The Journal of Advances in Parasitology
Research Article
Prevalence of Gastrointestinal Parasitic Infections in Different Existing Goat Breeds in Different Districts of Bangladesh
Muhammad Al Amran1, Saroj Kumar Yadav2*, Ferdoshe Akter3, Sudeb Sarkar3, Md Amir Hossain3, Subrata Malaker Joy4, Al-Ameen Khalid Samrat1
1Veterinary Collegiate Hospital (DAPVCH), Companiganj-3850, Noakhali, Bangladesh; 2Department of Medicine and surgery, Chittagong Veterinary and Animal Sciences University, Khulshi, Chit¬tagong-4202, Bangladesh; 3Upazilla Livestock Office, Bangladesh; 4Bangladesh Agriculture University, Mymensing, Bangladesh.
Abstract | A one-year long epizootiological survey was conducted to study the prevalence of gastrointestinal (GI) parasitic diseases in goats (Capra hircus) of three geographical areas of Bangladesh including Central Veterinary hospital (CVH) of Dhaka& S.A. Quaderi Teaching Veterinary Hospital (SAQTVH) of Chittagong, and Veterinary Hospital of Bangladesh Agricultural University (BAU), Mymensingh. Fecal samples were evaluated by routine coproscopical methods. The effects of topography, season, age and gender on GI parasitic infection were evaluated by chi-square test and t-test. An overall parasitic burden was nearly similar in all the study areas; 63.88% in samples from CVH, 62.13% in samples from BAU Veterinary Hospital and 59.43% in samples from S.A. Quaderi Teaching Veterinary Hospital. The highest prevalence of Trematodes infection was recorded in BAU Veterinary Hospital (40%) compared to CVH (6%) and S.A. Quaderi Teaching Veterinary Hospital (2%). Prevalence of Nematodes infection was the highest (66%) in goats of CVH. Among nematodes, the highest prevalence was recorded for Haemonchus (39.81%) in CVH. Prevalence of cestode was remarkably low in three study areas. GI parasitic infections were more common in female BB(63%), Jamunapari (64%), when the animals were more than 1 year old (61%) and among goats of Dhaka (68%) region. It was observed a heterogenous occurrence of some GI parasitic diseases according to different regions which might indicate the presence of some risk factors in the study areas. The present study could serve as a baseline study for further extensive experiments to evaluate region-specific risk factors.
Keywords | Black Bengal, Gastrointestinal parasites, Haemonchus, Jamunapari, Prevalence
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | September 28, 2017; Accepted | March 02, 2018; Published | March 28, 2018
*Correspondence | Saroj Kumar Yadav, Department of Medicine and surgery, Chittagong Veterinary and Animal Sciences University, Khulshi, Chit¬tagong-4202, Bangladesh; Email: [email protected]
Citation | Amran MA, Yadav SK, Akter F, Sarkar S, Hossain MA, Joy SM, Samrat AAK (2018). Prevalence of gastrointestinal parasitic infections in different existing goat breeds in different districts of bangladesh. J. Adv. Parasitol. 5(1): 11-21.
DOI | http://dx.doi.org/10.17582/journal.jap/2018/5.1.11.21
Copyright © 2018 Amran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In Bangladesh, livestock is one of the most potential sub-sectors of agriculture which plays an indispensable role in promoting human health and national economy of the country. Livestock not only assists to upgrade the financial condition but also makes a substantial contribution to human nutrition. However, livestock is an integral part of a farming system which has a better contribution to enhancing the economy of Bangladesh. Large ruminants (Cattle and Buffalo) and small ruminants (sheep and goat) constitute the major portion of livestock. The present population of livestock is 23.7 million cattle, 1.47 million Buffalo, 25.76 million goats and 3.3 million sheep (DLS, 2015-16). The total contribution of livestock sub-sector to Gross Domestic Product (GDP) in Bangladesh is approximately 7.23% and livestock in agricultural production 17.32% (Anonymous, 2007). It also generates 13% of foreign currency and provides 20% fulltime employment and 50% partial employment of rural population (Alam, 1993). In this country, 80% rural people are involved with livestock farming. Most animals are reared in houses under the traditional husbandry practices where small ruminant especially goat and sheep are mainly reared for several reasons including meat, wool and skin production (Hossain et al., 2004). The production and productivity of animals are greatly hampered by different diseases including gastrointestinal parasitic infections.Gastrointestinal parasitism is a world-wide problem (Regassa et al., 2006). The losses caused by parasitic infections are in the form of lowered general health condition, retarded growth rate, diminishing the working efficiency, decrease milk and meat production, abortion, cost associated with preventive measures and reduces the disease resistance capability, which may ultimately lead to higher mortality (Chavhan et al., 2008; Silvestre et al., 2000). Gastrointestinal parasitic infections especially Fascioliasis, Haemonchosis, Trichostrongylosis, Oesophagostomiasis, and Monieziosis impaired the growth and productivity of goats (Speedy, 1992). It was observed that 25% kids and 43% adult