Research Journal for Veterinary Practitioners
Research Article
Growth and Distribution of Intestinal Mucosa Associated Lymphoid Tissues and Peyer’s Patches in Native Chicken (Gallus gallus domesticus) of Bangladesh
Md. Shahriar Hasan Sohel1*, Kh. Nurul Islam2, Mohammad Lutfur Rahman2
1Laboratory of Veterinary Anatomy, Joint Graduate School of Veterinary Sciences, Gifu University, Gifu-City, Japan; 2Department of Anatomy and Histology, Faculty of Veterinary Medicine, Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh.
Abstract | A histomorphological study was conducted to observe the growth and development of intestinal mucosa-associated lymphoid tissues (MALTs) and Peyer’s patches (PPs) in native chicken of Bangladesh. A total of 120 chicks were reared up to 180 days and subsequently different segments of intestine were collected at day 1 (D1), day 30 (D30), day 90 (D90) and day 180 (D180). Tissues were stained with hematoxylin and eosin (HE). In the present study, intraepithelial lymphocytes (IELs) were abundantly found in all segments of the intestine except colo-rectum. The highest number of IELs were found at D180 duodenum (194.6 ± 4.50), jejunum (188.8 ± 7.61) and cecal tonsil (176.6 ± 5.52), whereas the lowest number was found at D1 colo-rectum (2.0 ± 0.70). The lamina propria of different segments of intestine contained isolatory and aggregated lymphocytes. The isolatory and aggregated lymphatic nodules were also found in lamina propria and the highest number was observed at D90 and D180. Moreover, the lymphocytes were scatteredly distributed in the submucosa in all stages of development. These results suggest that the postnatal growth and development of MALTs and PPs varies in different intestinal segments and these are greatly influenced by the aging of scavenger native chicken of Bangladesh. This variation of lymphocytes also indicates the size of microbial load and level of immune status, acquired due to the presence of antigens.
Keywords | Chicken, Intestine, Histology, Mucosa-associated lymphoid tissues, Peyer’s patches
Received | June 20, 2020; Accepted | July 09, 2020; Published | December 15, 2020
*Correspondence | Md. Shahriar Hasan Sohel, Laboratory of Veterinary Anatomy, Joint Graduate School of Veterinary Sciences, Gifu University, Gifu-City, Japan; Email: [email protected]
Citation | Sohel MSH, Islam KN, Rahman ML (2020). Growth and distribution of intestinal mucosa associated lymphoid tissues and peyer’s patches in native chicken (Gallus gallus domesticus) of Bangladesh. Res J. Vet. Pract. 8(4): 56-61.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2020/8.4.56.61
ISSN | 2308-2798
Copyright © 2020 Sohel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Due to frequent exposure to the antigens and commercial microbes containing feed, the intestinal tract of chickens is the major site of antigenic challenges as compared to other parts of the body. To combat this antigenic challenges intestinal mucosa being populated by some immune competent cells called mucosa-associated lymphoid tissues (MALTs) and peyer’s patches (PPs) (Sohel et al., 2020; Rahman et al., 2003). The appearance, number and organization of MALTs and PPs differ within the intestine, i.e. between jejunum and ileum with age (Holt et al., 2010; Christensen et al., 2006; Befus et al., 1980). Previously it has been reported that MALTs in the form of PPs or isolated lymphoid follicles (ILF) and lamina proprial lymphocytes are frequently found in parts of the lamina propria and submucosa, but absent in the muscularis mucosa. In addition, lymphoid tissues are much more abundant in the lamina propria and submucosa of the ilecocecal orifice as compared to the other parts of the intestine with changes of age (Christensen et al., 2006; Kajiwara et al., 2003; Befus et al., 1980).
