The Journal of Advances in Parasitology
Short Communication
Epidemiological Situation of Bovine Tropical Theileriosis (Theileria annulata Infection) in the Northwest Tunisia
Mohamed Anis Boussaadoun1, Mohamed Gharbi1*, Leila Sayeh2, Mohamed Chiheb Soudani3, Mohamed Aziz Darghouth1
1Laboratory of Parasitology, Ecole Natinale de Médecine Vétérinaire de Sidi Thabet, Institution de la Recherche et de l’Enseignement Supérieur Agricoles, Univ. Manouba, 2020 Sidi Thabet, Tunisia; 2Field Veterinary Surgeon, 7010 Sejenane, Tunisia; 3Field Veterinary Surgeon, 7000 Bizerte, Tunisia.
Abstract | The authors present a cross-sectional survey of clinical cases of tropical theileriosis in the humid region of Bizerte (Northwest Tunisia). A total number of 122 belonging to 107 extensive cattle farms were confirmed positive to two species of piroplasms (Theileria annulata and Babesia spp.) by Giemsa technique. The prevalence of co-infections (T. annulata-Babesia spp.) was 5.7% (7/122). The incidence of clinical cases was unimodal, it lasted from early June to mid-August, with a peak in mid-July. The majority (95/735; 12.92%) of clinical cases were observed in exotic breeds; by comparison, the prevalence among crossbred animals was 3.53% (26/735). In total, 14.42% (106/735) of cattle were recently introduced in the farm. The infection prevalence in cows was 10.2% (75/735) they were more infected by T. annulata than the other cattle categories. This study is the first report description concerning tropical theileriosis in Northwest Tunisia; further studies are needed to better characterize the epidemiology of theileriosis in the humid region of Tunisia.
Keywords | Theileria annulata, Cattle, Tropical theileriosis, Giemsa, Tunisia
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | September 01, 2015; Revised | November 24, 2015; Accepted | November 27, 2015; Published | December 11, 2015
*Correspondence | Mohamed Gharbi, Laboratory of Parasitology, Ecole Natinale de Médecine Vétérinaire de Sidi Thabet, Institution de la Recherche et de l’Enseignement Supérieur Agricoles, Univ. Manouba, 2020 Sidi Thabet, Tunisia, Tunisia; E-mail: [email protected]
Citation | Boussaadoun MA, Gharbi M, Sayeh L, Soudani MC, Darghouth MA (2015). Epidemiological situation of bovine tropical theileriosis (Theileria annulata infection) in the Northwest Tunisia. J. Adv. Parasitol. 2(4): 69-74.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.4.69.74
ISSN | 2311-4096
Copyright © 2015 Boussaadoun et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Tropical theileriosis (Theileria annulata infection) is a protozoan disease transmitted by ticks of the Hyalomma genus. It is one of the major constraints to cattle breeding development and intensification in several parts of the world (Darghouth et al., 2010). In North Africa, the infection prevalence increased with the introduction of exotic cattle breeds, since they are susceptible to T. annulata infection (Glass et al., 2005). In Tunisia, more than 2,500 clinical cases of tropical theileriosis need treatment each year (Bahri et al., 1995). Theileria annulata infection causes dramatic financial losses due to live weight decrease; drop in milk yield, abortions and deaths (Gharbi, 2006; Gharbi et al., 2011). In Tunisia, T. annulata is transmitted by H. scupense. The animals are infected by adult ticks during the summer season. Clinical cases are mainly present in the humid to the semi-arid regions (94%). Due to the scarcity of cattle, only 6% of clinical cases are declared in the arid region. Finally, no clinical cases are declared in the Saharan part of Tunisia (Gharbi et al., 2014). The epidemiology of tropical theileriosis is complex, involving several interactions between abiotic factors (temperature and humidity) that influence the dynamics of vector activity (Gharbi et al., 2013; Gharbi and Darghouth, 2014), breeding practices, the parasite and the vector (Preston et al., 2002). The control of this disease, currently based in Tunisia on the treatment of clinical cases with buparvaquone, vector control with acaricides and the cattle building upgrade, needs a good understanding of its epidemiology (Gharbi, 2006; Darghouth, 2008). If the epidemiology of tropical theileriosis has been well studied in semi-arid and sub-humid Tunisian areas (Darghouth et al., 1996), it remains less explored in the humid region (Northwest of Tunisia) where exotic cattle breeds were introduced during the late 70s.
