Advances in Animal and Veterinary Sciences
Research Article
Elettaria cardamomum Essential Oil Rescues Paracetamol-Induced Hepatorenal Damage via Modulating Oxidative Stress in Rats
Ayman A. Khattab1, Azza M. Tawfek2, Khaled Abo-EL-Sooud3*, Kawkab A. Ahmed4,
Abd El Nasser El-Gendy5, A.R. Ahmed2
1Egypt Center for Research and Regenerative Medicine, Military Forces, Cairo, Egypt; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 3Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza,12211, Egypt; 4Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 5Medicinal and Aromatic plants Research department, National Research Centre, Dokki, Giza, Egypt
Abstract | Hepatoprotective efficacy of cardamom (Elettaria cardamomum) seeds crude essential oil was evaluated in experimentally paracetamol (PCM) hepatotoxic rats. Sixty-five male Sprague Dawley rats divided into six groups. First group was kept as control negative, while the 2nd group was given PCM (500 mg/kg bw). The 3rd and 4th groups were orally administered silymarin + PCM (500mg/kg bw + 500 mg/kg bw), and cardamom oil + PCM (100 mg/kg bw + 500 mg/kg bw), respectively. The 5th and 6th groups were treated with silymarin (500 mg/kg bw) and cardamom oil (100 mg/kg bw), respectively in normal rats. All the previous dosages lasted for two weeks. Blood samples collected weekly from all tested groups. Assessment of hemogram, some serum biochemical parameters and histopathology of liver and kidney were carried out. Chemical compositions of cardamom oil was established by gas chromatography-mass spectrometry (GC-MS), revealed that the oil was rich in monoterpenoids, while the monoterpene hydrocarbon, was existing with comparatively small percent. Results of PCM-intoxicated rats revealed significant decrease in total protein and albumin with significant hyperglycemia and elevation in serum transaminases, urea and creatinine which were significantly ameliorated in groups receiving cardamom oil or silymarin. Administration of cardamom essential oil significantly ameliorates the hepato-renal profiles and elevates total antioxidant capacity. Moreover, the oil protects the liver and kidneys from histopathological alterations in PCM-intoxicated rats. Neither cardamom oil nor silymarin induce any alterations in serum biochemistry and total antioxidant activity in normal rats. The present results proved the ameliorative effect of cardamom essential oil against hepato-renal changes in PCM-intoxicated rats reasonably due to its potent antioxidant effect.
Keywords: Cardamom Oil, Paracetamol, Gas Chromatography-Mass Spectrometry, Antioxidant, Hepato-renal, Rats
Received | June 26, 2020; Accepted | July 05, 2020; Published | September 20, 2020
*Correspondence | Khaled Abo-EL-Sooud, Department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza,12211, Egypt; Email: k[email protected]
Citation | Khattab AA, Taweek AM, Abo-EL-Sooud K, Ahmed KA, El-Gendy AN, Ahmed AR (2020). Elettaria cardamomum essential oil rescues paracetamol-induced hepatorenal damage via modulating oxidative stress in rats. Adv. Anim. Vet. Sci. 8(s2): 24-33.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s2.24.33
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abo-EL-Sooud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Liver is considered one of the most important organs in the body as it’s responsible for many vital functions (Mayuren et al., 2010). So, liver must be kept in healthy condition for keeping normal physiological function. Drugs are the principal cause of hepatic injury that resulting in substantial clinical problem all over the world (Lakshmi et al., 2018). Paracetamol, which known as acetaminophen is considered one of the most used analgesics (Sheen et al., 2002). Paracetamol when taken in a therapeutic dose is known to be relatively nontoxic (Siemionow et al., 2016). Its toxic effect is resulted when taken in a single or repeated high dose, or after chronic ingestion (Tittarelli et al., 2017). Hepato- renal dysfunction is considered as one of the numerous problems in drug therapy (Cepa et al., 2018). Paracetamol-induced hepatotoxicity has been studied for several years (Hinson et al., 2010; Ahmed et al., 2019).
The metabolism of the ingested therapeutic dose of PCM is mainly accomplished by cytochrome P450 followed by glucuronidation or sulfation whereas N-acetyl-p-benzoquinone imine (NAPQI) is conjugated with reduced glutathione (GSH) levels are associated with liver damage (Hinson et al., 2010). Thus, excessive drugs accumulations become accessible for biotransformation and metabolism by cytochrome P450, which result in GSH depletion. Exhausted GSH levels allow NAPQI free to bind with other targeted cellular proteins which evoke cellular oxidative stress and indulge in the cellular necrosis process (Hamza and Al-Harbi, 2015). Hence, the use of antioxidants could offer protective and preventive effects against drug-induced hepatic injury.
Numerous herbal medicines were evaluated both in vitro and in vivo for their hepatoprotective efficacy all over the world. Moreover, several polyherbal formulations were developed and proved to be more effective than the known synthetic hepatoprotective drugs (Lakshmi et al., 2018). Application of essential oils (EOs) as a substitute to synthetic chemicals is an increasing claim nowadays. Medicinal plants including cardamom, which belong to the family of Zingiberaceae, has beneficial effects especially against human diseases due to their bioactive compounds (Mutlu-Ingok and Karbancioglu-Guler, 2017).