The native scavenger chicken (Gallus gallus domesticus) is the most important local chicken breed in Bangladesh (Faruque et al., 2010). Most of the farmer reared this chick
Table 1: Frequency of intraepithelial lymphocytes (IELs) per 10 microscopic fields (400x magnification) in different segments of intestine of native chicken.
| Segments of intestinal tract | Frequency of IELs in different ages of chicken (Mean ± SE) | |||
|
D1 |
D30 |
D90 |
D180 |
|
| Duodenum | 20.4 ± 2.33 | 49.4 ± 8.14 | 148.8 ± 7.87 | 194.6 ± 4.50 |
| Jejunum | 21.0 ± 2.23 | 90.2 ± 4.06 | 187.0 ± 7.42 | 188.8 ± 7.61 |
| Ileum | 15.6 ± 1.86 | 63.6 ± 3.95 | 101.4 ± 5.16 | 149.2 ± 4.21 |
| Cecum | 36.6 ± 2.92 | 85.4 ± 4.99 | 116.8 ± 8.45 | 170.2 ± 7.05 |
| Cecal tonsil | 39.6 ± 4.26 | 92.8 ± 3.52 | 132.8 ± 5.82 | 176.6 ± 5.52 |
| Colo-rectum | 2.0 ± 0.70 | 14.2 ± 3.33 | 25.0 ± 2.00 | 30.2 ± 1.24 |
en that attains a weight around 1.0~1.4 kg at 6 months of age (Uddin et al., 2011; Ershad, 2005). As stated by Rahman et al. (2003), these chickens are scavenging in nature. Due to scavenging nature, intestinal MALTs specially PPs of these chickens contain more immune-competent cells as compared with high yielding chickens (Islam et al., 2008; Khan et al., 2007). However, information about the growth and distribution of these MALTs and PPs in native chicken is scarce, since the most investigations were performed in high yielding chickens (Kozuka et al., 2010; Nagy and Olah, 2010; Moral et al., 1998). So, considering the above facts this study was planned to observe the postnatal growth and development of MALTs with giving emphasis on PPs in different intestinal segments with the aging of scavenger native chicken of Bangladesh.
MATERIALS AND METHODS
Rearing of Chicken and Collection of Tissues
Total 120 chicks were reared in scavenging system up to six months with proper feeding and watering. The chickens were routinely sacrificed at D1, D30, D90 and D180 by excess chloroform inhalation and then the intestinal tract, viz. duodenum, jejunum, ileum, cecum, cecal tonsil and colo-rectum were dissected out carefully.
Staining Technique
The collected tissues were fixed in 10% buffered formalin for 72 hours and then washed in running tap water for overnight. Then the tissues were dehydrated in ascending graded alcohol, cleaned by xylene followed by embedding in paraffin for preparing the block and finally the tissue sections were stained with HE as described previously by Griedly (1960).
Histomorpholoy And Data Analysis
For histomorphological observation, photomicrographs were taken from each age group of intestinal tissues by using AmScope image measuring software (x86, 3.7.3036 version). The mean value and standard error were calculated by using excel sheet (Microsoft Excel-2010) and results were expressed as Mean ± SE.
RESULTS
Duodenum
Histological studies revealed that duodenum, jejunum and ileum consisted of four layers, viz. tunica mucosa, submucosa, muscularis externa and serosa. The villi of the duodenum were lined by simple columnar epithelium. Lymphocytes were mostly found in the layers of tunica epithelium, lamina propria and submucosa (Figure 1). The frequency of intraepithelial lymphocytes (IELs) in the different age group of native chickens presented in Table 1. The highest frequency of IELs was found at D180 (194.6 ± 4.50) among different age groups (Figure 1). The diffuse and isolatory lymphocytes were noticed in the lamina propria and submucosa at all the stages and was being higher in population with increasing of the age. On the contrary, a few number of aggregated lymphatic nodules were found only at D90 and D180 (Figure 1).
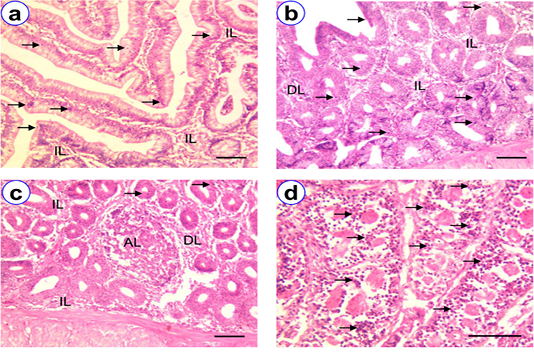
Figure 1: HE staining of duodenum at D30 (a), D90 (b), D180 (c~d) showing villi (V), intraepithelial lymphocytes (arrow), isolatory lymphocytes (IL), diffuse lymphocytes (DL), aggregated lymphocytes (AL); Scale bar = 50 µm (a~c), 100 µm (d).