The aim of this work was to describe the epidemiological feature of clinical cases of tropical theileriosis in cattle located in the humid bioclimatic region in Northwest Tunisia.
The present study was carried out during one tropical theileriosis season (from June to August 2007) in two regions of Bizerte Governorate, North-West of Tunisia (Bizerte South and Sejnane) (Figure 1). These two localities are humid with a minimum and maximum temperature of 9.9 and 26.1°C respectively. The mean monthly rainfall is between 4 (July) and 134 mm (December). This region is totaling 14,400 cattle heads of different breeds mainly maintained in small traditionally managed farms (Regional Direction of Agricultural development, 2007). Purebred cattle (Holstein, Tarentaise cattle, Brown Swiss) represent 22% of the total cattle population. The flora of this region is dominated by conifers and maquis shrub land.
A total number of 122 cattle suspected of tropical theileriosis from 107 extensive cattle farms totaling 735 overall cattle population, were included in the present study. The animals graze during the day and housed in traditional cattlesheds during the night. During the tick season, the farmers implement an empiric tick control program. The clinical suspicion was based on one or more of the following symptoms: hypogalactia (or agalactia), lymph node enlargement, hyporexia (or anorexia) and fever.
During the onset of the clinical symptoms, EDTA blood samples were collected once from all the animals and Giemsa stained blood smears were performed and examined with a microscope at 100x magnification with immersion oil. The following haemopathogens: Theileria annulata, Babesia spp. and Anaplasma spp. were screened. For each blood sample a total number of 50-field microscope were examined (corresponding to approximately 10 to 15,000 erythrocytes) (Darghouth et al., 1996).
The comparison of percentages was performed with Epi-info 2000 for Windows® (Schwartz, 1993). The correlation test between the farm size and the incidence of clinical cases was gathered with SPSS 13 for Windows®. The threshold value for the tests was 0.05. A total number of 122 animals were confirmed positive to piroplasm, among them, 114 animals (93.4%) were exclusively infected by T. annulata, 7 (5.7%) presented T. annulata - Babesia spp. co-infection and one cattle was exclusively infected by Babesia spp. (0.8%). The incidence of clinical cases was unimodal, the first case appeared early June, the peak was located in mid-July, the number of clinical cases decreased progressively to be naught late August (Figure 2). There was also no difference in prevalences between males and females (χ2=0.34; p= 0.55). This should be due to the presence of high tick burdens leading to the installation of an enzootic stability state with no difference between both sexes (Gharbi et al., 2014). Male calves were more infected (8/32; 25%) then female calves (5/24; 20.8%), heifers (22/123; 17.88%), bull calves (11/71; 15.5%) and finally cows (75/485; 15.46%) (χ2=2.61; p= 0.62). The disease prevalence did not significantly varied according to age groups (Table 1).
