The Journal of Advances in Parasitology
Research Article
Muscular Sarcocystosis and Non-Specific Rheumatic Diseases are growing Neglected Coming Challenge
Ahmed Mohamad Mandour, Mahmoud El-Hady Mohamad Mohamad Monib, Abeer El-Sayed Mahmoud*, Asmaa Hosney Deeb
Departments of Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt.
Abstract | Sarcocystosis is a zoonotic disease caused by coccidian/apicomplexan protozoa in humans and animals. The prevalence of muscular sarcocystosis among 100 patients with non-specific rheumatic diseases was determined. An indirect fluorescent antibody test (IFAT) using the Sarcocystis fusiformis antigen was used. The sero-prevalence among all examined patients was 52%. Patients with myositis have the highest percentage of positivity (39.5%). Statistical significance was between positive and negative cases in group A (non-specific rheumatic diseases) with highly significant results in the myositis subgroup. 39 out of 52 (75%) seropositive patients expressed eosinophilia (7-18%). There was no cross reaction with Toxoplasma positive serum. Muscular sarcocystosis may be an important cause of the non-specific rheumatic diseases especially myositis with increasing percentages over years in our locality. Further studies, for the source of infection, early diagnosis and treatment are necessary, as they remain unclear.
Keywords | Muscular sarcocystosis, Non-specific rheumatic diseases, Myositis, IFAT, Sarcocystis fusiformis antigen
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | August 01, 2016; Accepted | September 19, 2016; Published | January 22, 2017
*Correspondence | Abeer E Mahmoud, Department of Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt; Email: [email protected]
Citation | Mandour AM, Monib MEMM, Mahmoud AE, Deeb AH (2017). Muscular sarcocystosis and non-specific rheumatic diseases are growing neglected coming challenge. J. Adv. Parasitol. 4(1): 9-14.
DOI | http://dx.doi.org/10.14737/journal.jap/2017/4.1.9.14
ISSN | 2311-4096
Copyright © 2017 Mandour et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sarcocystosis is a cosmopolitan zoonotic disease. It is caused by Sarcocystis which is an intracellular apicomplexan/coccidian parasite, with more than 120 recognized species. Recurrent outbreaks of muscular sarcocystosis among tourists visiting endemic areas focused the international attention on this disease (Tappe et al., 2014; Fayer et al., 2015). Human sarcocystosis has two forms. In the first form humans act as intermediate hosts via ingestion of water or food contaminated with carnivores, reptilian or unidentified species. Faeces containing sporocysts lead to muscular sarcocystosis (no intestinal phase). It is found in skeletal, cardiac, smooth muscles and neural tissue (Abdul-Rahman et al., 2002; Fayer, 2004; Jehle et al., 2009; Prakas and Butkausas, 2012; Fayer et al., 2015). The second form is intestinal sarcocystosis which results from eating raw or undercooked meat containing mature Sarcocystis cyst. Infective oocysts are shed with stool (no muscular phase). It can be prevented by cooking or freezing meat. Intestinal sarcocystis is caused by S. hominis and S. suihominis which cause non-invasive self-limiting diarrheal symptoms, while muscular Sarcocystis is caused by S. lindemanni (Lingappa et al., 2015).
Muscular sarcocystosis is typically biphasic, infection develops early in the vascular endothelium, with difficult diagnosis characterized by a prodromal stage of one week with headache, fever and myalgia followed by two-week asymptomatic lastly the development of intramuscular cysts with myositis leading to long-lasting fever with severe myalgia, eosinophilia and an elevated creatine kinase (CK) level. Diagnoses are confirmed by finding Sarcocystis cyst in muscle biopsy (Tappe et al., 2013; Slesak et al., 2014; Fayer et al., 2015).
Non-specific rheumatic manifestations and myositis with undiagnosed causes are common. Symptoms are overlapped and there is no specific diagnostic test. Some of these are associated with parasitic infections as muscle sarcocystosis (Habeeb et al., 1996; Crum-Cianflone, 2008). Many cases have been recently reported and some are clinically diagnosed as eosinophilic myositis (Arness et al., 1999). Muscular sarcocystos is described in many parts of the world, 21% of the patients in Southeast Asia, 47% in U.S. military team in rural Malaysians, 19.8% in Malaysia, 60 cases in the United States and 26% patients in Iraq (Wong and Pathmanathan, 1992; Arness et al., 1999; Abdul-Rahman et al., 2002; Kiel, 2002; Al-Taee et al., 2009). Sero-positivity for sarcocystis among patients with rheumatic complains was recorded in many Egyptian governorates. It ranged from 13%-15.6% among rheumatoid arthritis (RA) patients and from 21.7%-30% among patients with chronic myositis (Azab et al., 1990; Habeeb et al., 1996; El-Nazer and Abdel-Azeem, 2000; Abdul-Rahman et al., 2002).