Cardamom is widely used for flavoring purposes in food, and in medicine it is used to treat gastrointestinal disorders (Jamal et al., 2006). Although, commonly known with their flavoring properties, their antibacterial, and antifungal properties have recently been of great interest. It has been reported that the antimicrobial activity of essential oil is generally due to phenolic and terpenoid compounds as well as aliphatic compounds (Lv et al., 2011). Little information has been reported on the antioxidant activity of aromatic oily liquid which can be obtained by steam distillation from native cardamom seeds.
In conduction with the previous literature, the present work was performed to investigate the anticipated hepatoprotective activity of cardamom oil as compared to silymarin as standard drug against PCM-induced hepato-renal toxicity in adult male albino rats. Such task was achieved throughout the assessment of hematological, serum biochemical and histopathological changes associated with the administration of PCM and/or the herbal extracts to albino rats.
MATERIALS AND METHODS
Animals
Sixty-five male Sprague Dawley rats weighing 180 ± 20 g (12~14 weeks old), purchased from the Laboratory Animals Breeding Unit, Faculty of Veterinary medicine, Cairo university were used. Rats were acclimatized for one week before the beginning of the experimental study. The animals were housed in separate cages and fed balanced commercial diet and offered water ad libitum. Experimental protocol was in accordance with the guidelines approved by the National Institute of Health for the Care and Use of Laboratory Animals and were comforted by Ethics of animal use in research Committee, Cairo University:
(Approval ID: CU/ ΙΙ/ F/ 72/ 18).
Chemicals
Paracetamol powder was purchased from (Sigma pharmaceuticals, Egypt) and were applied in a dose of 500 mg/kg (Bektur et al., 2013).
Silymarin was purchased from local market pharmacy (SEDICO, Egypt), and applied in a dose of 500 mg/kg. (Juma’a et al., 2009). All chemical and solvents were analytical authentic grade.
Plant Materials
Cardamom seeds were purchased from local market. The seeds were identified and authenticated and the voucher specimen of was deposited in the Herbarium of the department of flora, ministry of agriculture.
Essential Oil Extraction:
The essential oil was obtained by hydro distillation using Clevenger apparatus for 2 hrs. The oils were dried over sodium sulfate and stored in an amber flask at 4ºC according to Guenther (1961) and expressed as ml/100g, The extracted essential oil was dehydrated over anhydrous sodium sulphate and stored at freezer till used for Gas Chromatography-Mass Spectrometry (GC-MS) analysis.
Gas Chromatography-Mass Spectrometry Analysis
The GC-MS analysis of the essential oil of cardamom seeds was carried out using an instrument stands at the Department of Medicinal and Aromatic Plants Research, National Research Center with the following specifications. Instrument: a TRACE GC Ultra Gas Chromatographs (THERMO Scientific Corp., USA), coupled with a THERMO mass spectrometer detector (ISQ Single Quadruple Mass Spectrometer). The GC-MS system was equipped with a TR-5 MS column (30 m x 0.32 mm i.d., 0.25 μm film thickness). Analyses were carried out using helium as carrier gas at a flow rate of 1.0 mL/min and a split ratio of 1:10 using the following temperature program: 60oC for 1 min; rising at 4.0 oC/min to 240 oC and held for 1 min. The injector and detector were held at 210oC. Diluted samples (1:10 hexane, v/v) of 1 μL of the mixtures were always injected. Mass spectra were obtained by electron ionization (EI) at 70 eV, using a spectral
Table 1: The chemical composition of cardamom oil by GC-MS
| No | Compounds | RT | Peak Area (%) |
| 1 | L-Phellandrene | 4.24 | 0.05 |
|
2 |
α-Pinene |
4.43 | 0.46 |
| 3 | Sabinene | 5.40 | 1.13 |
| 4 |
2-α-PINENE |
5.56 | 0.15 |
| 5 |
α-Myrcene |
5.81 | 0.59 |
| 6 | 2,3-Dehydro-1,8-Cineole | 5.91 | 0.10 |
|
7 |
Octanal (CAS) | 6.42 | 0.07 |
| 8 |
α-Terpinene |
6.68 | 0.32 |
| 9 | D-Limonene | 7.07 | 1.66 |
| 10 | 1,8-Cineole | 7.22 | 27.10 |
|
11 |
α-Ocimene |
7.62 | 0.12 |
| 12 |
λ-Terpinene |
8.05 | 0.56 |
| 13 | Trans Sabinene Hydrate | 8.57 | 0.18 |
| 14 |
α-Terpinolene |
8.98 | 0.38 |
|
15 |
Linalool | 9.63 | 6.61 |
| 16 | 1-Terpineol | 10.63 | 0.08 |
| 17 | Linalyl Propionate | 12.57 | 0.19 |