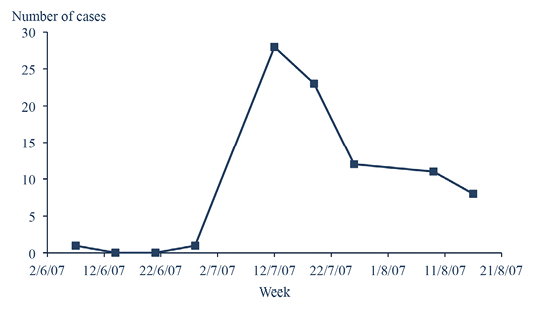
Figure 2: Weekly incidence of tropical theileriosis clinical cases in Bizerte region, Northwest of Tunisia
Positive/overall (%) OR [95% CI] |
|||||
Parameter |
Theileria annulata |
Babesia spp. |
T. annulata and Babesia spp. |
Overall |
|
Breed |
Holstein |
34/235 (14.46) 1.11 [0.6; 2.05] |
0/235 N.A. |
2/235 (0.85) 0.35 [0.04; 2.23] |
36/235 (14.4) 0.97 [0.54; 1.75] |
Brown Swiss |
53/293 (18.08) 1.59 [0.56; 4.85] |
1/293 (0.3) N.A. |
1/293 (0.3) 0.14 [0.01; 1.32] |
55/293 (18.7) 1.05 [0.61; 1.8] |
|
Tarentaise cattle |
5/41(12.19) 0.91 [0.28; 2.77] |
0/41 N.A. |
0/41 N.A. |
5/41 (12.19) 0.75 [0.23; 2.24] |
|
Crossbreed |
22/166 (13.25) |
0/166 |
4/166 (2.4) |
26/166 (15.66) |
|
Sex |
Male |
18/103 (17.47) 1.18 [0.65; 2.12] |
0/103 N.A. |
1/103 (0.97) N.A. |
19/103 (18.44) 1.16 [0.65; 2.05] |
Female |
96/632 (15.18) |
1/632 (0.15) |
6/632 (0.94) |
103/632 (16.29) |
|
Animal category |
Male calf |
8/32 (25) 1.94 [0.77; 4.77] |
0/32 N.A. |
0/32 N.A. |
8/32 (25) 1.8 [0.71; 4.4] |
Female calf |
5/24 (20.8) 1.53 [0.48; 4.54] |
0/24 N.A. |
0/24 N.A. |
5/24 (20.8) 1.42 [0.45; 4.19] |
|
Bull calf |
10/71 (14.08) 0.96 [0.44; 2.04] |
0/71 N.A. |
1/71 (1.4) N.A. |
11/71 (15.5) 0.99 [0.47; 2.05] |
|
Heifer |
20/123 (16.26) 1.13 [0.64; 2] |
0/123 N.A. |
2/123 (1.62) 1.99 [0.25; 12.75] |
22/123 (17.88) 1.17 [0.67; 2.03] |
|
Cow |
71/485 (14.6) |
1/485 (0.2) |
4/485 (0.82) |
76/485 (15.67) |
|
Age |
≤ 6 months |
13/56 (23.2) 1.59 [0.76; 3.29] |
0/56 N.A. |
0/56 N.A. |
13/56 (23.2) 1.44 [0.69; 2.96] |
6 months< <15months |
14/89 (15.73) 0.98 [0.49; 1.93] |
0/89 N.A. |
0/89 N.A. |
14/89 (15.73) 0.89 [0.45; 1.74] |
|
15 months≤ <4 years |
29/227 (12.77) 0.77 [0.46; 1.28] |
0/227 N.A. |
3/227 (1.3) 1.2 [0.21; 6.4] |
32/227 (14.09) 0.78 [0.48; 1.27] |
|
≥ 4 years |
58/363 (15.97) |
1/363 (0.27) |
4/363 (1.1) |
63/363 (17.35) |
|
Number of summer seasons in the farm |
0 1 ≥ 2 Overall |
99/622 (15.91) 1.02 [0.52; 2.01] 2/30 (6.66) 0.38 [0.06; 1.98] 13/83 (15.05) 114/735 (15.51) |
1/622 (0.16) N.A. 0/30 N.A. 0/83 1/735 (0.13) |
7/622 (1.12) N.A. 0/30 N.A. 0/83 (0.94) 7/735 (0.95) |
107/622 (17.2) 1.12 [0.58; 2.21] 2/30 (6.66) 0.38 [0.06; 1.98] 13/83 (16) 122/735 (16.59) |
Abortion |
4/30 (13.33) |
0/30 |
1/30 (3.33) |
5/30 (16.6) |
|
Lethality |
2/735 (0.27) |
0/735 |
0/735 |
2/735 (0.27) |
|
1: Association between the different parameters and prevalence of piroplasms in cattle
OR: Odds Ratio; N.A: Not Applicable
The clinical symptoms observed on animals with co-infections were in favor of tropical theileriosis, because babesiosis (Babesia spp. infection) is transmitted by Rhipicephalus annulatus which is active during the autumn and winter (Darghouth, 2004). This seasonal distribution was similar to that observed in semi-arid regions in Tunisia (Darghouth et al., 1999; Gharbi et al., 2006), as well as the activity of the tick vector (Bouattour et al., 1996; Gharbi et al., 2013).