goat die of gastrointestinal parasitism under both rural and farm condition (Rahman et al., 1975). Moreover, the country losses lots of money annually due to mortality, stunted growth, production loss, abortion and poor quality skin due to ectoparasitic infestation (Dewan et al., 1979). Despite significant losses by gastrointestinal parasitism, the problems are often neglected and overlooked a majority of the infected animals show a number of little obvious clinical signs throughout their productive life and their effects are gradual and chronic (Raza et al., 2010). Nevertheless, among the gastrointestinal parasitic diseases, haemonchosis caused by Haemonchus spp which is a predominantly a highly pathogenic and economically important disease of sheep and goats (Nwosu et al., 2007). It is a serious health problem, which causes lower production due to high morbidity, mortality, and cost of treatment and control measures. These parasites are common blood feeders that cause anemia and reduced productivity and can lead to death in heavily infected animals (Githigia et al., 2001). It has been estimated that each worm sucks about 0.05 ml of blood per day or responsible for continuous seepage of blood from feeding site resulting severe anemia and death of the individual (Urquhart et al., 2000). The prevalence of haemonchosis is higher in comparison to other gastrointestinal parasites. It is primarily a disease of tropical and subtropical regions of the world. The high humidity at least the microclimate of the feces and the herbage is essential for larval development and their survival. Different studies showed a positive correlation between the occurrence of H. contortus infection and climatic condition. Significantly highest infection rate was recorded during the rainy season (72.57%) followed by summer (66.46%) and winter (51.54%) seasons. The infection was recorded peak in the July (84.42%) and lowest in January (46.15%) (Shahiduzzaman et al., 2003).The geo-climatic conditions of the country also favor the growth, development, and survival of various parasites. An occurrence of gastrointestinal parasitic infections varies greatly depending upon the diverse intrinsic and extrinsic epidemiological and biological factors associated with them (Sardar et al., 2006). Epidemiological pattern of the parasitic diseases in the different agro-climatic zones of the country usually provides a basis for developing strategic and tactical control measures against them. Several epidemiological studies have been conducted on gastrointestinal parasitism of goats in different regions of the country but, a limited investigation was done on gastrointestinal parasitic diseases of goat in Chittagong. Moreover, the current study comprises three different veterinary hospitals of three different areas namely S.A. Quaderi Teaching Veterinary Hospital of Chittagong Veterinary and Animal Sciences University (CVASU), Central Veterinary Hospital (CVH), Dhaka and Veterinary Hospital of Bangladesh Agricultural University (BAU). The hospitals were selected due to their geographical location as well as heavy patient load in those hospitals.
Materials and Methods
Materials and Methodology for Prevalence Study
Study Area and period: The study was conducted in three districts and veterinary hospitals namely, Central veterinary Hospital (CVH), Dhaka, S.A. Quaderi Teaching Veterinary Hospital (SAQTVH), Chittagong Veterinary and Animal Sciences University, Chittagong and Teaching Veterinary Hospital, Bangladesh Agricultural University(BAU), Mymensingh. Takorgaon District Veterinary Hospital and Bera upazilla Livestock office and Pabna District. The investigation was conducted for a period of 12 months starting from July’ 2015 to June’ 2016.
Selection of animals and Survey Design: a) Goat of different breeds like Black Bengal, Jamunapari, a Crossbred goat was selected for this study as target animals.
b) To determine the age and breed susceptibility of different parasites, goats were categorized into two subgroups as one was less than one year(<1 yr) and another was more than one year age (>1 yr).
c) A total of 422 (four hundred twenty-two) fecal samples were collected from 422 individuals which were brought for treatment during the study period.
d) Random sampling was followed during sample collection.
A prototype questionnaire was used to record the information like owner’s name and address, animal Identification (ID), farm size, breed, age, sex, deworming history. In the present study, the minimum age of the goat was 25 days and the maximum was 60 months.
Sample collection and preservation: Only one type of biological samples (faces) were collected during this study where an individual animal was considered as a sampling unit. Approximately 5-10gm of a fecal sample from each individual animal was collected directly from the rectum. However, freshly voided fecal samples were also considered and subsequently, the collected samples were stored in plastic containers. Then, the container was filled with formalin (10%) and refrigerated at 40C temperature. During sample collection, labeling of the samples was strictly maintained to prevent the misinterpretation.