| 18 | L-4-Terpineol | 12.90 | 1.99 |
|
19 |
α-Terpineol |
13.60 | 4.62 |
| 20 | N-Octyl Acetate | 14.11 | 0.05 |
| 21 | Nerol (CAS) | 14.77 | 0.06 |
| 22 | Z-Citral | 15.43 | 0.14 |
| 23 | Linalyl Acetate | 15.59 | 2.09 |
| 24 | Geraniol | 15.87 | 1.37 |
| 25 | E-Citral | 16.75 | 0.21 |
| 26 | Ocimenyl Acetate | 18.35 | 0.16 |
| 27 |
α-Terpinenyl acetate |
19.82 | 47.40 |
| 28 | Neryl Acetate | 20.33 | 0.13 |
| 29 | Geranyl Acetate | 21.19 | 0.75 |
| 30 |
α-Terpinyl Propionate |
23.34 | 0.12 |
| 31 | Á-Selinene (CAS) | 25.43 | 0.08 |
| 32 |
λ-Muurolene |
26.42 | 0.08 |
| 33 | Nerolidol | 28.44 | 0.92 |
| 34 | Trans-Farnesol | 28.82 |
0.05 |
range of m/z 40-450 17. The identification of the chemical constituents of the EO were deconvoluted using AMDIS software (www.amdis.net) and identified by its retention indices (relative to n-alkanes C8-C22), mass spectrum matching to authentic standards (when available), and Wiley spectral library collection and NIST library database. Most of the compounds were identified using two different analytical methods: (a) KI, Kovats indices in reference to n-alkanes (C9-C22) (National Institute of Standards and Technology); and (b) mass spectra (authentic chemicals, Wiley spectral library collection and NSIT library).
Experimental Design
Rats were divided into six groups. Group I (control negative group): rats that fed only standard diet. Group II (control positive group) (n=15): rats treated with 500 mg/kg bw. PCM orally. Group III (n=15): rats treated with silymarin+ PCM (500 mg/kg bw for both), Group IV (n=15) rats treated with cardamoms+ PCM (100 mg/kg bw +500 mg/kg bw, respectively). Group V (n=5): rats treated with silymarin (500 mg/kg bw). Group VI (n=5): rats treated with cardamom (100 mg/kg bw.). All treatments were administered orally for 14 days.
Table 2: Effect of oral administration of cardamom oil on erythrogram parameters in paracetamol-induced hepatotoxicity in rats.
| Group | Weeks |
RBCs x 106 /ul |
Hb g/dl | PCV % | MCV fl | MCH pg | MCHC % |
|
Control |
1st |
5.01±0.17a |
15.70±1.14a |
28.83±3.70a |
57.77±9.49a |
31.17±2.46a |
54.87±6.63a |
|
2nd |
5.04±0.18a |
15.60±1.12a |
28.01±3.40a |
55.19±8.33a |
31.02±2.53a |
54.23±6.78a |
|
|
3rd |
5.01±0.17a |
15.70±1.14a |
28.83±3.70a |
57.77±9.49a |
31.17±2.46a |
54.87±6.63a |
|
|
Paracetamol |
1st |
3.97±0.26b |
12.60±0.82b |
22.27±2.02b |
56.07±1.62a |
31.70±0.44a |
54.67±1.50a |
|
2nd |
4.45±0.52a |
13.93±0.31a |
25.40±0.46a |
57.63±8.01a |
31.50±3.20a |
54.87±2.15a |
|
|
3rd |
4.96±0.15a |
14.97±0.75a |
28.83±0.75a |
58.10±0.66a |
30.17±1.29a |
51.90±1.65a |
|
|
Paracetamol + silymarin |
1st |
4.99±0.09a |
15.60±0.75a |
27.47±1.24a |
55.03±1.64a |
31.27±2.06a |
56.93±4.96a |
|
2nd |
5.03±0.11a |
15.60±0.92a |
27.93±1.31a |
55.50±2.19a |
30.97±1.30a |
55.93±4.40a |
|
|
3rd |
4.99±0.10a |
14.67±0.15a |
29.03±1.70a |
58.13±2.25a |
29.43±0.76a |
50.63±3.23a |
|
|
Paracetamol + cardamom |
1st |
5.01±0.11a |
15.63±0.74a |
27.30±0.46a |
54.50±2.08a |
31.20±1.85a |
57.27±1.97a |
|
2nd |
5.03±0.13a |
15.50±0.40a |
27.27±0.29a |
54.27±1.94a |
30.87±1.42a |
56.83±1.00a |
|
|
3rd |
5.07±0.08a |
16.00±0.26a |
32.37±0.40a |
63.83±0.40a |
31.57±0.12a |
49.43±0.25a |
Values represent mean ± SD
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
Clinicopathological Examinations
Blood samples were collected weekly for 3 weeks from the first 4 groups (5 rats from each group) and after 2 weeks from 5th and 6th groups. Blood samples were collected from retro-orbital venous plexus of rats under light ether anesthesia. Each sample was separated into 2 portions. The 1st portion was received on dipotassium EDTA for the standard hemogram (CBC) utilizing Animal Blood cell Counter (ABC Vet, France) according to Feldman et al. (2000). The 2nd portion of blood was collected in plain tubes for serum separation. Serum samples were analyzed for total antioxidant capacity (TAC) (Koracevic et al., 2001), glucose, total protein &albumin (Doumas and Biggs, 1972), serum creatinine (Tabacco et al., 1979), blood urea (Fabiny and Ertingshausen, 1971), as well as activities of aspartate aminotransferase (AST) (Tietz, 1986)., and alanine aminotransferase (ALT) (Reitman and Frankel, 1957). All the biochemical analyses of serum were estimated spectrophotometrically utilizing commercial test kits supplied by Stan Bio Laboratories incorporation, USA.
