The Journal of Advances in Parasitology
Research Article
Lip Leishmaniasis in Saudi Arabia: A Typical Presentation of Cutaneous Leishmaniasis
Doaa Abdelbadie Salem1*, Ebrahim Hassan Najmi2
1Department Of Medical Parasitology, Mansoura faculty of Medicine, Mansoura University, Egypt; 2Department Of Dermatology, Khamis Mushyte General Hospital, Asir, Kingdom of Saudi Arabia.
Abstract | Cutaneous leishmaniasis (CL) in Saudi Arabia is an increasing public health problem. The diverse presentation of cutaneous leishmaniasis make the diagnosis of CL is a challenge. Our aim was to reinforce the importance of considering lip leishmaniasis in the differential diagnosis of any lip lesions especially in endemic regions for leishmaniasis. This study was carried out in Khamis Mushyte General Hospital, KSA during the period of January 2014 to April 2015. History taking, examination of the lesions, and laboratory investigation were done. The diagnosis is confirmed by the demonstration of amastigotes in a Giemsa-stained smear. When the parasite cannot be detected on smear, histopathologic examination was done looking for granulomas +/− amastigotes. Out of 107 patients attended dermatology OPD with CL, 11 (10.28%) had of lip lesion, 9 of them were slit smear positive and 2 of them were slit smear negative and diagnosed by histopathologic examination.Median age of patients with lip lesions was 32 years with male to female ratio 7:4. Six cases with lower lip lesions and 5 affecting the upper lip. We concluded that lip Cutaneous leishmaniasis should always considering in differential diagnosis of any lip lesions especially in endemic region; to make the diagnosis early and initiate effective treatment to avoid secondary bacterial infection and development of disfiguring lesions.
Keywords | Cutaneous leishmaniasis, Epidemiology, Leishmania major, Leishmania tropica
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | January 26, 2016; Accepted | April 24, 2016; Published | May 20, 2016
*Correspondence | Doaa Abdelbadie Salem, Mansoura faculty of Medicine, Mansoura University, Egypt; E-mail: [email protected]
Citation | Salem DA, Najmi EH (2016). Lip leishmaniasis in Saudi Arabia: A typical presentation of cutaneous leishmaniasis. J. Adv. Parasitol. 3(3): 89-92.
DOI | http://dx.doi.org/10.14737/journal.jap/2016/3.3.89.92
ISSN | 2311-4096
Copyright © 2016 Salem and Najmi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Leishmaniasis is a chronic inflammatory disease caused by obligate intracellular kinetoplastid protozoan parasites, Leishmania spps. Which are transmitted through the bite of infected sandflies.
Globally, leishmaniasis threatens 350 million people around the world. 88 countries are endemic for human leishmaniasis, with an estimated yearly incidence of 1 million to 1.5 million cases of CL. The incidence is not uniformly distributed in endemic areas; Ninety percent of CL cases are found only in seven countries (Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria) (Reithinger et al., 2007). They cause a wide spectrum of disease ranging from asymptomatic to cutaneous, muco cutaneous or visceral manifestations depending on the species of the parasite and the type of immune response induced (Hepburn, 2003).
Cutaneous leishmaniasis in Saudi Arabia is an increasing public health problem due to rapid urbanization, intensive agriculture and human migration. Although the incidence of CL in the Asir Region, Saudi Arabia, has decreased over the last decade, it is still a significant health problem. (Health Statistical Year Book, 2012).
Leishmania tropica is exclusively endemic to the South Western region in Saudi Arabia, where it is transmitted by Ph. sergenti and causes anthroponotic CL. (El Hassan, 2014). Diagnosis of patients with typical lesions is not difficult. However, it can be presented with atypical sites of the body and mimicked several other skin diseases. The lip is one of the atypical sites for CL where lesions primary involving lip (Bari and Rahman, 2008).
The aim of this study was to reinforce the importance of considering lip cutaneous leishmaniasis in the differential diagnosis of any lip lesions especially in endemic regions for leishmaniasis.
Materials and methods
Patients and Samples
Our observational study was carried out on 107 cases of cutaneous leishmaniasis, attended dermatology OPD in Khamis Mushyte general hospital; between January 2014 to June 2015. Eleven cases (10.28%) of them had lip lesion.
Khamis Mushayte Governorate lies in the southwestern region in Saudi Arabia, situated at 18.31° North latitude, 42.73° East longitude and 1965 meters elevation above the sea level. Its population exceeds 387,553 inhabitants.
This study was approved by our hospital ethics and research committee and informed consent was taken from each patient before enrollment in this study. All patients were subjected to history taking, clinical examination, laboratory investigations, localization of the lesions and treatment received (type, dose, route and duration). The diagnosis was confirmed by the demonstration of amastigotes in a Giemsa-stained slit smear. In negative slit smear, histopathologic examination was done looking for granulomas +/− amastigotes.
