The Journal of Advances in Parasitology
Research Article
Detection of Malaria in Healthy Blood Donors using PCR in an Endemic Area in Yemen
Ragaa Ali Othman1*, Hanan Elsayed Eldeek1, Amal Mohammed Almatary1, Ayat Abdelrahman Sayed2, Asmaa Alsakaf3
1Department of Parasitology; 2Department of Biochemistry, Faculty of Medicine, Assiut University, Egypt; 3Department of Parasitology, Faculty of Medicine, Ministry of Health, Yemen.
Abstract | The transmission of malaria by blood transfusion is one of the most important problems when selecting blood donors in transfusion centres particularly in endemic areas. Traditionally, malaria diagnosis has been made using thick blood smears, but more sensitive techniques are required especially in blood donor screening. The present study aimed at evaluating the diagnostic performance of nested PCR for detection of malaria parasites in healthy blood donors compared with light microscopy. Two hundred blood samples were collected randomly from healthy blood donors presented to the blood bank at the Almukalla Hospital, Hadramout Governorate, Yemen. Light microscopic examination of thin and thick blood smears and nested PCR were performed on the blood samples. Out of the 200 blood samples,148 were taken from males (74%) and 52 from females (26%), with an average age of 29.8+8.4. Twenty-eight out of the 200 samples (14%) were detected positive for Plasmodium by light microscopy, 2 (1%) of them were identified as Plasmodium vivax and 26 (13%) as Plasmodium falciparum. Thirty-three out of the 200 samples (16.5%) were detected positive by nested PCR, which were all identified as Plasmodium falciparum including 5 samples detected negative by light microscopy. It was concluded that nested PCR is the most appropriate method for population-based screening of blood donors for malaria parasites. It enabled the identification of P. falciparum in a considerable proportion of clinically healthy donors, highlighting the potential risk for transfusion-transmitted malaria. This tool can be adopted for the screening of malaria in haemotherapy centres, especially in malaria-endemic areas.
Keywords | Malaria, Blood donor, Microscopy, Nested PCR
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | July 16, 2015; Revised | August 05, 2015; Accepted | August 06, 2015; Published | August 10, 2015
*Correspondence | Ragaa Ali Othman, Assiut University, Egypt; Email: [email protected]
Citation | Othman RA, Eldeek HE, Almatary AM, Sayed AA, Alsakaf A (2015). Detection of malaria in healthy blood donors using PCR in an endemic area in Yemen. J. Adv. Parasitol. 2(2): 40-47.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.2.40.47
ISSN | 2311-4096
Copyright © 2015 Othman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Malaria is recognized as a serious public health problem occurring in tropical regions such as Africa, Asia, Central and South America (WHO, 2011). Malaria is transmitted not only by the bite of the female Anopheles mosquito but also by congenital transmission, by blood transfusion and the sharing of needles and syringes (Piñeros-Jiménez et al., 2011). According to the World Malaria Report published by the World Health Organization, 207 million malaria cases occurred among 3.3 billion at-risk people from 103 countries in 2012, resulting in an estimated 627,000 deaths (WHO, 2011).
Yemen is an Asian country where 65% of its population is at risk of malaria, with 43% at high risk (Omar et al., 2014). Plasmodium falciparum is the predominant species which is responsible for 90% of the malaria cases followed by Plasmodium vivax and Plasmodium malariae (Abdulsalam et al., 2010).
Infected blood donors are considered a potential hazard for blood recipients. The transmission of malaria via blood transfusion is the second manner of transmission and is associated with acute manifestations (Kazemi et al., 2005). The history of transfusion-transmitted malaria dates back to 1882, when Gerhardt empirically demonstrated the transmission of malaria in humans by infected blood (King, 1938). However, the first case of accidental transmission by blood transfusion was reported in 1911 (Singh and Sehgal, 2010). The risk of transfusion-transmitted malaria (TTM) has been associated with the difficulty in identifying infected potential donors, most with low numbers of parasites circulating in the blood (incomplete immunity), as well as the ability of this parasite to remain viable in stored blood bags, even after the storage process (Kitchen and Chiodini, 2006; Chattopadhyay et al., 2011). Thus, the transfusion practice constitutes a major challenge in malaria-endemic areas because many potential blood donors are infected. This situation could jeopardize the attainment of blood and blood products for transfusion demand in areas where the refusal of donation is high (Kitchen and Chiodini, 2006). Another difficulty is the lack of parasite-diagnostic methods that are sensitive, specific and easily reproducible in endemic areas and are focused on laboratory screening of blood donors (Seed et al., 2005; Noubouossie et al., 2012).