Jejunum
Histologically, the villi of the jejunum of native chicken were lined by simple columnar epithelium, which were longer than that of duodenum (Figure 2b). The lamina epithelia, lamina propria and submucosa of jejunum were the most common sites for the presence of lymphocytes. The number of IELs in the different age group of native chickens illustrated in Table 1 and revealed that, among the different age group, the highest mean number of IELs was observed at D180 (188.8 ± 7.61) than the others. The isolatory and diffuse lymphocytes were mostly found in the core of the villi, lamina propria and submucosa of all age groups (Figure 2). Besides, these number of lymphocytes were increased with the aging of all aged groups of chickens. Whereas, very few number of aggregated lymphocytes were also observed in the lamina propria and submucosa of all age groups except D1.
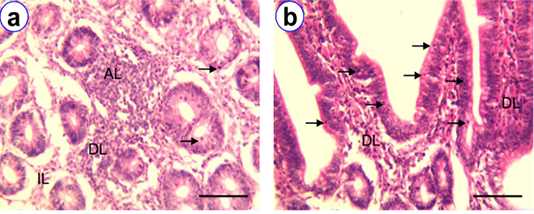
Figure 2: HE staining of jejunum at D30 (a) and D180 (b) showing intraepithelial lymphocytes (arrow), isolatory lymphocytes (IL), diffuse lymphocytes (DL), aggregated lymphocytes (AL); Scale bar = 100 µm (a~b).
Ileum
The lining epithelium of ileum was similar to duodenum and jejunum, but the apical part of most villi was blunt whereas the basal part was wider. The villi of the ileum of native chicken were long and slender. Numerous goblet cells were noticed in the lining of epithelial cell. The major sites for the presence of lymphocytes were in the mucosa and submucosa. We found the highest mean number of IELs at D180 (149.2 ± 4.21) than the others (Table 1). The isolatory and diffuse lymphocytes in lamina propria were mostly found at D90 and D180 as compared with D1 and D30 (Figure 3). A few number of aggregated lymphocytes were also found in the lamina propria of ileum at D90 and D180 (Figure 3). But this type of lymphocytes was absent at D1 and D30 ileum.
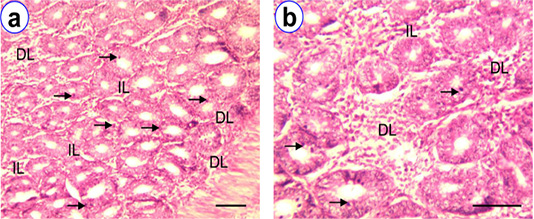
Figure 3: HE staining of ileum at D90 (a~b) showing intraepithelial lymphocytes (arrow), isolatory lymphocytes (IL), diffuse lymphocytes (DL); Scale bar = 50 µm (a), 100 µm (b).
Cecum
The well developed villi were found in proximal parts, whereas the distal parts were less developed villi. The lining epithelium of villi was simple columnar with goblet cells. Lymphoid tissues mostly distributed in the mucosa, lamina propria and submucosa and core of the villi. The frequency of IELs in different age group of native chickens presented in Table 1. Among different age group the highest frequency of IELs in cecum was observed at D180 (170.2 ± 7.05). A few number of IELs were also observed at D1 and D30. The isolatory, diffuse and aggregated lymphocytes and lymphatic nodules were noticed in the core of villi, lamina propria, and submucosa of all the age groups, however, their frequency of occurrence were found to be higher with their aging (Figure 4).