Examination of samples: In addition to gross examination of fecal samples (color, consistency, blood or mucus, etc.), three different types of qualitative tests, namely direct smear, flotation, and sedimentation techniques were used to examine the fecal samples (Hendrix, 2006). Zinc Sulphate solution was used as floatation fluid. At least, two smears were prepared from each sample for each test to identify the morphological characteristics of eggs, cyst, Oocysts etc. (Hendrix, 2006; Soulsby, 1982).
Statistical Analysis
Questionnaire data were entered into the Excel spreadsheet. Descriptive analysis was performed by means of frequency (N, %) of positive and negative sample test results overall and stratified by different explanatory variables. Invariable analysis was conducted using Chi square test and t-test for the selected explanatory variables and those having P-value ≤0.05 were considered significant. Data management and analysis were performed using Microsoft Excel and STATA version 12 (Stata Corp, College Station, Texas).
Results
Results Belong to a Prevalence of Gastrointestinal Parasitic Infections Described with Following Headlines:
• Descriptive statistics of different variables.
• The overall prevalence of gastrointestinal parasites.
• Prevalence on the basis of a type of parasite (Trematodes, Nematodes, and Cestodes).
• Prevalence of different genus of gastrointestinal parasites in goat.
• Median, maximum, minimum, 25th and 75th quartile values of continuous variables (age and body weight) stratified by presence and absence of parasite.
• Relationship of different variables with a presence of gastrointestinal parasites in goats.
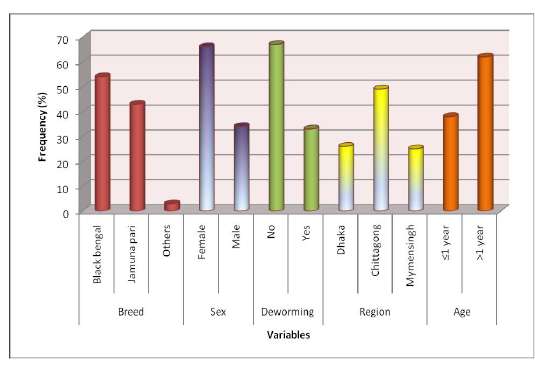
Descriptive statistics of different variables: Samples were collected from different breeds of goat, namely Black Bengal and Jamunapari and some crossbred which constituted 54%, 43%, and 3% samples, respectively. Among all study population 66% was a female goat and the rest 34% were male. Samples were collected from varying aged animals of which 38% were less than or equal 1 years old and the rest (62%) were more than one year old. 67% animals of the study population were not dewormed ever. We collected samples from three different study areas, namely S. A. Quaderi Teaching Veterinary Hospital, Chittagong, Central Veterinary Hospital, Dhaka and Agricultural University Veterinary Hospital, Mymensingh which provided 49%, 26% and 25% samples in the study, respectively. Descriptive statistics of variables are presented in Figure 1.

Figure 1: Experimental Design (at a glance)
Age and body weight of the subjects were collected. Before categorization, mean age of the animals were calculated. Mean age of the animals were nearly similar in Chittagong and Mymensingh (21.73±1.12 and 25.88±1.19, respectively). The mean age of the animals from Dhaka region was 13.28 (SE 0.89). Mean body weight of the animals from Chittagong and Dhaka region was nearly similar (Table 1)
Table 1: Descriptive statistics of continuous variable
| Continuous | Mean | SE | 95% CI | |||||||
| Variables | Ctg | Dhk | Msingh | Ctg | Dhk | Msingh | Ctg | Dhk | Msingh | |
| Age (month) | 21.73 | 13.28 | 25.88 | 1.12 | 0.89 | 1.19 | 19- | 11- | 23-28 | |
| 23 | 15 | |||||||||
| Body weight | 18.77 | 17.15 | 10.87 | 0.66 | 0.78 | 0.41 | 17- | 15- | 10-11 | |
| (kg) | 20 | 18 | ||||||||
Ctg = Chittagong; Dhk = Dhaka; Msingh = Mymensingh; SE = Standard error; CI = Confidence interval
but the lower mean weight was estimated at animals from Mymensingh (10.87±0.41). Narrower confidence intervals for all estimates were observed which might indicate an adequate sample size for the study.
Overall prevalence of gastrointestinal parasites: Nearly similar prevalence of gastrointestinal parasites in selected study areas was recorded (Figure 2). The highest prevalence of overall gastrointestinal parasitic infection in goats was recorded in samples from Central Veterinary Hospital, Dhaka (63.88%). A slightly lower prevalence was estimated in samples of Bangladesh Agricultural University Veterinary Hospital (Mymensingh) (62.13%). In S. A. Quaderi Teaching Veterinary Hospital overall prevalence was estimated as 59.43%. Around 40% goats were free from parasites in the study areas.