Microscopy is considered the gold standard for malaria diagnosis although its wide application is limited (Thongdee et al., 2014). Microscopy offers significant advantages to other methods, but its limitations include detection of low parasite loads, result interpretation, mixed infections and limited usefulness in non-endemic regions due to frequently inadequate training and experience of laboratory personnel (Wilson, 2013).
For two decades, the sensitivity of malaria diagnostic tests has considerably been improved with the introduction of molecular assays (Canier et al., 2015). Methods based on molecular biology have been used to detect different types of Plasmodium by PCR, such as nested PCR (Batista-dos-Santos et al., 2012). The performance of nested PCR as a malaria diagnostic tool is excellent with respect to its high accuracy, sensitivity, specificity, and ability to discriminate Plasmodium species (Canier et al., 2015). PCR technique can detect parasites below the threshold levels of microscopy; when performed under optimal conditions, PCR can detect parasitaemia as low as 0.01-1 parasite/μL of blood (Tangpukdee et al., 2009). The results depend directly on the quality of the genetic material (DNA) of the parasite obtained during extraction and amplification as well as on the quality of the reagents, and the test requires a long analysis time. Despite the increased sensitivity, PCR has not been established as a routine diagnostic method in laboratories or blood banks (Lima et al., 2011). One limitation of the PCR-based methods is that the parasite DNA can remain in the blood stream long after infection has been cleared and therefore differentiating an active infection from a recently cleared infection is difficult (Thongdee et al., 2014). Another major limitation is the volume of blood samples collected and analysed, obviously limited in mass screening studies (usually 5-30 µL). Indeed, larger volumes of blood may allow the detection of very low parasite density, and could reveal a higher than expected proportion of malaria parasite carriers, including mixed infections (Canier et al., 2015).
The objective of the present study was to evaluate the diagnostic performance of nested PCR for the detection of malaria infection in healthy blood donors compared with light microscopy.
MATERIALS AND METHODS
Study Area
The study was conducted over the period from May to November 2014 in Hadramout Governorate, the largest Yemeni governorate, accounting for half of the country’s surface area with a total population of 1,028,556 according to the last available census for the year 2004 (Ministry of Planning and International Cooperation Yemen, 2004). Hadramout is located in the east of the republic of Yemen and at 777 km from the capital Sana’a, bounded to the north by the Empty Quarter, and the middle Mahra Governorate, in the south by the Arabian Sea and to the west by Shabwa. Hadramout was selected as an endemic area for malaria in our study.
Blood Collection
Two millilitres of venous blood sample were collected from each of the two hundred participants who were randomly selected from blood donors presented to the blood bank at the Almukalla Hospital, Hadramout Governorate. The subjects were of both sexes and their age ranged from 20-55 years. All participants completed data forms for history of malaria infection, or fever and chills and also anti-malaria drug consumption during the last 2 years. The blood samples were collected in EDTA anticoagulant tubes. Thin and thick blood smears were prepared and sent with the stored blood samples to the Parasitology Department, Faculty of Medicine, Assiut University, Assiut, Egypt in order to perform the study techniques.
Ethical Approval
The study was approved by the Ethical Review Committee of the Yemen Ministry of Public Health. Moreover, the results of examination were given to the participants, free of charge.
Light Microscopic Examination
All samples were air-dried, fixed in methanol and stained for 15–30 minutes in 1:10 diluted Giemsa stain (BDH Ltd); a (pH 7.2). The stain was washed off with tap water and the smears were examined by oil immersion lens×1,000 magnification by 2 independent experienced parasitologists. Thick and thin films were interpreted as negative only after examination for at least 100 oil immersion fields. Parasitaemia was determined as: No. infected RBCs ÷ Total No. RBCs counted) × 100 = Percent Infected RBCs.