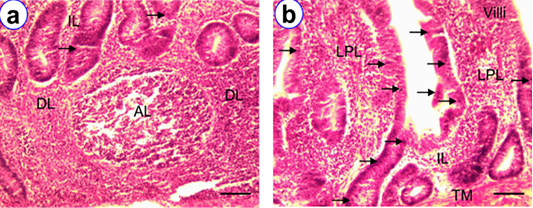
Figure 4: HE staining of cecum at D180 (a~b) showing villi (V), intraepithelial lymphocytes (arrow), isolatory lymphocytes (IL), diffuse lymphocytes (DL), aggregated lymphocytes (AL), lamina proprial lymphocytes (LPL); Scale bar = 50 µm (a~b).
Cecal Tonsil
The broad tubular shape cecal tonsils were in the proximal one third of the paired tubular cecum. In histological observations, there were four layers in cecal tonsil i.e., tunica mucosa, submucosa, muscularis and serosa. We observed higher frequency of IELs at D180 (176.6 ± 5.52) as compared to other age groups. The isolatory, diffuse and aggregated lymphocytes, including lymphatic nodules were mostly noticed in the lamina propria at D180 (Figure 5). The hightest overall number of lymphatic nodules were found in cecal tonsil (Figure 5) as compared with other segments of intestine.
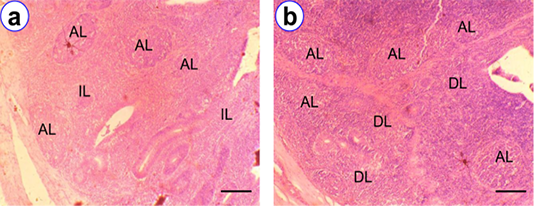
Figure 5: HE staining of cecal tonsil at D90 (a) and D180 (b) showing isolatory lymphocytes (IL), diffuse lymphocytes (DL), aggregated lymphocytes (AL); Scale bar = 50 µm (a~b).
Colo-Rectum
The colo-rectum was the terminal part of intestine of native chicken. The most common sites for the presence of lymphocytes in colo-rectum were the lamina epithelia, lamina propria and the submucosa. Among the various age groups the mean frequency of IELs were found higher at D180 (30.2 ± 1.24) than the other groups (Table 1). However, the frequency of this IELs was comparatively less in number than the other segments of intestine. In addition, isolatory lymphatic nodules and aggregated lymphatic nodules were pointed out in the lamina propria of colo-rectum (Figure 6).
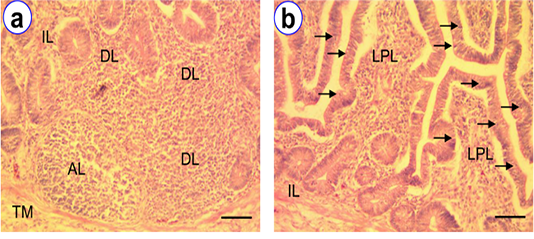
Figure 6: HE staining of colo-rectum at D180 (a~b) showing intraepithelial lymphocytes (arrow), isolatory lymphocytes (IL), diffuse lymphocytes (DL), aggregated lymphocytes (AL); Scale bar = 50 µm (a~b).
DISCUSSION
The present study showed the growth and distribution of MALTs in intestine giving emphasis of PPs in native chicken of Bangladesh. The scavenger native chickens are always in contact with various microorganisms due to their feeding habits (Javaregowda et al., 2016). All the times, their ascending and descending routes are exposed with different types of antigens, specially during intake of food particles from here and there (Mahmud et al., 2015; Rahman et al., 2003). To combat with that invading microorganisms, lymphocytes with various distributional pattern appears in the intestinal mucosa as a part of their natural defense mechanism. Their number, distribution and location in different layers of mucosa and submucosa are not unique in different segments of intestine (Khan et al., 2007). The histological features of different intestinal segments in native chickens were consisted of four layers, viz. tunica mucosa, submucosa, muscularis and serosa, where the villi were lined by simple columnar epithelium with goblet cells. These findings were alike with the results of Khambualai et al. (2009), Hodge (1974), Randall and Reece (1996) and Dellmann and Eurell (1998). In this study, the lamina epithelia, lamina propria and submucosa were the most common sites for the presence of lymphocytes in the duodenum, jejunum, ileum and cecum. Previously, similar findings were also reported in different hybrid chickens (Islam et al., 2008; Vervelde and Jeurissen, 1993). Furthermore, we observed higher frequency of IELs at D180, but previously it was reported at D90 in native chicken (Rahman et al., 2003) and in hybrid chicken (Befus et al., 1980) and this discrepancy may be due their feeding habits.