Little information is known about Sarcocystis for practicing clinicians and patients (Esposito et al., 2012). Its diagnosis is difficult due to nonspecific clinical manifestations depends on muscle biopsy which is invasive and not reliable (Mandour, 1969; Ndiritu et al., 1996; Fayer, 2004). Moreover, fetal growth retardation was recorded in pregnant woman. The patients with musculoskeletal affection are chronic patients and those dependent on systemic steroids or non-steroidal anti-inflammatory drugs. Both drug regimens have well known side effects (Slesak et al., 2015). Therefore, proper diagnosis of muscular sarcocystosis could relieve them from continuous pain and therapy side effects (Azab and El-Shennawy, 1992). So, there is rising demand for serological diagnosis of sarcocystosis (Arness et al., 1999). IFAT as a specific and sensitive serologic test was developed for its diagnoses (Al-Taee et al., 2009; Durate et al., 2003; Crum-Cianflone, 2008). IFAT was used in the diagnosis of Sarcocystis infection in animals and humans (Azab et al., 1990; Habeeb et al., 1996; Al-Taee et al., 2009). Its sensitivity was 88.70 % - 94.44 % in different animals (Sovobodova and Nevole, 1990). The aim of the present study is to throw some light on the magnitude of the sero-prevalence of muscular sarcocystosis in patients with non-specific rheumatic diseases by (IFAT) in Assiut Governorate, Egypt.
Materials and methods
Patient Groups
This study involved a total of one hundred parasitic free patients by stool examination (direct wet mount and formal-ethyl concentration technique) and urine examination to exclude the presence of other parasites causing eosinophilia and/or cross-reacting Sarcocystis cysts antigens. The history of allergy was excluded as a cause of eosinophilia. They were admitted in the Department of Rheumatology and Rehabilitation, Assiut University Hospital, Assiut, Egypt. Patients had actual musculoskeletal complaints, especially myalgia and myositis, but no intestinal complaint regardless of their age and sex. The duration of complaints ranged from months to several years. They were classified into two groups:
Group (A): This group involved a total of 38 patients with non-specific rheumatic diseases. They were divided according to the Department diagnosis into two subgroups:
1) Eighteen patients presenting with myositis in different muscles, with predilection to the quadriceps muscle (Bohan and Peter, 1975).
2) Twenty patients with musculoskeletal manifestations such as arthralgia mostly in big joints especially the knees.
Group (B): This group involved a total of 62 patients were with autoimmune connective tissue disease. They were divided according to the Department diagnosis into three subgroups:
1) Rheumatoid Arthritis (RA) 26 diagnosed patients (Arnett et al., 1988).
2) Juvenile Idiopathic Arthritis (JIA) 11 patients with chronic arthritis with no apparent etiology (Huang, 2012).
3) Systemic Lupus Erythematosus (SLE) 25 diagnosed patients (Hochberg, 1997).
Controls and Samples
Positive control 20 serum samples from naturally infected cattle with macroscopic cysts of Sarcocystis fusiformis were collected. Negative controls were tested by using Phosphate Buffer Saline (PBS). Human serum from 20 patients confirmed to be positive for Toxoplasma was tested to detect cross reactivity (Mohamed, 2013). Several investigations in the Department of Rheumatology and Rehabilitation were conducted to exclude other rheumatic diseases, traumatic and degenerative disorders. Differential blood count for eosinophilia was done. Serum was collected from all patient groups and stored at -20°C until use.
Indirect Fluorescent Antibody Test (IFAT)
IFAT was used to test the presence of Sarcocystis antibodies. Sarcocystis fusiformis cystizoite antigen (Ag) was prepared from macroscopic esophageal cysts of buffaloes as Ag coated slides and stored at -20°C. The tested serum samples were diluted by PBS starting from 1:8 and the end point was 1:32. 25 µl of the diluted serum was added to the fixed antigen on the slide. The anti-Human Conjugate was polyvalent fluorescin isothiocyanate labelled antihuman immunoglobulin G (IgG) for chronic infection with 0.02 Evan’s blue as a counterstain (Immco Diagnostics, USA). The mounting buffer (purchased from Sigma Company) was added and cover slips were applied to slides. The slides were examined with the fluorescence microscope. The slide was considered positive with prominent florescence crescent-shape cystezoites in dark background illumination (Habeeb et al., 1996; Abdul-Rahman et al., 2002; Mohamad, 2013).