Histopathological Examinations
Liver and kidneys specimens from all groups (3 rats from each group) at last week of experiment were collected and fixed in neutral buffered formalin 10%, routinely processed and embedded in paraffin wax. Paraffin blocks were sectioned at 4-5 µm thickness and stained with Hematoxylin and Eosin (Bancroft and Stevens, 2008) for histopathological examination by light microscope (Olympus BX50, Japan).
Statistical Analysis
Data are expressed as mean ± SD. Variables were statistically analyzed by one-way ANOVA followed by a Tukey post hoc test, was used to compare multiple groups, and all comparisons were significant when p ≤ 0.05 using software COSTAT (version 6.400, Cohort software, USA).
RESULTS
Chemical Compositions of Cardamom Essential Oil
The chemical composition of cardamom essential oil was analyzed by GC-MS showed that the monoterpene hydrocarbon, was present with relatively low concentrations but the oil was rich in monoterpenoids. The main constituents of the oil were α-terpinenyl acetate (47.40%), 1,8-cineole (29.2%), α-terpineol (4.62%) linalyl acetate (2.09%) and D-limonene (1.66%) in cardamom (Table 1).
Hemogram Results
Data of erythrogram and leucogram in PCM-intoxicated are illustrated in Tables (2 & 3) respectively, all the hematological parameters were found to be not significantly different from control group. Paracetamol induced normocytic normochromic anemia during the 1st week of administration. Moreover, neither cardamom nor silymarin in normal rats induce any alterations in erythrogram and leucogram in normal rats (Table 4).
Biochemical Results
Assessment of changes in protein profile, activities of ALT, AST, glucose in addition to some renal biomarkers (urea and creatinine) are presented in Table 5. Results of PCM-intoxicated rats revealed significant decrease in total protein and albumin in with significant hyperglycemia and elevation in serum transaminases urea and creatinine which were significantly ameliorated in groups receiving
Table 3: Effect of oral administration of cardamom oil on leucogram parameters in paracetamol-induced hepatotoxicity in rats.
| Group | Weeks |
TLCx 103 /µL |
Neutrophil % | Lymphocyte% | Monocyte % | Eosinophil % |
|
Control |
1st |
9.27±1.01a |
36.67±10.26a |
54.00±6.00a |
4.67±2.31a |
4.67±2.31a |
|
2nd |
9.21±1.02a |
36.14±10.47a |
53.00±6.00a |
4.61±2.34a |
4.61±2.33a |
|
|
3rd |
9.27±1.01a |
36.67±10.26a |
54.00±6.00a |
4.67±2.31a |
4.67±2.31a |
|
|
Paracetamol |
1st |
8.93±0.47a |
37.33±4.62a |
56.67±2.08a |
3.67±1.53a |
2.33±1.53a |
|
2nd |
9.43±0.57a |
36.00±5.29a |
54.33±5.51a |
6.00±0.00a |
3.67±1.53a |
|
|
3rd |
9.20±0.50a |
35.33±6.11a |
54.33±5.51a |
6.00±0.00a |
4.33±1.53a |
|
|
Paracetamol + silymarin |
1st |
8.77±0.40a |
34.00±2.00a |
57.33±2.08a |
5.67±0.58a |
3.33±0.58a |
|
2nd |
9.20±0.44a |
33.33±4.16a |
59.33±0.58a |
4.33±2.08a |
3.00±1.73a |
|
|
3rd |
8.90±0.44a |
36.00±2.00a |
52.67±2.08a |
6.00±0.00a |
5.33±0.58a |
|
|
Paracetamol + cardamom |
1st |
8.63±8.90a |
36.00±36.00a |
58.00±56.50a |
3.33±4.33a |
2.67±3.25a |
|
2nd |
8.73±9.16a |
40.00±36.50a |
52.67±55.08a |
5.33±5.08a |
2.00±3.33a |
|
|
3rd |
9.20±9.14a |
36.67±36.17a |
55.00±54.00a |
5.33±5.50a |
3.00±4.33a |
Values represent mean ± SD
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
Table 4: Effect of oral administration of cardamom oil and silymarin on Complete Blood Count in normal rats
| Group |
RBCs x 106 /ul |
Hb g/dl |
PCV % | MCV fl | MCH pg | MCHC % |
TLC x 103 /µL |
Neutrophil % | Lymphocyte % | Monocyte % | Eosinophil % |
| Control |
5.01 ±0.17a |
15.7 ±1.14a |
28.83 ±3.70a |
57.77 ±9.49a |
31.17 ±2.46a |
54.87 ±6.63a |
9.27 ±1.01a |
36.67 ±10.26a |
54.00 ±6.00a |
4.67 ±2.31a |
4.67 ±2.31a |
| Silymarin |
5.07 ±0.15a |
14.53 ±0.35 a |
28.30 ±1.31a |
55.87 ±1.04a |
28.70 ±0.70a |
51.43 ±1.66a |
9.47 ±0.57a |
31.33 ±5.03a |
60.00 ±3.00a |
5.67 ±0.58a |
3.00 ±1.73a |
| Cardamom |
5.10 ±0.20a |
14.83 ±0.40a |
29.53 ±2.65a |
57.80 ±2.90a |
29.10 ±0.36a |
50.40 ±3.15a |
9.13 ±0.25a |
32.67 ±3.06a |
56.33 ±2.08a |
6.00 ±0.00a |
5.00 ±1.00a |
Values represents means ± SD
Means with different letters within the same column are significantly different p ≤ 0.05
Table 5: Effect of oral administration of cardamom oil on some biochemical parameters in paracetamol-induced hepatotoxicity in rats.