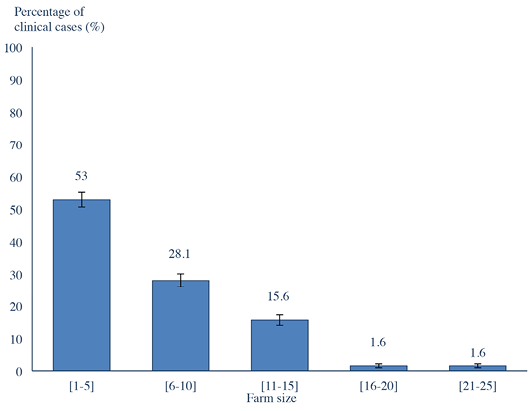
The majority of clinical cases (109/122; 89.3%) were from Sejnane delegation, especially El Maâlia locality (71/122; 58.2%) (χ2= 96.15; p<0.0001) (Figure 4). These results can be explained by differences in risk factors such as the technicality of breeders or poorly built cattle enclosures and size of the herd of cattle at risk in these areas. The majority of clinical cases (99/122; 81.1%) (χ2=94.3; p<0.0001) were from farms containing less than or equal to 10 animals. There was a significant negative correlation between the farm size and the incidence of clinical cases (Pearson correlation coefficient = - 0.81; p<0.0001) (Figure 5). Tropical theileriosis is more frequent in small cattle farms characterized by poor technical skills and low financial incomes affecting the cattle premises quality (presence of crevices and cracks) favorable to domestic tick H. scupense and on herd health (Darghouth, 2000). Indeed, the nymphs hibernate in the crevices and cracks, these shelters are also the egg laying site of engorged females (Gharbi and Darghouth, 2014). When considering the cattle population in the region, pure bred cattle were the most infected, the prevalence of the disease in Brown Swiss breed was 11.25% (54/480), 11.25% (36/320) in Holstein and only 0.34% (26/7500) in the crossbreed (χ2=623.55; p<0001). This difference could be explained by genetic resistance of crossbreed cattle (Glass et al., 2005). The majority of diseased animals (106/122; 86.88%) (χ2=135.21; p<0.0001) were recently introduced in the farm (Figure 3). These animals were naive against T. annulata and when introduced to the farms they are infected by the ticks (Darghouth et al. 1996).
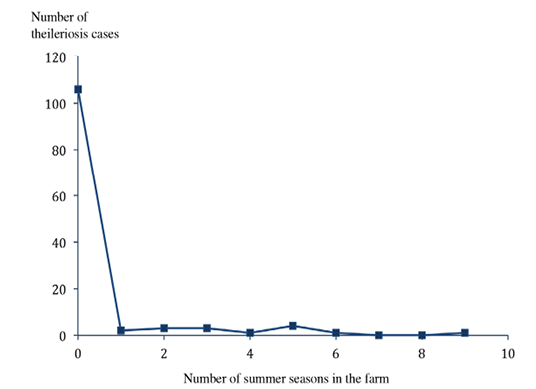
Figure 3:Number of tropical theileriosis clinical cases with regard to the number of summer seasons spent in the farm
These results show that the disease is mainly due to the introduction of exotic cattle breeds. Tropical theileriosis is a problem during any incomplete transition from traditional breeding to exotic breeds without acquiring the necessary technical package. This observation therefore illustrates the need of technical support to breeders by state regional structures concerned to ensure rational transition to the conditions for the breeding of improved breeds. The maximum incidence of clinical cases was observed in animals aged less then or equal to six months, this distribution would indicate the presence of an unstable enzootic state (Darghouth et al., 1996; Darghouth, 2000). Furthermore, the presence of many cases of disease in cows also indicate the presence of an unstable enzootic state as seems to confirm the presence of cows born in stables and yet affected by the disease.