The PPs was not observed in different age groups of duodenum, which support the previous study conducted by Rahman et al. (2003). However, the isolatory and aggregated lymphocytes were observed in all the age groups of scavenger native chicken and the highest numbers were found in the lamina propria, submucosa and core of villi, whereas Rahman et al. (2003) were observed the highest numbers in the lamina propria and core of villi which were almost similar to our findings, therefore, we considered the differences as insignificant. In general, we observed PPs more or less in all segments of the intestine except duodenum, which is partially similar to the previous study on development of PPs in hybrid chicken studied by Kajiwara et al. (2003) in which reported that PPs were not only observed in Meckel’s diverticulum and ileocecal junction but also in the other segments of intestinal tract of adult chickens. This partial dissimilarity is may be due their different rearing system of native chickens.
Vervelde and Jeurissen (1993) and Sohel et al. (2019) stated that, in hybrid chicken, the major part of the digestion and absorption take place through the mucosa of intestine, as the mucosa is exposed to harmful materials and the microbes, the lymphocytes and PPs were infiltrated in the various segments of small intestine, which was more in duodenum and jejunum than the other segments of intestine. In scavenger native chicken, our study on the distribution of infiltrated lymphocytes were alike with the findings of hybrid chicken.
In case of ileum, Islam et al. (2008) reported the highest frequency of isolatory, aggregated, IELs and PPs was found at D180 hybrid chicken, which agreed with this present study in native scavenger chicken, however, Rahman et al. (2003) reported the highest frequency at D90 native chicken. On the other hand, in the cecum, we observed highest number of isolatory, aggregated, IELs and PPs at D90 and D180, which are not similar with the previous study in hybrid chicken where authors observed highest at D30 and D180 (Del Cacho et al., 1993; Vervelde and Jeurissen, 1993). In case of cecal tonsil, remarkable amounts of IELs were found at D30, may be it was due to production of more immunity in the early stage as the chicks were lack of maternal colostrum like mammals. Unlike IELs, the distribution pattern of lamina proprial lymphocytes with various arrangements of lymphocytes was also higher in this organ probably due to the conventional nature of these chickens and this observation was not similar to the study of Honjo and Hirota (1993) and Mahmud et al. (2015). This dissimilarity was due to the fact that they used germ free chickens. The frequency of IELs in the colo-rectum was less than the other segments of intestine which was similar with the previous report in broiler chickens (Sohel et al., 2019) and hybrid chickens (Holt et al., 2010; Vervelde and Jeurissen, 1993). The maximum number of isolatory and diffuse lymphocytes were distributed in the core of the lamina propria among all stages of growth and development. In addition, isolatory lymphatic nodules and aggregated lymphatic nodules were pointed out in the lamina propria of colo-rectum. These similar results were reported in deshi chicken (Rahman et al., 2003).
CONCLUSION
The histomorphological study illustrated the growth and distribution of intestinal MALTs and PPs in native chicken of Bangladesh. The lymphocytes were present in all the segments of intestine, but their number, density and pattern of arrangement were clearly variable which indicate the size of microbial load in mucosa and level of immune status, acquired due to the presence of antigens. The postnatal growth and development of MALTas and PPs in different intestinal segments were greatly influenced with the aging of scavenger native chicken and the age related changes clarify that the antibody level is comparatively better in the later stages of native chicken. Although, further study is necessary to find out the type of lymphocytes and their producing antibodies in different intestinal segments of native chicken.
ACKNOWLEDGEMENTS
The authors are thankful to The University Grant Commission (UGC) for providing funding to conduct this research work. Special thanks to members of the department of Anatomy and Histology, Chattogram Veterinary and Animal Sciences University for their kind co-operation to complete this research work.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
authors contribution
Sohel MSH designed the study, carried out lab work and wrote the manuscript. Islam KN edited the manuscript. Rahman ML supervised all the activities of this study.
REFERENCES






