatalysts for leading transestrification, resolution of chiral substrates and compounds with basic amide structure, acetylation, bond making abilities as selective points within drug complexes (Gotor-Fernández et al., 2006).
For drug assembly lipases take part in quickening the transfer of acyl groups between a donor (ester) and acceptor (such as amines) resulting in establishment of racemized products through ping pong course of action (Päiviö and Kanerva, 2013). Successful production of up-to-the-minute category of Profens that fit in to the Non steroidal anti-inflammatory drugs is synthesized with help of these lipases that in their action is more efficient than it its parent molecule in S-enantiomer was done in late nineties and revived currently by Zhang et al. (2005) with better output. Furthermore now in the field of nanomedicine researchers like (Khan et al., 2017) has demonstrated the dilapidation of artificial polyesters after successful drug administration by lipases of lactobacillus origin. Currently Trousil et al. (2017) have shown the improved released of Rifampicin after dilapidation of polymers by bacterial lipases.
Lipases elicit the discharge of Necrosis factors that degrade the growth of abnormally developed cancerous cells (Hasan et al., 2006). Liposome are also extensively investigated and are still an emerging research horizon for the drug transportation and deliverance at targeted sites, they are accompanied with drug together with lipases to shun bio-inactivation and detoxification during the route and within the body of organisms. Streck et al. (2016) used mechainism of phase switching for such lipid supported drug transportation through injecting the drug Benznidazole. Nutraceuticals are the derivative compounds of edible sources with eminent therapeutic benefits owing to their diet related favor, are reported to obtained with help of new lipases of bacterial origin (Jaeger and Eggert, 2002)
Biosensing
The term biosensing was introduced in 1999 and it can be defined as checking the existence or determining the amount of a certain biomolecule with help of a biosensor within living organism for investigating medical reasons (Loo, 2015). Biosensors are combination of a bimolecular probe with quantifying unit that can display results in digital way. This sector has now gained a tremendous legendary in every field influenced by humans and their environment. For health care purposes and routine monitorings lipases are used for tracking Triglycerides (TG) intensity plus the amount of cholesterol and fats within blood (Herrera-López, 2012).
(Califano et al., 2014) has well described the model of silicon supported lipase biosensor that can serve medical purposes well and this model has employed MAPLE technique for settling of lipases. Beside investigation of TG solely in blood this function can also be performed for the same purpose in food sector, rather than determination in biological fluids only. This holds multipurpose applications in improving the quality of edibles. Such a sample based lipases biosensor is developed by (Escamilla-Mejía et al., 2015). In addition to measuring TG and cholesterol lipases bases biosensing can also detect level of some insect killing chemicals like organopesticides via pH fluctuation produced in biosensor meanwhile investigating samples (Kartal et al., 2007). Recently this has proven useful in measuring many pesticide concentrations within aquatic habitats as a hall mark of water quality and maintaining quality and monitoring ecology of water (Reddy et al., 2014).
Macromolecular science
Massive and heavy weighted large scale polymers, polyesters and biodegradable stuff is manufactured by unification of recurring smaller subsets of molecules. Plastic and fibers are the biggest hallmark of this sector but they are a necessary evil as they are beneficial for mankind on the other hand they are hazardous for our surroundings. To maintain greener environmental conditions degradation of these artificially synthesized products is necessary; this is primarily done with help of decomposers that include fungi and bacteria. They release extracellular enzymes that shatter these macromolecules; these enzymes are lipases in particular as they are catalytic in characteristic. Polyurethane (PAE) and polycaprolactone (PCL) is reported to be degraded by lipases of bacterial origin (Tokiwa et al., 2009).