The abortion rate (16.6%) and lethality (0.27%) were probably underestimated. Indeed, they were calculated on the basis of cases reported by breeders. These values were significantly lower than those reported in Tunisia by Darghouth et al. (1997), Khayeche (1998) and Bouattour (2001) which were 30 to 40% and 10 to 13.5%, respectively.
Knowledge of epidemiological indicators is important to define the epidemiological situations and allow an appropriate control programs implementation. Definitively, the failure of control programs against tropical theileriosis is mainly due to the presence of bad cattle premises where exotic cattle are introduced in enzootic tropical theileriosis.
This study is the first to be carried out in humid bioclimatic regions in Tunisia, further studies are needed to better characterize the enzootic states and phenology of the vector in this region.
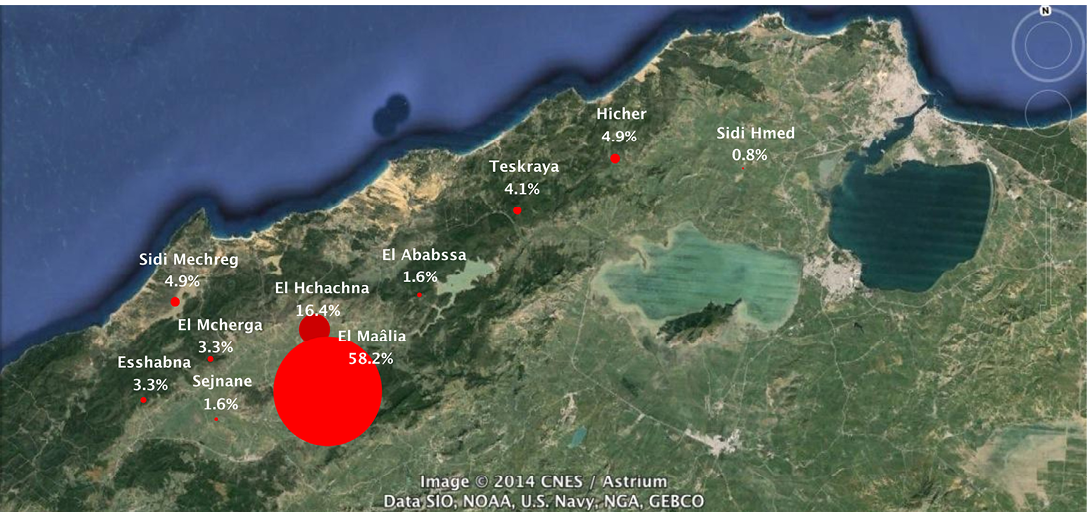
Figure 4: Geographic distribution and prevalence of tropical theileriosis clinical cases in Bizerte gouvernorate (Northwest Tunisia)
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
ACKNOWLEDGEMENTS
This study was supported by Ministry of Higher Education, Scientific Research and Information, Technology and Communication, Tunisia and the Deutsche Forschungsgemeinschat project “Molecular epidemiology network for promotion and support of delivery of live vaccines against T. parva and T. annulata infection in Eastern and Northern Africa’’ (Grant No. SE 862/2-1) and IRESA project “Etude des contraintes relatives à la filière lait bovin dans la région de Bizerte: vers un nouveau bassin laitier. The authors would like to thank all cattle farmers included in this study and all the staff of Laboratory of Parasitology, National School of Veterinary Medicine, Sidi Thabet, Tunisia: Limam Sassi, Mohamed Jedidi, Taoufik Lahmar and Béchir Guesmi.
Authors’ Contribution
MAB: collected and analyzed the samples and wrote the manuscript;
MG: wrote the manuscript;
MCS and LS: collected the samples;
MAD: designed the study and supervised the work
REFERENCES