Statistical Analysis
The collected data were analyzed using the program SPSS (Statistical Package for Social Sciences), version 20 for Windows (IBM Enterprize, Armonk, New York, USA). The statistical method was the descriptive method using chi-square test.
Ethical Clearance
This study was approved by the Institutional Ethics Review Board of the Faculty of Medicine, Assiut University, Assiut, Egypt. Oral consent was obtained from the patients before they were recruited into the study.
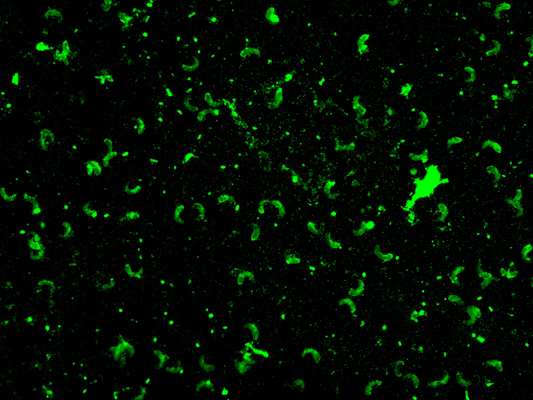
Figure 1: IFAT positive case (x 40)
Table 1: Detection of Sarcocystis antibodies by IFAT in each group
|
Result |
No. of patients |
p value |
|||
|
+Ve |
-Ve |
||||
|
Group A: non-specific rheumatic disease (myositis, arthralgia) |
Patient count |
21 |
17 |
38 |
0.001* |
|
% |
55.3 |
44.7 |
- |
||
|
Group B: autoimmune connective tissue disease (RA, SLE, JIA) |
Patient count |
31 |
31 |
62 |
0.587 |
|
% |
50.0 |
50.0 |
|||
Results
Sarcocystis antibodies were detected in patients’ serum by IFAT as prominent florescent crescent-shape cystizoite in a dark background in 52 out of 100 (52%) of all groups (Figure 1). A titer of 1:8 -1:32 was used. Group A (non-specific rheumatic diseases) was higher in positivity than group B (autoimmune connective tissue disease) (55. 3%) and (50%), respectively (Table 1). In group A myositis positivity was (39.5%) which is the highest percentage in these results in all subgroups, followed by SLE (22.6%), RA (17.7%), arthralgia (15.8), and finally JIA subgroup (9.6 %) (Table 2 and 3). Statistical significance was detected between positive and negative cases in group A (Table 1 and 2) with highly significant results in the myositis subgroup (Table 2). The differential blood count of 39 Sarcocystis seropositive out of 52 (75%) showed eosinophilia that ranged from 7-18%. All positive control bovine serum samples were positive (100% sensitivity) by the test, and there was no cross reaction with Toxoplasma positive serum.
Table 2: Detection of Sarcocystis antibodies by IFAT in Group A
|
Result |
P. value |
|||
|
+Ve |
-Ve |
|||
|
Myositis |
Patient count |
15 |
3 |
0.001** |
|
% |
39.5 |
7.9 |
||
|
Arthralgia |
Patient count |
6 |
14 |
0.037* |
|
% |
15.8 |
36.8 |
||
Table 3: Detection of Sarcocystis antibodies by IFAT in Group B
|
Result |
P. value |
|||
|
+Ve |
-Ve |
|||
|
RA |
Patient count |
11 |
15 |
0.374 |
|
% |
17.7 |
24.2 |
||
|
SLE |
Patient count |
14 |
11 |
0.496 |
|
% |
22.6 |
17.7 |
||
|
JIA |
Patient count |
6 |
5 |
0.754 |
|
% |
9.7 |
8.1 |
||
Chi-squared test was used; *: Significant difference at p value ≤0.05 between positive and negative cases inside the same group; **: Highly significant at (P ≤0.001)
Discussion
In the present study, the seropositivity of muscular sarcocystosis was 52 out of 100 (52%) in all examined groups. The highest positivity in our results was (39.5%) in the myositis subgroups, SLE (22.5%), RA (17.7 %), arthralgia (15.8) and JIA (9.7 %). Statistical significance was between positive and negative cases in group A with highly significant results in myositis subgroup. The results of the present study were higher than those obtained by other researchers. In Iraq, Al-Taee et al. (2009) by IFAT, detected 26 out of 100 (26%) in humans and (45%-82%) in different animals. In Cairo, Egypt Azab et al. (1990) detects 3 out of 20 (15%) patients with myositis by IFAT. Habeeb et al. (1996) in Zagazig, Egypt recorded sero-positivty (20%) among patients with chronic myositis and (10%) among patients with musculoskeletal complaints by IFAT. In Upper Egypt, El-Nazer and Abdel-Azim (2000) recorded 21.7% among patients with chronic myositis and 15.6% among rheumatoid arthritis patients using Western Blot. In Assiut Governorate, Egypt Abdul-Rahman et al. (2002) showed sero-positivity of 46% by Western Blot in patients’ with non-specific rheumatic disease and rheumatoid arthritis all patients have myositis. This increasing rate in Egypt especially in our locality suggests that human muscular sarcocystosis is a much more growing problem than it was previously thought. The low sero-positivity among patients with rheumatoid arthritis (RA) may be due to immunosuppression or the use of steroid (El-Nazer and Abdel-Azim, 2000).