Biodiesel manufacturing
Compared to the diesel fuel consumed in general, it is renewable, environment friendly and safer to use. Its fundamental ingredients are fatty acids together with methyl esters derived from sources of animal and plant origin. Lipases are engaged in its production because of their ability of carrying out transesterifications as catalysts. Meka and Tripathi (2007) have reported its optimum production with 2% catalyst using 1 ratio of safflower oil with 6 ratio of alcohol acquiescing 96.8% of biofuel. De Regil and Sandoval (2013) have compared the superior quality of lipase aided fuel synthesis with the long-established procedures due to esterifications of fats and alcohols with an overall advantage of unproblematic step down of product.Gog et al. (2012) have mentioned eighty to ninety percent better performance of customized bacterial lipases for biodiesel manufacture. Fan et al. (2012) has described the dominance of lipases to chemical catalysts in their mode of action without damaging the needed product and biodegradability. In addition to obtaining oils from typical plant sources unusually Algae and microalgae can be utilized for yielding higher product percentages. Taher et al. (2011) used immobilized lipases for it and reported them convenient with paimless separation as compared to dissolved ones. Du et al. (2004) have experimentally achieved ninety four percent yield of biodiesel using Novozyme 435 from crude oil of soyabean.
Making beauty products
Almost every human being uses some kind of cosmetic as beauty making or improving product, the objective of their use maybe different and varies with each individual, but the main thing is their enormous use in our daily lives in a way to improve ourselves. Beauty products range from general soaps, hand washes and body washes to creams, lotions, perfumes, make up, shampoos and powders. Records show this is very powerful industry whose sales cross billion every year and gradually this accessory has become a necessity of everyone in modern age.
According to the product type these products are mixtures of various categories of chemical components like surfactants, thickeners, moisturizers, antioxidants, preservatives, vitamins, cooling agents, stimulators, conditioners, scrubbing agents, oils and fatty acids along with alcohols. Lipases enzyme serve an active agent in manufacturing these products. They are used to regulate the activities of glands present within the layers of skin whose secretions affect the physical appearance of skin like more oil secretion from sebaceous gland will result in more oily skin furthermore they are also use to tan or whiten the complexion and monitor and intervene in keratinization (Ansorge-Schumacher and Thum, 2013).
For commercial production of these chemical components lipases proves to be the most significant group of enzymes and their immobilized form is most economical as well technically correct to use for this purpose to obtain fatty acids plus ester for sole cosmetic purposes. Commercialized processed involve the use of lipases.
Detergents
Their fat hydrolyzing capacity makes them an excellent part of detergents as stain removers. Alkaline lipases of bacterial origin are of special interest in this regard because alkalinity together with high temperature works best (Cherif et al., 2011) in his post doctoral research has investigated this detergent action of bacterial lipase from staphylococcus sp. abbreviated as (SL-1) against various fatty substrates like olive oil, with optimization of pH, temperature and stability to formulate lipase based detergents. Niyonzima and More, (2015) has experimented various bacterial lipases for their lipolytic strokes, when any cloth or crockery item with marks of fats and oils is prone to lipases action it removes the stains by hydrolyzing fats from larger to smaller units along with their detachment from the surface. They have also mentioned that lipases constitute 50 percent of detergents in scientifically up to dated countries as green chemicals. Chauhan et al. (2013) has reported 62 percent increased in the efficiency of detergents with incorporation of bacterial lipases. Examination of this action is done in three repeats of washing and exposure to air. The important parameters in these observations are temperature, metal ion’s concentration and time of soaking and exposure to detergent plus lipases as an additive (Aehle, 2004).
Paper industry
Paper is used everywhere for a lot of purposes, the annual production of paper has crossed 28.9 million tons this year, according to the ∑ The worlds count. About 50 percent of the waste from every business comes in the form of wasted papers. Owing to its demand and usage wasted papers are recycled again so that raw materials are managed wisely without exhausting them. In making paper and paper products along with recycling the older ones in every process enzymes are used. Lipases are used for deinking and solving pitch problem and since their substrate is glycerol backbone they transform triglycerides to fatty acids that are more water resistant and stable. On the other hand recycling older papers involve removing ink from the previously used papers through hydrolysis by lipases that helps to attain the desired whiteness of papers (Leduc et al., 2011). Lipases are proved to be useful for removing laser ink from printed papers with approximately one percent increment in the whiteness of papers and this removal through enzymes is more effectual as compared to the chemicals used in long-established methods, that involve the use of chemicals (Lee et al., 2013).