Microscopy in Slit Smear and Tissue Biopsy
Slit smear: The lesion was cleaned with 70 % alcohol swab and allow to dry completely, gently pinched edge of the lesions between index finger and thumb for 1–2 min, exerting enough pressure to blanch it. A clean cut about 5 mm long and 3 mm deep was made with a sterile scalpel into the dermis and blot away any blood dot. The blade of the scalpel was turned 90°, and using the blunt side of blade, the sides of the cut were scraped in an outwards direction to obtain tissue juice and cells. The aspirate was thinly spread on clean glass slide using a circular motion working outwards to avoid parasites in those parts of the smear that have started to dry. When the smear was dried, slide was fixed by covering it with a few drops of absolute methanol, for 2-3 minutes. Stain the smear using the Giemsa technique. When the smear is dry, a drop of immersion oil was spread on it and examined first with 10X and 40X objectives to detect macrophages which may contain amastigots (the parasites can also be found outside the macrophage cell) then 100X oil immersion objectives to identify amastiotes. (Kumar et al., 2006).
Biopsy: Negative skin smears were followed by skin biopsy for histopathological diagnosis. Biopsy samples were taken from the borders of ulcers (lesions) using a 3-mm disposable punch and fixed in 10% formalin, dehydrated and then embedded in paraffin. The embedded tissue was sectioned into very thin (2–7 μm) sections using a microtome and layered on a glass slide then air dried, fixed in methanol , stained with Giemsa and examined microscopically using a 100× oil immersion lens (Kumar et al., 2006).
Statistical Analysis
Data were analyzed using IBM SPSS statistics version 21. Qualitative data were described using number and percent. Continuous variables were presented as mean ± SD (standard deviation). The significance of difference of age between lip and non lip leishmaniasis was determined using independent sample T test. Fisher Exact test was used for qualitative variables. The statistical significance was determined as p value < 0.05.
Results
A total of 107 cases of CL, attended dermatology OPD with mean age 26.8±16.4 years, median age 36 years and age ranging from 2 years to 63 years were diagnosed and notified with CL. We reported 96 of these patients had non lip CL with mean age 26.7±16.7 years, on the other hand only eleven cases (10.28%) of these patients with CL had lip leishmaniasis with mean age 27.5±13.7 years. By comparing both groups we did not find any statistical significant difference between patients with lip and non lip lesions as regard age, gender and nationality (p> 0.05). The age, gender and nationality distribution of both groups were illustrated in Table 1.
Table 1: Data of reported cases of cutaneous leishmaniasis
|
Parameters |
Total |
Non lip Leishmaniasis |
Lip Leishmaniasis |
|
Age: year |
|||
|
Mean±SD |
26.8±16.4 |
26.7±16.7 |
27.5±13.7* |
|
Age distribution |
|||
|
Less than 12 years |
29(27.1%) |
27(25.2%) |
2 (1.9%) |
|
From 12-30 years |
38(35.6%) |
35(32.7%) |
3 (2.8%) |
|
From 31-50 years |
28(26.2%) |
22(20.6%) |
6 (5.6%) |
|
>50 years |
12(11.2%) |
12(11.2%) |
0 (0%) |
|
Gender |
|||
|
Male |
72(67.3%) |
65(60.7%) |
7(6.5%)** |
|
Female |
35( 32.7) |
31( 29%) |
4(3.7%) |
|
Male/female ratio |
2.05:1 |
2.1:1 |
1.8:1 |
|
Nationality |
|||
|
Saudi |
80(74.8%) |
70 (65.4%) |
10(9.3%)*** |
|
Non Saudi |
27(25.2%) |
26(24.3%) |
1(0.9%) |
*p=0.9; **p=0.51; ***p=0.18
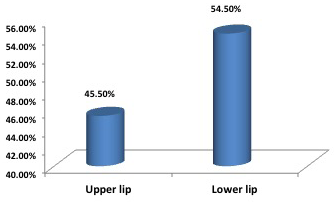
Out of the eleven patients with lip CL, 6 cases with lower lip lesions (54.5%) and 5 with upper lip lesions ( 45.5%) (Figure 1). Nine cases of lip lesions were diagnosed by slit smear (Figure 2 A and B) and two cases of them were slit smear negative and diagnosed by histopathology examination.
Discussion
Cutaneous leishmaniasis is known for its clinical diversity especially in the regions where it is endemic. In many literatures, there has been an increase in the number of reports for new and rare variants of CL. Lesions may appear at unusual sites and in unusual number or the disease may present with atypical morphologies. These unusual sites reported include the genital area, penis, eyelids, lips, nostrils, hair-bearing scalp and nipple (Raja et al., 1998; Rahman and Bari, 2002; Iftikhar et al., 2003; Bari and Rahman, 2008).
Lip leishmaniasis, as cutaneous type of leishmaniasis not as part of mucocutaneous type, has been infrequently mentioned in the medical literature (Veraldi et al., 2002). The interesting issue in our study cases of lip leishmanias is that the lesion started and confined to the lip without history of previous leishmaniasis and hence the rarity.