Prevalence on the basis of a type of parasite (trematodes, nematodes, and cestodes): Both nematodes (38%) and trematodes (40%) were highly prevalent in samples from Bangladesh Agricultural University Veterinary Hospital (Mymensingh). Samples from the other two study areas (Central Veterinary Hospital, Dhaka, and S. A. Quaderi Teaching Veterinary Hospital) were more positive for nematodes (66% and 49%, respectively) but the prevalence of trematodes was not remarkable (6% and & 2%, respectively) in the mentioned areas. Prevalence of cestode was nearly similar in all three study areas (lowest 3% and highest 8%) (Figure 4).
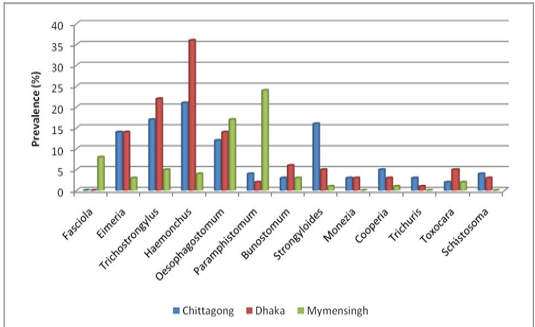
Prevalence of different genus of gastrointestinal parasites in goat: The current study revealed highest prevalence for Haemonchus (20.28%) and lowest prevalence for Toxocara (0.9%) in S .A. Quaderi Teaching Veterinary Hospital of Chittagong. In Central Veterinary Hospital, Dhaka highest prevalence was recorded for Haemonchus (39.81%) and lowest for Trichuris (0.01%). On the ot
er hand, in Bangladesh Agricultural University Veterinary Hospital (Mymensingh) the most prevalent parasite was Paramphistomum (24%) and lowest prevalence was estimated for Strongyloides and Cooperia (01%) (Figure 3).
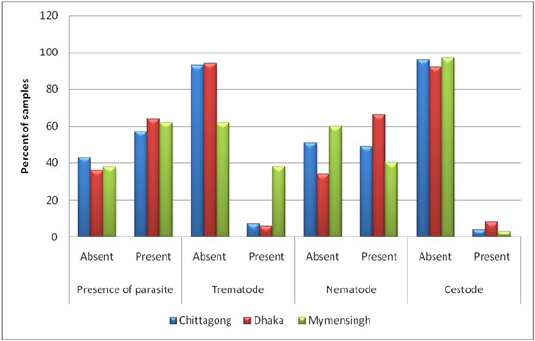
Figure 3: Prevalence on the basis of type of parasite (Trematodes, Nematodes and Cestodes) stratified by study areas.
Considerably higher prevalence was recorded for Strongyloides (15.56%), Trichostrongylus (15.09%), Oesophagostomum (9.90%) and Eimeria (6.60%) than Paraphistomum, Bunostomum, Schistosoma and Moniezia infection in the population of S. A. Quaderi teaching Veterinary Hospital. Besides Haemonchus, a higher prevalence was estimated for Trichostrongylus (24.07%), Eimeria (13.80%), Oesophagostomum (13.80%) and Strongyloides (6.48%) than Paramphistomum, Bunostomum, Schistosoma, Moniezia and Toxocara infection in samples from Central Veterinary Hospital, Dhaka. On the other hand, Oesophagostomum (17%), Fasciola (8%) and Trichostrongylus (5%) were more prevalent in samples from Bangladesh Agricultural University Veterinary Hospital (Mymensingh) in comparison with the prevalence of Bunostomum, Haemonchus, Eimeria and Toxocara (Figure 4).
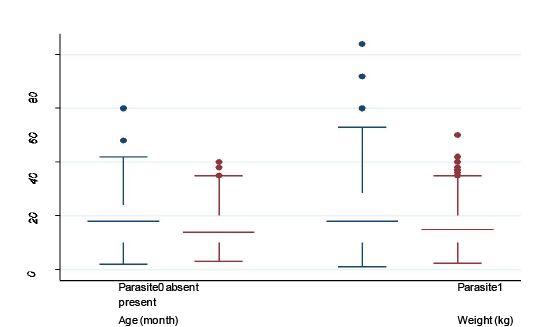
Median, maximum, minimum, 25th and 75th quartile values of continuous variables (age and body weight) stratified by presence and absence of parasite: box plot was created to show the difference in age and body weight in animals with and without parasitic infection
In the present study minimum age was 4 month and maximum age was 42 month for goats without parasitic infection, whereas, minimum and maximum age for the goats with parasitic infection was 3 months and 55 months, respectively.