Conclusion and Recommendations
Lipases are catabolic enzymes that split lipids and are of multiple origin. Lipases exemplify immense point and location selectivity for bond fissure and formulation during catalysis. Bacterial lipases are although bit expensive but the compensation with their easy step approach, purification and improved production. The specificity of lipases is far-fetched for their point selection and kinds of reactions they undergo on their discernible substrates. Although decades of the precise researches have been devoted to lipases but the potential of this distinctive group of enzymes is yet to be paid and there is a great need to make human beings beneficiary of their services through biotechnology.
Refrences
Aehle, W., 2004. Enzymes in industry, production and applications. Wiley-VchVerlag, Weinheim.
Ahn, J.H., Pan, J.G. and Rhee, J.S., 2001. Homologous expression of the lipase and ABC transporter gene cluster, tliDEFA, enhances lipase secretion in Pseudomonas spp. Appl. Environ. Microbiol., 67(12): 5506-5511. https://doi.org/10.1128/AEM.67.12.5506-5511.2001
Alkan, H., Baysal, Z., Uyar, F. and Dogru, M., 2007. Production of lipase by a newly isolated Bacillus coagulans under solid-state fermentation using melon waste. Appl. Biochem. Biotechnol., 136: 183–192.
Ansorge-Schumacher, M.B. and Thum, O., 2013. Immobilised lipases in the cosmetics industry. Chem. Soc. Rev., 42(15):6475-6490. https://doi.org/10.1039/c3cs35484a
Aravindan, R., Anbumathi, P. and Viruthagiri, T., 2007. Lipase applications in food industry. Indian J. Biotechnol., 6 (4):141-158.
Bakir, Z.B. and Metin, K., 2016. Purification and characterization of an alkali-thermostable lipase from thermophilic Anoxybacillus flavithermus HBB 134. J. Microbiol. Biotechnol., 26 (6):1087-1097. https://doi.org/10.4014/jmb.1512.12056
Bapiraju, K., Sujatha, P., Ellaiah, P. and Ramana, T., 2004. Mutation induced enhanced biosynthesis of lipase. African J. Biotechnol., 3 (11):618-621.
Barbaro, S., Trevors, J. and Inniss, W., 2001. Effects of low temperature, cold shock, and various carbon sources on esterase and lipase activities and exopolysaccharide production by a psychrotrophic Acinetobacter sp. Can. J. Microbiol., 47 (3):194-205 https://doi.org/10.1139/cjm-47-3-194.
Bharathi, D., Rajalakshmi, G. and Komathi, S., 2018. Optimization and production of lipase enzyme from bacterial strains isolated from petrol spilled soil. J. King Saud Univ., https://doi.org/10.1016/j.jksus.2017.12.018
Borkar, P.S., Bodade, R.G., Rao, S.R. and Khobragade, C., 2009. Purification and characterization of extracellular lipase from a new strain: Pseudomonas aeruginosa SRT 9. Braz. J. Microbiol., 40(2):358-366. https://doi.org/10.1590/S1517-83822009000200028
Califano, V., Bloisi, F., Aronne, A., Federici, S., Nasti, L., Depero, L. E.,Vicari, L. R., 2014. Biosensor applications of MAPLE deposited lipase. Biosensors., 4(4):329-339. https://doi.org/10.3390/bios4040329
Chang, S.-W., Lee, G.-C. and Shaw, J.-F., 2006. Codon optimization of Candida rugosa lip 1 gene for improving expression in Pichia pastoris and biochemical characterization of the purified recombinant LIP1 lipase. J. Agric. Food Chem., 54(3): 815-822. https://doi.org/10.1021/jf052183k
Chang, S.-W., Shaw, J.-F. and Shieh, C.-J., 2003. Optimization of enzymatically prepared hexyl butyrate by lipozyme IM-77. Food Technol. Biotechnol., 41(3):237-242.