Two cases of mucosal leishmaniasis in two boys in Saudi Arabia. In one patient, a primary lesion was identified on the patient’s neck. In the second patient, no lesion or scar of old lesion or even history of leishmaniasis was reported. The causative agent was typed biochemically and proved to be Leishmania tropica. A study conducted in Iran revealed that, L. tropica was detected from the lesions present in the gingiva and lower lip of two cases having mucosal nodules and erosion (Shirian et al., 2013).
Diagnosis of lip leishmaniasis is usually based on the basis of symptoms, signs and the residence in endemic region. Most of patients presented with chronic 2×3-mm, firm, non tender nodules of 6 to 8 months duration on the lip that gradually enlarged. The lip nodules were asymptomatic; they did interfere with eating and drinking. Some lesions are presented as lip crusted ulcer with indurated borders. Patients were coming from endemic regions.No lymph nodes were enlarged. No evidence of cutaneous leishmaniasis was seen anywhere else. Systemic examination revealed no abnormality. Slit skin smear was positive for most of cases and negative slit smears were diagnosed by histopathology examination.
The patients were treated by intralesional injection of 1 ml of sodium stibgluconate once weekly for 4 to 6 weeks.
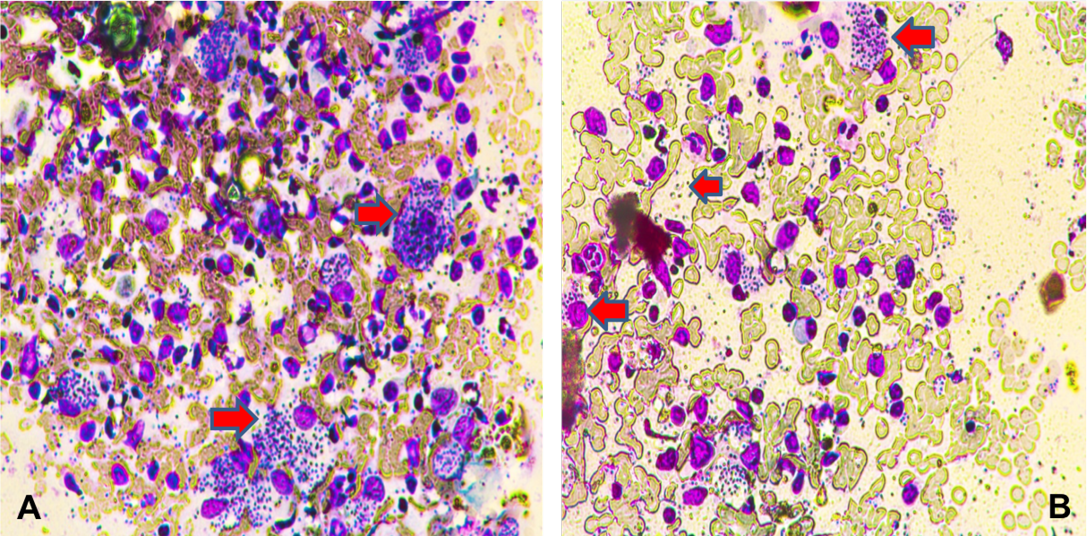
Figure 2: Section of intestine showing sloughing of villli and heterophillic infiltration)Lip skin slit smear shows amastigot form of Leishmania inside intact macrophages (A); some macrophages were ruptured and releasse the amastigote form into the srrounding (B) (X400)
Tablet of ketoconazole 400 mg once daily for 4 weeks was added to one resistance case. We added sessions of cryotherapy for some cases.
The follow up of treated cases was done by clinical examination and repeated slit smear or biopsy after 4-6 weeks. By the end of treatment course, clinical response to drugs is rapid, complete reepithelization of the lesions is observed in most of cases and became negative by slit smear. Only one case was resistant for the treatment and received, 400 mg ketoconazol tablet daily for another 4 weeks and become negative after that. In 3 cases, we added sessions of cryotherapy.
The reason for this unusual clinical type is unknown; however Lesions at unusual sites are considered to be due to chance bites of sandflies at these sites or may be due to local extension of the lesions over the lip. Also, altered host immune response such as defective macrophage function, macrophage resistant strains of Leishmania and decreased production of lymphokines, especially interferon, specific subtypes of parasite.
Conclusion
Lip cutaneous leishmaniasis should always considering in differential diagnosis of any lip lesions especially in endemic region; to make the diagnosis early and initiate effective treatment to avoid secondary bacterial infection and development of disfiguring lesions.
Acknowledgement
To all patients who were enrolled in this study
Conflict of interest
No conflict of interest.
authors’ contribution
Doaa salem presented the idea of the research, contributed in parasitological diagnosis (by taking preparation and examination of slit skin smear) and processing, writing and correction of the manuscript and statistical analysis of the data. Ebrahim Hassan Najmi contributed in clinical examination of the cases, helping in writing of the manuscript.
References