Minimum and maximum body weights in both groups of goats (with and without parasitic infection) were nearly similar (4 kg and 38 kg in without infection and 4 kg and 39kg in with GI parasitic infection) (Figure 5). However, there were some outliers who had extreme age and body weight (shown as dots). Parasite-infected 25% goats were of 10 months old with 10 kg body weight and 75% goats
were of 30 months old with 20 kg body weight. Median age and median body weight for both groups of goats were nearly similar (Figure 4).
Table 2, We conducted a t-test analysis to identify if mean age and mean body weight was significantly different in groups of goats with and without gastrointestinal parasitic infection (Table 2). Mean age and body weight were slightly different in two groups of goats and the p-value of the t-test was not significant, indicates a less remarkable influence of age and body weight on the occurrence of parasitic infection in goat.
Table 2: comparison of means of continuous variables (age and weight) between the groups of animals with and without gastrointestinal parasitic infection using t-test
| Variables | Parasitic infection | Mean | SE | 95% CI | P-value |
| Age | Present | 21.39 | 0.99 | 19-23 | 0.18 |
| Absent | 19.43 | 0.99 | 17-21 | ||
| Weight | Present | 16.70 | 0.57 | 15-17 | 0.28 |
| Absent | 15.73 | 0.68 | 14-17 |
Relationship of different variables with a presence of gastrointestinal parasites in goats: Chi-square test was performed to identify the association of different variables with the presence of gastrointestinal parasites in goats. Prevalence was higher in females (63%) in comparison with males (56%), however, variables were not significantly associated (p-value = 0.16) (Table 3). It can be concluded that the prevalence was similar in different breeds as the slight difference among the breeds were not statistically significant (p-value = 0.42). A Higher prevalence (63%) was observed in a group of animals who were not dewormed in comparison with the dewormed animals. The highest prevalence was observed in Dhaka region (68%) followed by Mymensingh (62%) and Chittagong (56%), an association was not statistically significant though. After categorization, age was not significantly associated with the parasitic infection status of the animal, concordant with the previous analysis shown in Table 2.
We conducted Chi-square tests to assess the association of different variables with the presence of commonly isolated parasitic genus. The analysis revealed that there is a statistically significant varying prevalence of Haemonchus, Oesophagostomum, Paramphistomum, and Trichostrongylus according to different regions (Table 4). Very low prevalence of Haemonchus was recorded in Mymensingh (4%) in comparison with other two regions (21% in Chittagong and 36% in Dhaka). On the other hand, a very high prevalence of Paramphistomum was recorded in Mymensingh region (35%) in comparison to other regions (Table 4).
Prevalence of Haemonchus and Paramphistomum was significantly varied with breeds. High prevalence of Haemonchus was observed in Jamunapari (28%) but the prevalence of Paramphistomum was lowest in this breed (10%) in comparison with black Bengal and other non-descriptive breeds (Table 4).
Table 3: Relationship of different variables with the presence of gastrointestinal parasite
| Variables | Level | Number of observation (N) | Parasite present (%) | Parasite absent (%) | p-value |
| Sex | Female | 268 | 170 (63) | 98 (37) | 0.16 |
| Male | 140 | 79 (56) | 61 (44) | ||
| Breed | Black | 225 | 131(58) | 94 (42) | 0.42 |
| Bengal | |||||
| Jamunapari | 171 | 110 (64) | 61 (36) | ||
| Others | 10 | 6 (60) | 4 (40) | ||
| Deworming | Yes | 137 | 78 (57) | 59 (43) | 0.24 |
| No | 272 | 171 (63) | 101 (37) | ||
| Region | Chittagong | 204 | 115 (56) | 89 (44) | 0.12 |
| Dhaka | 101 | 69 (68) | 32 (32) | ||
| Mymensingh | 103 | 64 (62) | 39 (38) | ||
| Age | ≤ 1 year | 149 | 90 (60) | 59 (40) | 0.96 |
| >1 year | 155 | 94 (61) | 61 (39) |
Table 4: Relationship of different variables with presence of Haemonchus, Oesophagostomum, Paramphistomum and Trichostrongylus
| Variables | Level | Haemonchus n (%) positive | Oesophagostomum n (%) positive | Paramphistomum n (%) positive | Trichostrongylus n (%) positive |
| Region | Chittagong | 44 (21) | 25 (12) |
9(4) |
33 (17) |
| Dhaka | 38 (36) | 15 (14) |
3(3) |
24 (22) | |
| Mymensingh | 4 (4) | 25 (24) | 36(35) | 7 (7) | |
| Breed | Black Bengal | 36 (16) | 38 (17) | 35(15) | 33 (15) |
| Jamunapari | 49 (28) | 26 (15) | 10(6) | 28 (16) | |
| Others | 1 (10) | 1 (10) |
3(25) |
3 (25) | |
| Sex | Female | 60 (22) | 47 (17) | 31(11) | 49 (18) |
| Male | 26 (18) | 18 (13) | 17(12) | 15 (11) | |
| Age | ≤ 1 year | 42 (26) | 24 (15) |
3(2) |
28 (18) |
| >1 year | 43 (17) | 41 (16) | 45(18) | 35 (14) |
Bold and Italic numbers within shaded boxes = significant relationship (p-value <0.05)
Except for Trichostrongylus, infection with other para sites showed no significant relationship with sex (Table 4). More female (18%) were infected with Trichostrongylus in comparison with male (11%).