| Group | Weeks | Total protein (g/dl) |
Albumin (g/d) |
Globulin g/dl) |
A/G Ratio | ALT (U/l) | AST (U/l) |
Glucose (mg/dl) |
Urea (mg/dl) |
Creatinine (mg/dl) |
|
Control |
1st |
8.43 ±0.35a |
3.33 ±0.21a |
5.10 ±0.56a |
0.66 ±0.11a |
39.40 ±4.51d |
138.90 ±1.39c |
94.60 ±1.59bc |
39.63 ±1.10d |
0.42 ±0.03b |
|
2nd |
7.17 ±0.35a |
3.17 ±0.15a |
4.00 ±0.35a |
0.80 ±0.09a |
73.20 ±5.37b |
186.83 ±5.08b |
80.90 ±2.72b |
42.67 ±1.29b |
0.65 ±0.01b |
|
|
3rd |
7.17 ±0.35a |
3.17 ±0.15a |
4.00 ±0.35a |
0.80 ±0.09a |
73.20 ±5.37b |
186.83 ±5.08b |
80.90 ±2.72b |
42.67 ±1.29b |
0.42 ±0.03b |
|
|
Paracetamol |
1st |
7.43 ±0.35b |
2.73 ±0.21b |
4.70 ±0.26a |
0.66 ±0.11a |
103.73 ±1.10a |
236.95 ±3.6a |
142.13 ±13.70a |
59.73 ±1.99a |
0.50 ±0.03a |
|
2nd |
6.43 ±0.21b |
2.40 ±0.20b |
4.37 ±0.57a |
0.8 0 ±0.09a |
95.37 ±6.70a |
262.40 ±0.69a |
104.77 ±9.47a |
49.73 ±1.31a |
0.73 ±0.05a |
|
|
3rd |
7.17 ±0.15a |
3.13 ±0.06a |
4.03 ±0.12a |
0.80 ±0.09a |
71.87 ±1.54a |
162.67 ±8.50a |
100.57 ±4.45a |
45.63 ±1.16b |
0.45 ±0.01a |
|
|
Paracetamol + silymarin |
1st |
8.20 ±0.53ab |
3.33 ±0.15a |
4.87 ±0.67a |
0.70 ±0.13a |
80.77 ±1.33b |
170.70 ±5.00b |
109.80 ±6.24b |
49.20 ±0.56b |
0.49 ±0.02a |
|
2nd |
7.23 ±0.21a |
3.27 ±0.06a |
3.97 ±0.23a |
0.82 ±0.06a |
84.73 ±4.21ab |
168.03 ±3.69c |
93.63 ±3.20ab |
38.80 ±2.82c |
0.58 ±0.02c |
|
|
3rd |
7.10 ±0.85a |
3.07 ±0.12a |
4.03 ±0.87a |
0.78 ±0.16a |
58.27 ±1.63b |
143.67 ±1.53b |
104.90 ±3.80a |
48.27 ±1.57a |
0.45 ±0.02a |
|
|
Paracetamol + cardamom |
1st |
8.23 ±0.23ab |
3.23 ±0.21ab |
5.00 ±0.17a |
0.65 ±0.05a |
55.70 ±3.12c |
134.47 ±4.15c |
82.47 ±6.57c |
46.30 ±0.95c |
0.41 ±0.04b |
|
2nd |
6.90 ±0.10ab |
3.23 ±0.15a |
3.60 ±0.10a |
0.90 ±0.06a |
87.83 ±5.95ab |
167.03 ±2.19c |
88.00 ±1.39b |
44.73 ±0.81b |
0.56 ±0.05c |
|
|
3rd |
7.20 ±0.30a |
3.30 ±0.10a |
3.90 ±0.36a |
0.85 ±0.10a |
67.53 ±3.58a |
130.77 ±4.20c |
90.97 ±6.18a |
44.93 ±0.75bc |
0.46 ±0.05a |
Values represent mean ± SD
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
cardamom oil or silymarin at p ≤ 0.05.