Patients investigated in the present study had actual musculoskeletal complaints from months to several years, especially myalgia, but they had no intestinal complaints (Tappe et al., 2013; Slesak et al., 2014; Fayer et al., 2015). Sarcocystosis should be considered in the differential diagnosis when a patient developed fever and myalgia especially after travelling to endemic area (Slesak et al., 2015). There are several considerations that such apparent antibodies may be the result of past or present intestinal infection. The first is the failure to detect antibodies in cats or dogs with intestinal infections (Markus et al., 1974). Markus (1979) found few cellular reactions in the intestines of Sarcocystis infected carnivores, indicating that no antibodies could be formed. The presence of positive reactions among vegetarians and persons with intestinal infections gave negative anti-body titre (Aryeetey and Piekarski, 1976; Piekarski et al., 1978). The ease with reinfection takes place means that there is no immunity against intestinal infection. From the above previous results extra intestinal infection is only necessary for antibody production, so the serological diagnosis is specific for extra-intestinal sarcocystosis (Azab et al., 1990).
In the present study, eosinophilia was in 39 seropositive cases out of 52 (75%) ranging from (7-18%). It indicates that muscular sarcocystosis may be a cause of eosinophilia. Sarcocystis might be an overlooked cause of unexplained eosinophilia and eosinophilic myositis (Arness et al., 1999; Abdul-Rahman et al., 2002). Tappe et al. (2013) reported that eosinophilia is a hallmark of acute and not chronic disease. The conjugate used in the present study was antihuman IgG, which detects chronic cases; this may explain why not all positive cases showed eosinophilia.
In the present study, Sarcocystis fusiformis from cattle was used as a source of antigen. This was used by several researchers, since there was a remarkable degree of cross reaction among Sarcocystis sp. from widely divergent host origins (Tadros et al., 1981). Moreover, it is more available, macroscopic, collected easily and yields a considerable amount of antigen. S. fusiformis antigen is used in ELISA, IFAT and Western blot for the detection of muscular sarcocystosis in humans (Habeeb et al., 1996; El-Nazer and Abdel-Azim, 2000; Abdul-Rahman et al., 2002).
In the present study, all the positive control bovine serum samples were positive (100% sensitivity), and there was no cross reaction with patient Toxoplasma positive serum (Aryeetey and Piekarski, 1976; Tadros et al., 1981; Tenter, 1988; Azab et al., 1990; Habeeb et al., 1996; Tappe et al., 2014). The Sarcocystis antigen is sensitive and specific and Toxoplasma gondi is confirmed to be an antigen distinct from Sarcocystis as many studies argued that the two parasites are phylogenetically closely related Eimeriid coccidian parasites. Moreover, Sarcocystis known positive sera were tested for rheumatoid factor, anti-nuclear and anti-DNA antibodies; which mostly occur in the sera of patients with connective tissue diseases especially myositis; no cross reaction with any of such factors was detected, so the test can be used with much confidence for assaying the infection in patients with myositis (Azab et al., 1990).