Young animals were found more susceptible to Haemonchus (26%) in comparison with animals with more than one year of age (17%). The reverse was observed for Paramphistomum (2% in young and 18% in adults) (Table 4).
Discussion
Prevalence Study
Overall prevalence of gastrointestinal parasitic infections: The generally predominance of gastrointestinal parasitic infections in goats showed consistency with the observation of Zeryehun et al. (2012), Hassan et al. (2011), Gadahi et al. (2008) who recorded 61.4% in small ruminants in Ethiopia, 63.41% in Black Bengal goat in Chittagong district, Bangladesh and 63.50% in sheep and goat in and around Rawalpindi and Islamabad, Pakistan, respectively. The earlier observation was partially consistent with the reports of Khajuria et al. (2012), Dagnachew et al. (2011), Biu et al. (2009) and Asif et al. (2008), who reported 67.24 % in Jammu province, Kashmir, 47.67% in Ethiopia, 58.0% in the University of Maiduguri research farm in Nigeria and 65.7% in Pakistan, respectively. Poorer prevalence of gastrointestinal parasitic infections was noticed by Rehman et al. (2006), Muraleedharan (2005) who recorded 41.16% in Pakistan and 46.12% in India, respectively. On the other hand, observation of this study was greatly varied from Islam et al. (2008) and Lima et al. (2003) who recorded 74.55% in different regions of Bangladesh and 82.00% in Brazil, respectively. Variation in the occurrence of gastrointestinal parasites infection might be due to geo-climatic conditions, sample size, breed, age, sex, a plane of nutrition, stress, availability of intermediate host, vegetation, grazing pattern, rearing and husbandry measures, deworming, genetic resistance etc. (Hansen and Perry, 1990).
Prevalence on the Basis of a Type of Parasite (Trematodes, Nematodes, and Cestodes)
Prevalence of trematodes in goats of a veterinary hospital of BAU was partially in accordance with the observation of Opara et al. (2005) who recorded 13% in southeast Nigeria. Nematodes infection of this study in CVH was partially in agreement with the result of Opara et al. (2005) who observed 78.4% in Nigeria. Moreover, Prevalence of nematodes in goats of S. A. Quaderi Teaching Veterinary Hospital was partially consistent with the results of Ijaz et al. (2008) who recorded 42.67% in goats in Lahore, Pakistan. Variation in the occurrence of nematodes and trematodes might be due to geo-climatic conditions including sample size, availability of intermediate hosts and rearing pattern of the goats in the study areas (Alim et al., 2012). On the other hand, an infection rate of cestodes in goats of this current study showed harmony with the results of Opara et al. (2005) who recorded 8.7% in Nigeria. Lower prevalence of cestodes of this study might be due to less dissemination of eggs in the faces from the gravid segments (Radostits et al., 1994).