Paracetamol treated rats showed significant decrease in TAC compared to control, in the meantime cardamom and silymarin dosages significantly elevate total antioxidant capacity at p ≤ 0.05 (Table 6). The ameliorative effects of cardamom and silymarin against hepato-renal changes in PCM-intoxicated rats was observed as their serum biochemical parameters behaved rather similar to control negative group. Moreover, neither cardamom nor silymarin in normal rats induce any alterations in serum biochemistry and in TAC activity normal rats (Tables 7).
Table 6: Effect of oral administration of cardamom oil on Total Antioxidant Capacity parameters in paracetamol-induced hepatotoxicity in rats.
| Group | Weeks | TAC (mmol/l) |
|
Control |
1st |
1.87 ±0.06b |
|
2nd |
1.87 ±0.06b |
|
|
3rd |
1.38 ±0.02a |
|
|
Paracetamol |
1st |
1.27 ±0.06c |
|
2nd |
1.37 ±0.06c |
|
|
3rd |
138 ±0.02a |
|
|
Paracetamol + silymarin |
1st |
2.27 ±0.06a |
|
2nd |
2.00 ±0.00a |
|
|
3rd |
1.37 ±0.02a |
|
|
Paracetamol + cardamom |
1st |
2.17 ±0.06a |
|
2nd |
2.07 ±0.06a |
|
|
3rd |
1.36 ±0.03a |
Values represent mean ± SD
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
Histopathological Findings
Liver Tissue
Liver of control rats revealed normal histological structure of hepatic lobule from central vein surrounded by radiating hepatocytes forming hepatic cords (Fig. 1a). On the contrary, liver of PCM treated rats revealed remarkable histopathological alterations described by hepatocellular vacuolization, sinusoidal leukocytosis (Fig. 1b) and hepatocellular necrosis associated with inflammatory cells infiltration (Fig. 1c). Moreover, Kupffer cells activation, apoptosis of hepatocytes (Fig. 1d) and portal fibrosis (Fig. 1e) were also recorded in all examined sections. Marked amelioration of the histopathological picture was noticed in liver of rats co-treated with PCM+ silymarin, the examined sections showed no histopathological changes except Kupffer cells activation was noticed in some sections (Fig. 1f). Moreover, liver of rats treated with silymarin revealed no histopathological alterations (Fig. 1g). Regression of the histopathological lesions was noticed in liver of rats co-treated with PCM + cardamom, as slight activation of Kupffer cells and binucleation of hepatocytes (Fig. 1h). No histopathological alterations were recorded in liver of rats treated with cardamom alone (Fig. 1i).
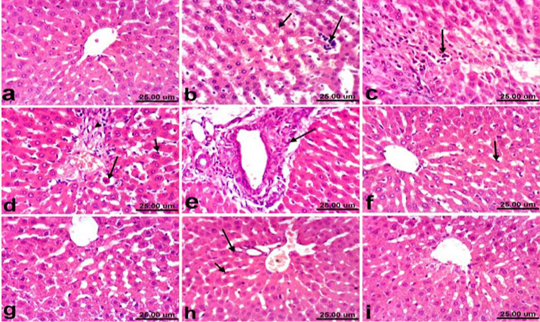
Figure 1: Liver of rat a) from control, normal group showing the normal histological structure of hepatic lobule from central vein surrounded by radiating hepatocytes. b), c), d) and e) treated with paracetamol, b) showing hepatocellular vacuolization (short arrow) and sinusoidal leukocytosis (long arrow). c) showing focal hepatocellular necrosis associated with inflammatory cells infiltration (arrow). d) showing Kupffer cells activation (short arrow) and apoptosis of hepatocytes (long arrow). e) portal fibrosis (arrow). f) co-treated with paracetamol + silymarin showing Kupffer cells activation (arrow). g) treated with silymarin showing no histopathological alterations. h). co-treated with paracetamol + cardamom showing slight activation of Kupffer cells (short arrow) and binucleation of hepatocytes (long arrow). i) treated with cardamom showing no histopathological alterations. (H & E, scale bar 25 um).