In the present study, titre of 1:8 -1:32 was used, as after that titre the number of positive cases decreased. The muscle involvement in man means chronic infection; therefore, it must not be associated with high level of specific antibodies and the absence of false positive reactions. A serum dilution of 1:8 can be accepted as a screening test in order not to misdiagnose chronic cases (Azab et al., 1990; Markus, 1979). This explains why the positive reactions at low titre can be accepted as a screening titre to avoid misdiagnosis of chronic cases. This finding is in agreement with the findings reached by (Sibalic, 1975; Rieter et al., 1981; Azab et al., 1990) as they recorded positive titre ranging from 1:20- 1:40. Al-Taee et al. (2009) 1:10 is appropriate to diagnose sarcocystsis. On the other hand, Habeeb et al. (1996) recorded positive titre at 1:16-1:128.
An environmental survey to detect the source of muscular sarcocystosis infection, animal reservoirs and effective anti-parasitic drug is needed for prevention strategies especially in endemic areas (Tappe et al., 2014; Fayer et al., 2015). It remains to be determined whether environmental factors, such as climate change or increasing reptile populations (i.e. possible final hosts) play a role in this disease (Tappe et al., 2013). Physicians should be aware of this disease; advice should be given regarding individual prevention measures, such as the consumption of cooked food, well-peeled fruit and pre-packed or boiled/filtered water only. Treatment with cotrimoxazole may be a therapeutic approach in the early phase of the disease to prevent muscle invasion, whereas steroids seem effective in treating severe myalgia/myositis in the later phase. Public health education regarding transmission, combined with proper diagnosis, is crucial (Tappe et al., 2014).
Muscular sarcocystosis may accompany and/or complicate many serious clinical diseases and clinicians may be unaware that discharging sinuses in the lower extremity and gluteal regions can be caused by Sarcocystis (Agarwal and Srivastava1, 1983). Sarcocystis may play a role in idiopathic cardiomyopathy (Habeeb et al., 1996). Sarcocystosis should be considered as opportunistic and systemic infection in which the patient served as both a definitive and intermediate host. The excreted sporulated oocysts could infect the same host through a fecal-oral course (Velásquez et al., 2008). Laryngeal squamous cell carcinoma is accompanied by sarcocystosis and negative Toxoplasma gondii in Southeast Asia (Larbcharoensub et al., 2011). Glomerulonephritis was found to have sarcocystis infestation in skeletal muscle biopsy. The occurrence of vasculitis and other manifestations like glomerulonephritis is suggested as a result of rupture of sarcocystis cysts at irregular intervals with resultant release of antigens into the circulation, thus forming antigen and antibody complexes that in turn get deposited in vessel walls or glomerular capillary basement membrane and causing damage. This case stresses the need for proper evaluation for parasitic infections in patients with unexplained glomerular disease. Sarcocystis has been reported to cause glomerulonephritis in animals (Balakrishna et al., 2013). A painful mass in the right lumbar region in female patients in India was caused by muscular sarcocystosis (Lingappa et al., 2015). Moreover, fatal encephalitis and myositis associated with Sarcocystis infections in three flocks of racing pigeons were reported by (Olias et al., 2009).
Data herein throw the light to use IFAT in a wide survey to detect positive cases in many other patient groups and searching for an actual treatment such as natural plant extracts that can treat muscular sarcocytosis with fewer hazards. In developing countries, mass awareness programs regarding good hygienic habits and proper disposal of excreta are essential to aid in prevention (Lingappa et al., 2015). In conclusion, the results of the present study draw the attention of parasitologists and rheumatologists to the fact that Sarcocystis may be an important cause of non-specific rheumatic diseases especially with myositis and the increasing percentage of positivity over years especially in our locality. Patients with musculoskeletal affections must be tested for eosinophila and IFAT using S. fusiformis as an alternative method to the invasive muscle biopsy. This test should be applied as a screening test especially in suspected patients and in endemic areas all over the world. Further studies, for the source of the infection, early diagnosis and treatment are necessary, as they remain unclear.
Acknowledgment
The authors would like to express their deep thanks to Professor Dr. Sonia M. Rashad, Department of Rheumatology and rehabilitation, Faculty of Medicine, Assiut University, Assiut, Egypt, for her support and help during this study and collection of samples. The financial support of this work is from the Grant Office, Faculty of Medicine, Assiut University, Assiut, Egypt.
Conflict of Interest
There is no conflict of interests.
Authors’ Contributions
Idea by A.M. Mandour; A.H. Deeb: performed the laboratory works, surveys and collection of papers; A.E. Mahmoud: collection of papers, data analysis and writing the manuscript; S.M. Rashad: performance of patient examinations; A.M. Mandour and M.E. M. M. Monib: Revising the manuscript A.
References