Prevalence of Different Genus of Gastrointestinal Parasites in Goats
The highest prevalence of Haemonchus spp in goats was in accordance with the results of Uddin et al., (2006) and Tehrani et al. (2012) who recorded 39.79% in goats of Bandarban district of Bangladesh and 33.08% in sheep at Urmia respectively. The earlier result of this study greatly varied from Coelho et al. (2012), Shahiduzzaman et al. (2003), Gadahi et al. (2008), Rajapakse et al. (2008), Lima et al. (2003), and Woldemariam (2003) who recorded 61. 65.63% in different regions in Bangladesh, 64.19% in Iran, 81% in Srilanka, 75.13% in Brazil and 95-100% in Ethiopia, respectively. Slightly lower prevalence of gastrointestinal parasites was recorded by Pathak et al. (2008) and Almalaik et al. (2008) in different regions of the world. Comparatively higher prevalence of Haemonchus spp in CVH and TVH of CVASU in compare to other. Favorable climatic conditions for the development of the free-living stages of the GI nematodes (Kantzoura et al., 2012).Prevalence of Paramphistomum spp infection of this study was varied with the observation of Uddin et al. (2006) who recorded 65.28%, 56.66% in different regions in Bangladesh. The earlier observation also varied from the report of Pathak et al. (2008) who recorded 80.68% of Paramphistomiasis in India. Lower prevalence of Paramphistomum spp infection of this study might be due to geo-climatic conditions (Gupta et al., 1987) or improved husbandry practices (Alim et al., 2012).Prevalence of Trichostrongylus spp in goats of CVH was consistent with the record of Coelho et al. (2012), Almalaik et al. (2008), Lima et al. (2006) and Waruiru et al. (2005) who reported 20.79%, 24.4% in Turkey, 24.32% in Brazil and 29% in Kenya, respectively. Khajuria et al. (2012), Nuruzzaman et al. (2012), recorded 13.67 % in Jammu province and 16.00% in Thakurgaon district, Bangladesh, 13.25% respectively which supported the prevalence observed in TVH, CVASU. An occurrence of Trichostrongylus spp infection in a veterinary hospital of BAU was partially consistent with the findings of Howlader et al. (2012) and Gadahi et al. (2008) reported 6.67% in Sylhet district of Bangladesh and 4.51% in Pakistan. However, observation of this study varied from the report of Rajapakse et al. (2008), Kumsa et al. (2006) and Woldemariam (2003) who recorded 59%, 48.8%, 40.2%, 83-100% and 40%, respectively in corners of the world. Comparatively higher prevalence of Trichostrongyltus spp of current investigation might be due to high susceptibility and survival of pre-parasitic phages (Soulsby, 1982) aswell as poor husbandry practices (Alim et al., 2012). Prevalence of Strongyloides spp infection in CVH and Veterinary hospital of BAU of this study was found consistent with the report of Gadahi et al. (2008), Nwosu et al. (2007), Yadav et al. (2006), Jithendran et al. (2001) who recorded 3.22% , 4.1%, 1.15% ,4.8% in Iran, Nigeria, Jammu district in Pakistan, India respectively. However, the observation of this study showed a discrepancy with reports of Hassan et al. (2011), Lima et al. (2003), Waruiru et al. (2005), Nwosu et al. (1996) who recorded 51.74% in Chittagong district, Bangladesh, 72.8%, 51.6%, 83% in Brazil, Kenya and Nigeria, respectively. Variation in an occurrence of such infection in goat might be due to free-living nature of the parasite and different bionomics of the parasites (Urquhart et al., 1996; Soulsby, 1982). Prevalence of Fasciola spp infection of this study in the veterinary hospital of BAU was consistent with the observation of Khajuria et al. (2012) and Jithendran et al. (2001), who reported 8.2 % in middle Jammu province and 9.6% in India. Observation of this study showed a discrepancy with the report of Uddin et al. (2006) and Waruiru et al. (2005) who recorded 15.42% and 31.5% Kenya. Lower prevalence of Fasciola spp was recorded by Kantzoura et al. (2012), Gadahi et al. (2008), Mungube et al. (2006), Koinari et al. (2012) and Yadav et al. (2006) which partially supported the observed results of Fasciola spp in TVH, CVASU and CVH, Dhaka. Comparatively lower prevalence of Fasciola spp infection of this study might be due to geo-climatic condition or poor same size or less availability of specific snail host in the study area (Kakar and Kakarsulemankhel, 2008; Bachal, 2002). Prevalence of Oesphagostomum spp in three study areas was relatively consistent with result of Waruiru et al. (2005) who reported 13%. The earlier observation greatly varied with the observation of Mohanta et al. (2007) who recorded 92%, 24.17% in different regions in Bangladesh. The observation also varied from the report of Rajapakse et al. (2008), Waruiru et al. (2005), and Woldemariam (2003) who recorded 88%, 13%, 33-83% in different corners of the world. In the present study, Oesophagostomum spp infection was observed low which might be due to the relatively long life cycle and low resistance to desiccation of the pre-infective stages of this genus (Pfukeny et al., 2007; Rivera et al., 1983).The prevalence of trichuriasis spp infection in goats of this study was partially consistent with the observation of Kantzoura et al. (2012), Koinari et al. (2012), and Nwosu et al. (2007) who recorded 2.9% and 3.6% respectively in Greece, Papua New Guinea, and Thailand. The earlier observation was inconsistent with the reports of Radfar et al. (2011), Gadahi et al. (2008), Rajapakse et al. (2008), Asif et al. (2008) who reported 44.75% (Iran), 35.48 (Pakistan), 59% (Srilanka), 62.5% (Pakistan), respectively. Variation in the occurrence of Trichuris spp infection in this study might be due to geo-climatic conditions of the study areas as well as husbandry practices (Alim et al., 2011).