Chauhan, M., Chauhan, R.S. and Garlapati, V.K., 2013. Evaluation of a new lipase from Staphylococcus sp. for detergent additive capability. BioMed. Res. Int., 2013 https://doi.org/10.1155/2013/374967
Cherif, S., Mnif, S., Hadrich, F., Abdelkafi, S. and Sayadi, S., 2011. A newly high alkaline lipase: an ideal choice for application in detergent formulations. Lipids Health Dis., 10(1):221. https://doi.org/10.1186/1476-511X-10-221
De Azeredo, L.A., Gomes, P.M., Sant’anna, G.L., Castilho, L.R. and Freire, D.M., 2007. Production and regulation of lipase activity from Penicillium restrictum in submerged and solid-state fermentations. Curr. Microbiol., 54(5): 361-365. https://doi.org/10.1007/s00284-006-0425-7
De Regil, R. and Sandoval, G., 2013. Biocatalysis for biobased chemicals. Biomolecules., 3(4): 812-847. https://doi.org/10.3390/biom3040812
Du, W., Xu, Y.Y., Zeng, J. and Liu, D.H., 2004. Novozym 435-catalysed transesterification of crude soya bean oils for biodiesel production in a solvent-free medium. Biotechnol. Appl. Biochem., 40 (2):187-190 https://doi.org/10.1042/BA20030142.
Escamilla-Mejía, J.C., Rodríguez, J.A., Álvarez-Romero, G.A. and Galán-Vidal, C.A., 2015. Monoenzymatic Lipase Potentiometric Biosensor for the Food Analysis Based on a pH Sensitive Graphite-epoxy Composite as Transducer. J. Mex. Chem. Soc., 59 (1):19-23.
Falony, G., Armas, J.C., Mendoza, J.C.D. and Hernández, J.L.M., 2006. Production of Extracellular Lipase from Aspergillus niger by Solid-State Fermentation. Food Technol. Biotechnol., 44 (2).
Fan, X., Niehus, X. and Sandoval, G., 2012. Lipases as biocatalyst for biodiesel production. Methods Mol. Biol., 471-483. https://doi.org/10.1007/978-1-61779-600-5_27
Ferreira-Dias, S., Sandoval, G., Plou, F. and Valero, F., 2013. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron. J. Biotechnol., 16(3):12-12. https://doi.org/10.2225/vol16-issue3-fulltext-5
Gog, A., Roman, M., Toşa, M., Paizs, C. and Irimie, F. D., 2012. Biodiesel production using enzymatic transesterification–current state and perspectives. Renew Energy., 39(1):10-16. https://doi.org/10.1016/j.renene.2011.08.007
Gotor-Fernández, V., Brieva, R. and Gotor, V., 2006. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B: Enz., 40 (3):111-120. https://doi.org/10.1016/j.molcatb.2006.02.010
Gulati, R., Saxena, R. and Gupta, R., 2000. Fermentation and downstream processing of lipase from Aspergillus terreus. Process Biochem., 36 (1-2):149-155. https://doi.org/10.1016/S0032-9592(00)00201-6
Gupta, R., Gupta, N. and Rathi, P., 2004. Bacterial lipases: an overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol., 64 (6):763-781. https://doi.org/10.1007/s00253-004-1568-8
Hasan, F., Shah, A. A. and Hameed, A., 2006. Industrial applications of microbial lipases. Enzyme Microb. Technol., 39 (2):235-251. https://doi.org/10.1016/j.enzmictec.2005.10.016
Herrera-López, E.J., 2012. Lipase and phospholipase biosensors: a review. Methods Mol. Biol.:525-543. https://doi.org/10.1007/978-1-61779-600-5_30
Houde, A., Kademi, A. and Leblanc, D., 2004. Lipases and their industrial applications. Appl. Biochem. Biotechnol., 118 (1-3):155. https://doi.org/10.1385/ABAB:118:1-3:155
Imandi, S.B., Karanam, S.K. and Garapati, H.R., 2010. Optimization of process parameters for the production of lipase in solid state fermentation by Yarrowia lipolytica from Niger seed oil cake (Guizotia abyssinica). J. Microbial. Biochem. Technol., 2: 28-33.