Table 7: Effect of oral administration of cardamom oil and silymarin on biochemical parameters in normal rats
| Group | TAC (mmol/l) |
Total protein (g/dl) |
Albumin (g/d) |
Globulin g/dl) |
A/G Ratio | ALT (U/l) |
AST (U/l) |
Glucose (mg/dl) |
Urea (mg/dl) |
Creatinine (mg/dl) |
| Control |
1.87 ±0.06b |
7.17 ±0.35a |
3.17 ±0.15a |
4.00 ±0.35a |
0.80 ±0.09a |
73.20 ±5.37a |
186.83 ±5.08a |
80.90 ±2.72a |
42.67 ±1.29a |
0.65 ±0.01a |
| Silymarin |
2.06 ±0.12a |
7.03 ±0.15a |
3.17 ±0.06a |
3.87 ±0.15a |
0.82 ±0.04a |
75.20 ±4.13a |
195.27 ±6.02a |
85.01 ±1.50a |
46.43 ±4.29 a |
0.66 ±0.03a |
| Cardamom |
2.03 ±0.06a |
7.33 ±0.68a |
3.27 ±0.06a |
4.07 ±0.67a |
0.82 ±0.13a |
78.23 ±2.4a |
194.67 ±6.03a |
84.67 ±1.53a |
48.4 ±3.27a |
0.67 ±0.01a |
Values represents means± SD
Means with different letters within the same column are significantly different p ≤ 0.05
Renal Tissue
Regarding kidneys, examined sections from control rats revealed normalized histological structure of renal tissue which consists from renal cortex and medulla (Fig. 2a). Meanwhile, kidneys of rats treated with PCM showed varia variable histopathological alterations which manifested by vacuolar degeneration of renal tubular epithelium, slight atrophy of glomerular tufts associated with distension of Bowman’s space, thickening of the parietal layer of Bowman’s capsule and focal inflammatory cells infiltration (Fig. 2b). However, kidneys of rats co-treated with PCM + silymarin showed restored histological structure and apparent normal renal tissue (Fig. 2c). Moreover, kidneys of rats treated with silymarin revealed no histopathological alterations (Fig. 2d). On the other hand, improved histopathological picture was noticed in kidneys of rats co-treated with PCM + cardamom. Slight vacuolization of epithelial lining some renal tubules was the only histopathological finding observed in sections from this group (Fig. 2e). In addition, kidneys of rats treated with cardamom revealed no histopathological alterations (Fig. 2f).
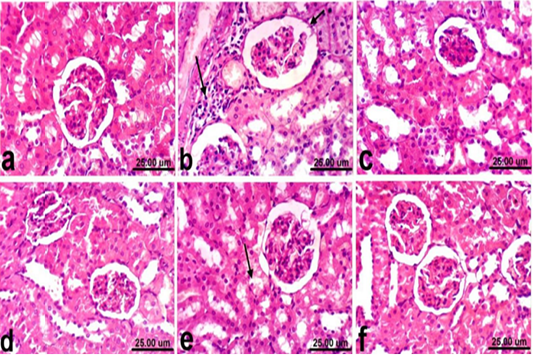
Figure 2: Kidney of rat a) from control, normal group showing the normal histological structure of renal parenchyma from renal cortex and medulla. b) Treated with paracetamol showing thickening of the parietal layer of Bowman’s capsule (short arrow) and focal inflammatory cells infiltration (long arrow). c) Co-treated with paracetamol+ silymarin showing apparent normal renal tissue. d) Treated with silymarin showing no histopathological alterations. e) co-treated with paracetamol + cardamom showing slight vacuolization of epithelial lining some renal tubules (arrow). f) Treated with cardamom showing no histopathological alterations. (H & E, scale bar 25 um).
DISCUSSION
Drugs are the major cause of liver failure that increasing every year (Lakshmi et al., 2018). Hepato-renal dysfunction is considered one of the major inevitable drugs complications (Cepa et al., 2018). Paracetamol is one of the most popular pain killer (analgesic) that used as an alternative for NSAIDs, which is commonly used without a prescription especially in children (Soha, 2017). It’s usually considered safe when used in its therapeutic dose, but overdose is justly common and frequently associated with hepatic and renal injury in both humans and experimental animals (Samuel et al., 2015). The present study attempted to explore the hepatoprotective activity of cardamom (Elettaria cardamomum) compared to silymarin against PCM-induced hepato-renal toxicity in adult male albino rats via the assessment of hematological, serum biochemical and histopathological changes associated with the administration of PCM and/or the herbal extracts to albino rats.
The main constituents of the cardamom oil were α-terpinenyl acetate (47.40%), 1,8-cineole (29.2%), α-terpineol (4.62%) linalyl acetate (2.09%) and D-limonene (1.66%) in cardamom. The results of GC-MS analysis indicated that chemical composition profiles obtained for cardamom oil were very similar to the previous results of different researchers with minor differences (Savan and Kucukbay, 2013). These five constituents of the essential oil possessed variable antioxidant activity when tested in the DPPH assay for non-specific hydrogen atom or electron donating activity (Mutlu-Ingok and Karbancioglu-Guler, 2017). Our data of present study strongly suggest that oral administration of cardamom essential oil might protect the liver against toxicity induced by PCM (500 mg/kg, p.o.) in male Sprague Dawley rats. The hepatotoxicity induced by PCM manifested biochemically by significant elevation of serum transaminases enzyme activities, urea and creatinine concentrations and decrease in TAC level.
Regarding the hemogram, PCM toxicity revealed normocytic normochromic anemia during the 1st week which may be related to destruction of mature RBC, reduction in the rate of erythropoiesis or inhibition of erythropoietin released from the kidney (Samuel et al., 2015).