Prevalence of Moniezia spp revealed by current study was consistent with the observation of Jithendran et al. (2001), Waruiru et al. (2005), Yadav et al. (2006), Mohanta et al. (2007), who recorded 2.7% sheep and goats of Himachal Pradesh of India, 2.5% in sheep and goats of Kenya, 0.96% in R.S. Pura, Bishnah and Samba tehsils of Jammu district, 2.66% in Mymensingh district of Bangladesh and 2.23% in Thailand. However, results of current study was not consistent with the reports of Nwosu et al. 1996, Pathak et al. (2008), Lima et al. (2003) and Koinari et al. (2012) who observed 31% in Nigeria, 11.25% in Bandarban district of Bangladesh, 17.04% in the Veterinary College of Durg district, Chhattisgarh, 8.4% in Brazil and 9.1% in sheep and goats in Papua New Guinea. The Variation on the prevalence for Moniezia might be due to geo-climatic location, stocking density, deworming and less dissemination of eggs from the gravid segments (Radostits et al., 1994). Prevalence of Eimeria spp of this current study was in harmony with the reports of Koinari et al. (2012), Yadav et al. (2006) who observed 17.3% in sheep and goats in Papua New Guinea and 6.73 % in of Jammu district. The earlier results showed a discrepancy with the result of Regassa et al. (2004) and Koudela et al. (1998), Coelho et al. (2012) and Terefe et al. (2012) who recorded the higher prevalence of such infection in different corners of the world. Adult showed lower susceptibility to Eimeria spp infection which might be the cause of the lower prevalence of such infections in the study animals.
Relationship of Different Variables with Manifestation of Gastrointestinal Parasites in Goats
Age wise prevalence of parasitic infection: In the current study, effects of age on the incidence of gastrointestinal parasitic diseases were observed. Prevalence of parasitic infection in goats more than 12 months of age of three study areas was consistent with the report of Hassan et al. (2011) and Uddin et al. (2006) who noticed that older animals or goats are more infected by GI parasites than younger animals. However, the result of this study based on age category showed discrepancy with the result of Raza et al. (2010) who reported that younger animals (<12 months) are more susceptible to parasitic infection than older ones. In this study, higher prevalence of parasitic infection in adult goat might be due to keeping them for a longer period of time in breeding purposes or supply inadequate feed against their high demand. Besides this, maximum adult female animals were in pregnancy during this investigation which might be accounted for higher prevalence in an adult. (Alim et al., 2011; Raza et al., 2010).
Sex wise prevalence of gastrointestinal parasites: The observed prevalence of gastrointestinal parasitic infection in female goats was consistent with the records of Uddin et al. (2006), Shahiduzzaman et al. (2003) and Tehrani et al. (2012) who observed that females showed more susceptibility to GI parasites infection than the males. Higher prevalence of GI parasitic infections in female animals of this study might be due to the variation in sample size, lowered resistance to female animals, temporary loss of acquired immunity near parturition stress, genetic resistance of the host. (Bachal et al., 2002).
Region wise prevalence of gastrointestinal parasites in goats: Prevalence of the gastrointestinal parasite’s infection varied in the three different regions of current study. Comparatively higher prevalence of parasitic infection was found in CVH of Dhaka than other two hospitals which might be due to sampling method, deworming history, rearing pattern of the goats.The overall prevalence GI parasitic infection was more in S.A. Quaderi Teaching Veterinary hospital was consistent with the result of Hassan et al. (2011) who reported 63.41% prevalence in Chittagong regions. Variation in the occurrence of GI parasitic infection in 3 different hospitals might be due to geo-climatic conditions, sample size, rearing pattern and husbandry practices.
Limitation of the study: This study was carried out to determine the prevalence of helminth parasites seasonally but the study doesn’t reveal why some parasites were more predominant and others were not. This study is limited to certain parameters and some of the parts of the study were left untouched due to time and cost factors so that future researchers can elaborate this study by approaching the untouched portion.
CONCLUSION
The study was performed aiming to determine the prevalence of gastrointestinal parasitic infections of a goat. The study revealed the comparatively higher prevalence of Haemonchus spp, Oesophagostomum spp, Trichostrongylus spp in TVH of CVASU and CVH of Dhaka and also revealed the comparatively higher prevalence of Paramphistomum spp and Oesophagostomum spp in goats. The result of this current study will give epidemiological forecasting in the occurrence of such diseases in goat which will help the clinician in a diagnosis of such infections. However, this study will make the way to take further study related to these diseases which will help to take necessary preventive and control measures against them. A well planned widespread investigation should be taken in future giving special emphasis on diversified topography and seasons of this country.
Acknowledgements
Special thanks to owner of Animals and Department of pathology and parasitology of Chittagong and veterinary animal sciences University.
Conflict of interest
No conflict interest.
Authors Contribution
All authors contributed equally.
REFERENCES