Jaeger, K.E. and Eggert, T., 2002. Lipases for biotechnology. Curr. Opin. Biotechnol., 13 (4):390-397. https://doi.org/10.1016/S0958-1669(02)00341-5
Jaeger, K., Dijkstra, B. and Reetz, M., 1999. Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Ann Microbiol., 53 (1):315-351. https://doi.org/10.1146/annurev.micro.53.1.315
Kartal, F., Kilinç, A. and Timur, S., 2007. Lipase biosensor for tributyrin and pesticide detection. Int. J. Environ. Anal. Chem., 87 (10-11):715-722. https://doi.org/10.1080/03067310701327741
Kempka, A.P., Lipke, N.R., Pinheiro, T.L.F., Menoncin, S., Treichel, H. and Freire, D.M.G., 2008. Response surface method to optimize the production and characterization of lipase from Penicillium verrucosum in solid-state fermentation. Bioproc. Biosyst. Engin., 31: 119–125.
Khan, I., Dutta, J. R. and Ganesan, R., 2017. Lactobacillus sps. lipase mediated poly (ε-caprolactone) degradation. Int. J. Biol. Macromol., 95:126-131. https://doi.org/10.1016/j.ijbiomac.2016.11.040
Kumar, M.P. and Valsa, A.K., 2007. Optimization of culture media and cultural conditions for the production of extracellular lipase by Bacillus coagulans. Indian J. Biotechnol., 6(1): 114-117.
Kuo, T. M. and Gardner, H. 2002. Lipid biotechnology, CRC Press. https://doi.org/10.1201/9780203908198
Kwoun Kim, H., Jung, Y.-J., Choi, W.-C., Ryu, H. S., Oh, T.-K. and Lee, J.-K., 2004. Sequence-based approach to finding functional lipases from microbial genome databases. FEMS Microbiol. Lett., 235 (2):349-355 https://doi.org/10.1111/j.1574-6968.2004.tb09609.x.
Lanser, A.C., Manthey, L.K. and Hou, C.T., 2002. Regioselectivity of new bacterial lipases determined by hydrolysis of triolein. Curr. Microbiol., 44 (5):336-340. https://doi.org/10.1007/s00284-001-0019-3
Leduc, C., Lanteigne-Roch, L.-M. and Daneault, C., 2011. Use of enzymes in deinked pulp bleaching. Cellul Chem. Technol., 45 (9):657.
Lee, C.K., Ibrahim, D. and Omar, I.C., 2013. Enzymatic deinking of various types of waste paper: Efficiency and characteristics. Process Biochem., 48 (2):299-305 https://doi.org/10.1016/j.procbio.2012.12.015.
Li, C.-Y., Chen, S.-J., Cheng, C.-Y. and Chen, T.-L., 2005. Production of Acinetobacter radioresistens lipase with repeated fed-batch culture. Biochem. Eng. J., 25 (3):195-199. https://doi.org/10.1016/j.bej.2005.05.002
Liu, D., Schmid, R. and Rusnak, M., 2006. Functional expression of Candida antarctica lipase B in the Escherichia coli cytoplasm—a screening system for a frequently used biocatalyst. Appl. Microbiol. Biotechnol., 72 (5):1024-1032. https://doi.org/10.1007/s00253-006-0369-7
Loo, F.C. 2015. Nanopore and Plasmon Resonance-based Biosensing for Disease and Anti-cancer Drug Screening. The Chinese University of Hong Kong (Hong Kong).
Mahler, G., Kok, R., Cordenons, A., Hellingwerf, K. and Nudel, B., 2000. Effects of carbon sources on extracellular lipase production and lipA transcription in Acinetobacter calcoaceticus. J. Ind. Microbiol. Biotechnol., 24(1):25-30. https://doi.org/10.1038/sj.jim.2900764
Mala, J.G.S., Edwinoliver, N.G., Kamini, N.R. and Puvanakrishnan, R., 2007. Mixed substrate solid state fermentation for production and extraction of lipase from Aspergillus niger MTCC 2594. J.Gen. Appl. Microbiol., 53: 247–253.