Serum biochemical results illustrated that the daily dosage of PCM (500 mg/kg) for two weeks caused elevations of serum liver enzymes (ALT & AST) and significant decrease in total protein, albumin in comparison to control group. These results are in agreement with the previous experiments (Fatemi et al., 2010; Dadkhah et al., 2015). Damaged structural integrity of the liver by PCM leads to release of cytoplasmic enzymes or to changes in hepatocyte membrane permeability and/or increased synthesis or decreased catabolism of aminotransferases (Lebda et al., 2013). Our results are confirmed by the histopathological findings which revealed remarkable hepatocellular vacuolization, sinusoidal leukocytosis and hepatocellular necrosis associated with inflammatory cells infiltration. Moreover, Kupffer cells activation, apoptosis of hepatocytes (Fig. 1d) and portal fibrosis were also recorded in all examined sections of liver in PCM treated rats. These alterations may be due to formation of large quantity of NAPQI, the PCM metabolites that promotes histopathological alteration of hepatocytes. These results were consistent with that reported by Hinson et al. (2010); Hamza and Al-Harbi, (2015).
Concerning protein profile our statistical analysis revealed significant decrease in both total protein and albumin in PCM treated rats, this is agreed with Lebda et al. (2013) and Hamza and Al-Harbi, (2015). They ascribed this to the decline in the number of cells responsible for albumin synthesis in the liver through necrosis. On the other hand, significant hyperglycemia was noticed in rats treated with acetaminophen. This is may be related to hepatotoxicity, or due to direct effect of PCM toxic metabolites on pancreatic beta cell ultrastructure or due to body response to stress enzymes (MacFie et al., 2009). Regarding TAC results PCM overdose leads to decreased TAC which may resulted from generation for more NAPQI, which is consequently adducted with mitochondrial proteins, leading to ROS formation and oxidant stress (Du et al., 2016; Ahmed et al., 2019). However, treatment with cardamom and silymarin improved TAC capacity which may be due to the ameliorating effect of both silymarin and cardamom as both have the antioxidant effect (Madrigal-Santillán et al., 2014; Aboubakr and Abdelazem, 2016; Ahmed et al., 2019).
Rats treated with PCM showed significant increase in serum urea and creatinine comparable to control group and this is supported by histopathological picture of the kidneys that showed vacuolar degeneration of renal tubular epithelium, slight atrophy of glomerular tufts associated with distension of Bowman’s space, thickening of the parietal layer of Bowman’s capsule and focal inflammatory cells infiltration.
This may be due to nephrotoxicity that results from formation of reactive oxygen species and increased oxidative stress due to depletion of GSH levels (Canayakin et al., 2016). On the other hand, the administration of cardamom ameliorated PCM induced acute liver and kidney injuries in rats, as evidenced by histologic, hematologic and biochemical findings. Similar protective effects were also observed in rats receiving silymarin. The protective effects of cardamom and silymarin in the restoration of normal liver and kidney functions is confirmed by histopathological findings which revealed that the examined liver sections of rats co-treated with PCM + silymarin, showed no histopathological changes except Kupffer cells activation was noticed in some sections. Regression of the histopathological lesions was noticed in liver of rats co-treated with PCM + cardamom, mild changes were observed as slight activation of Kupffer cells and binucleation of hepatocytes. These effects could be attributed to protection of membrane integrity which may be due to the antioxidant potential of cardamom and silymarin (El-Segaey et al., 2007, Khare et al., 2012, Aboubakr and Abdelazem, 2016; Ahmed, et al. 2019). Moreover, kidneys of rats co-treated with PCM + silymarin showed restored histological structure and apparent normal renal tissue. On the other hand, improved histopathological picture was noticed in kidneys of rats co-treated with PCM + cardamom. Slight vacuolization of epithelial lining some renal tubules was the only histopathological finding observed in sections from this group. These effects could be attributed to improvement on glomerular function of kidney and maintaining positive nitrogen balance in addition to its antioxidant and free radical scavenging properties (Bektur et al., 2013; Elkomy et al., 2016).
CONCLUSION
The present research demonstrated that cardamom essential oil diminished acute PCM-mediated hepatorenal damage by inducing in vivo antioxidant activity and ameliorating the histopathological changes as well as biochemical and hematological parameters in rats. It is conclusive to use cardamom oil as a protective drug against oxidative stress mediated toxicities in future. Further studies are needed to explore the exact mechanism of antioxidant properties of this promising oil.
acknowledgements
The authors express sincere thanks to Dr Khaled Amer the major general of Egypt Center for Research and Regenerative Medicine, Military Forces, Cairo, Egypt. Many thanks to departments of Clinical Pathology, Pharmacology, and Pathology Faculty of Veterinary Medicine, Cairo University, and National Research Centre, Dokki, Giza, Egypt.
conflict of interest
The authors declare no conflicts of interest.
authors contribution
All authors contributed equally to the manuscript.
REFRENCES