Martinez, D.A. and Nudel, B.C., 2002. The improvement of lipase secretion and stability by addition of inert compounds into Acinetobacter calcoaceticus cultures. Can. J. Microbiol., 48 (12):1056-1061. https://doi.org/10.1139/w02-108
Meka, P. K. and Tripathi, V., 2007. Synthesis of biodiesel fuel from safflower oil using various reaction parameters. J. Oleo Sci., 56 (1):9-12. https://doi.org/10.5650/jos.56.9
Mohamad, N.R., Marzuki, N.H.C., Buang, N.A., Huyop, F. and Wahab, R.A., 2015. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip., 29 (2):205-220. https://doi.org/10.1080/13102818.2015.1008192
Nawani, N. and Kaur, J., 2000. Purification, characterization and thermostability of lipase from a thermophilic Bacillus sp. J33. Mol. Cell. Biochem., 206 (1):91-96.
Niyonzima, F.N. and More, S.S., 2015. Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J. Basic Microbiol., 55 (10):1149-1158. https://doi.org/10.1002/jobm.201500112
Päiviö, M. and Kanerva, L.T., 2013. Reusable ω-transaminase sol–gel catalyst for the preparation of amine enantiomers. Process Biochem., 48 (10):1488-1494. https://doi.org/10.1016/j.procbio.2013.07.021
Pandey, A., 2003. Solid-state fermentation. Biochem. Eng. J., 13 (2):81-84.
Pandey, A., Benjamin, S., Soccol, C.R., Nigam, P., Krieger, N. and Soccol, V.T., 1999. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem., 29 (2):119-131.
Pinheiro, T.D.L.F., Menoncin, S., Domingues, N.M., Oliveira, D.D., Treichel, H., Di Luccio, M. and Freire, D.M.G., 2008. Production and partial characterization of lipase from Penicillium verrucosum obtained by submerged fermentation of conventional and industrial media. Food S. Technol. (Campinas)., 28 (2):444-450. https://doi.org/10.1590/S0101-20612008000200028
Priya, K.U. and Reddy, B.I., 2015. Isolation, optimization and partial purification of lipase enzyme. Biotechnol. Appl. Biochem., 6: 2156-2171.
Rajendran, A., Palanisamy, A. and Thangavelu, V., 2009. Lipase catalyzed ester synthesis for food processing industries. Braz. Arch. Biol. Technol., 52 (1):207-219 https://doi.org/10.1590/S1516-89132009000100026.
Ran, C., He. S., Yang. Y., Huang. L. and Zhou, Z., 2015. A Novel lipase as aquafeed additive for warm-water aquaculture. PLoS ONE, 10(7): e0132049.
Rathi, P., Saxena, R.K. and Gupta, R., 2001. A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem. 37:187–192.
Reddy, K.G., Madhavi, G. and Swamy, B.K., 2014. Mobilized lipase enzymatic biosensor for the determination of chlorfenvinphos and malathion in contaminated water samples: a voltammetric study. J. Mol. Liq., 198:181-186. https://doi.org/10.1016/j.molliq.2014.06.019
Reimerdes, E., Franke, K. and Sell, M., 2004.. Influencing functional properties of egg yolk by using phospholipases. In: Conference on Food Structure and Food Quality.
Rooney, D. and Weatherley, L., 2001. The effect of reaction conditions upon lipase catalysed hydrolysis of high oleate sunflower oil in a stirred liquid–liquid reactor. Process Biochem., 36 (10):947-953. https://doi.org/10.1016/S0032-9592(01)00130-3
Shah, K. and Bhatt, S., 2011. Purification and characterization of lipase from Bacillus subtilis Pa2. J. Biochem. Technol., 3 (3).
Sirisha, E., Rajasekar, N. and Narasu, M.L., 2010. Isolation and optimization of lipase producing bacteria from oil contaminated soils. Adv. Biolog. Res., 4(5):249-252.
Sharma, R., Soni, S., Vohra, R., Jolly, R., Gupta, L. and Gupta, J., 2002. Production of an extracellular alkaline lipase from a new Bacillus sp. RSJ1 and its application in ester hydrolysis. Indian J. Microbiol., 42 (1):49-54.
Shieh, C.-J. and Chang, S.-W., 2001. Optimized synthesis of lipase-catalyzed hexyl acetate in n-hexane by response surface methodology. J. Agric. Food Chem., 49 (3):1203-1207. https://doi.org/10.1021/jf001050q
Shu, Z.-Y., Jiang, H., Lin, R.-F., Jiang, Y.-M., Lin, L. and Huang, J.-Z., 2010. Technical methods to improve yield, activity and stability in the development of microbial lipases. J. Mol. Catal. B: Enzym., 62 (1):1-8. https://doi.org/10.1016/j.molcatb.2009.09.003
Streck, L., Sarmento, V.H., Machado, P.R., Farias, K.J., Fernandes-Pedrosa, M.F. and Da Silva-Júnior, A.A., 2016. Phase transitions of isotropic to anisotropic biocompatible lipid-based drug delivery systems overcoming insoluble benznidazole loading. Int. J. Mol. Sci., 17 (7):981. https://doi.org/10.3390/ijms17070981
Taher, H., Al-Zuhair, S., Al-Marzouqi, A.H., Haik, Y. and Farid, M.M., 2011. A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enz. Res., 2011. https://doi.org/10.4061/2011/468292
Takahashi, S., Ueda, M. and Tanaka, A., 1999. Independent production of two molecular forms of a recombinant Rhizopus oryzae lipase by KEX2-engineered strains of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol., 52 (4):534-540. https://doi.org/10.1007/s002530051556
Thakur, S., 2012. Lipases, its sources, properties and applications: A review. Int. J. Sci. Eng. Res., 3 (7):1-29.
Thakur, V., Tewari, R. and Sharma, R., 2014. Evaluation of production parameters for maximum lipase production by P. stutzeri MTCC 5618 and scale-up in bioreactor. C. J. Bio., 2014. https://doi.org/10.1155/2014/208462
Tokiwa, Y., Calabia, B.P., Ugwu, C.U. and Aiba, S., 2009. Biodegradability of plastics. Int. J. Mol. Sci., 10 (9):3722-3742. https://doi.org/10.3390/ijms10093722
Trousil, J., Filippov, S.K., Hrubý, M., Mazel, T., Syrová, Z., Cmarko, D., Svidenská, S., Matějková, J., Kováčik, L. and Porsch, B., 2017. System with embedded drug release and nanoparticle degradation sensor showing efficient rifampicin delivery into macrophages. Nanomed. Nanotechnol. Biol. Med., 13 (1):307-315. https://doi.org/10.1016/j.nano.2016.08.031
Ugo, A.K., Amara, A.V., Igwe, C.N. and Kenechuwku, U., 2017. Microbial lipases: A prospect for biotechnological industrial catalysis for green products- a review. Ferment. Technol., 6: 144.
Vakhlu, J., 2006. Yeast lipases: enzyme purification, biochemical properties and gene cloning. Electron. J. Biotechnol., 9 (1). https://doi.org/10.2225/vol9-issue1-fulltext-9
Vargas, G.D., Treichel, H., De Oliveira, D., Beneti, S. C., Freire, D.M. and Di Luccio, M., 2008. Optimization of lipase production by Penicillium simplicissimum in soybean meal. J. Chem. Technol. Biotechnol., 83 (1):47-54. https://doi.org/10.1002/jctb.1776
Veerapagu, M., Narayanan, A.S., Ponmurugan, K. and Jeya, K., 2013. Screening selection identification production and optimization of bacterial lipase from oil spilled soil. Asian J. Pharm. Clin. Res., 6 (3):62-67.
Waites, M.J., Morgan, N.L., Rockey, J.S. and Higton, G. 2009. Industrial microbiology: an introduction, John Wiley & Sons.
Zhang, H.Y., Wang, X., Ching, C.B. and Wu, J.C., 2005. Experimental optimization of enzymic kinetic resolution of racemic flurbiprofen. Biotechnol. Appl. Biochem., 42 (1):67-71. https://doi.org/10.1042/BA20040163
Zhao, X., Shi-Jian, D., Tao, G., Xu, R., Wang, M., Reuhs, B. and Yang, Y., 2010. Influence of phospholipase A2 (PLA2)-treated dried egg yolk on wheat dough rheological properties. F. Sci. Technol., 43 (1):45-51 https://doi.org/10.1016/j.lwt.2009.06.027.
To share on other social networks, click on any share button. What are these?