Table 1: Primers for nested PCR of Plasmodium 18S rRNA gene
|
Species |
Primer |
Sequence 5´-3´ |
Size of PCR product (bp) |
|
Plasmodium sp. |
rPLU5 |
CCTGTTGTTGCCTTAAACTTC |
1,100 |
|
rPLU6 |
TTAAAATTGTTGCAGTTAAAACG |
||
|
P. falciparum |
rFAL1 |
TTAAACTGGTTTGGGAAAACCAAATATATT |
205 |
|
rFAL2 |
ACACAATGAACTCAATCATGACTACCCGTC |
||
|
P. vivax |
rVIV1 |
CGCTTCTAGCTTAATCCACATAACTGATAC |
120 |
|
rVIV2 |
ACTTCCAAGCCGAAGCAAAGAAAGTCCTTA |
Nested Polymerase Chain Reaction (Nested PCR)
DNA extraction from EDTA blood samples was carried out using the QIAamp DNA Mini spin columns according to the manufacturer protocol (http://www.qiagen.com), quantified, and stored at -20°C. Nested PCR assay was carried out as previously described elsewhere (Snounou et al., 1993; Mekonnen et al., 2014).
DNA samples were amplified using both genus-specific and species-specific primers (Table 1) targeting the Plasmodium spp. 18S small subunit ribosomal RNA genes. The primers used were manufactured in laboratories of the analysis for life invitrogen by life technologies, Texas, USA.
The PCR reactions were carried out in a 25 μL reaction volume containing 12.5 µL of 2x green PCR Master Mix, 200 nM primers, with 5µL of DNA solution (for the 1st round reactions) and 5 µL of the PCR products in the 2nd round, and up to 25 μL nuclease free water on a Veriti® Thermal Cycler (Life Technologies). The thermo-cycler was programmed as follows: Hot start at 95°C for 10 min; initial denaturation at 95°C for 45 sec; annealing at 58°C for1 min; extension at 72°C for 2 min. A total of 40 cycles were used followed by a final extension 72°C for 10 mins for the 1st round reactions. For the second round reaction the condition is as follows: 30 cycles of, respectively, 25 sec at 94°C for denaturation, 25 sec at 56°C for annealing temperature, and 60 sec at 72°C extension. This was followed by a final extension of 10 min at 72°C. The amplified product was detected by electrophoresis on 1% agarose gel stained with ethidium bromide and visualized on an ultraviolet trans-illuminator. Positive and negative controls were included in each run (positive controls were persons who are evident clinically and microscopically having malaria infection with high parasitaemia, while the negative controls were healthy individuals with no history of malaria, living in non-endemic areas) .
Data Analysis
Categorical variables were described by number and percentage (N & %), whereas continuous variables were described by mean and standard deviation (Mean ±SD). Sensitivity, specificity, positive predictive value (PPV), negative predictive (NPV) and accuracy rate were calculated. All analyses were performed with the SPSS 20.0 software.
Results
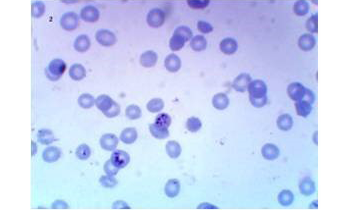
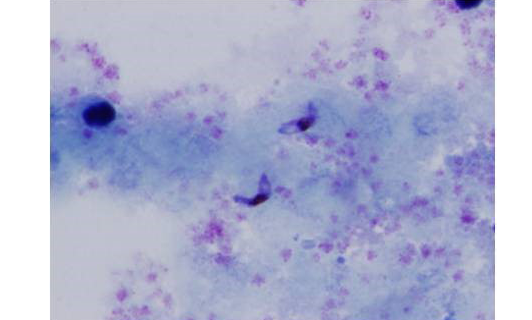
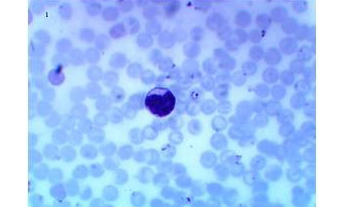
A total of 200 donor samples were evaluated, all in a governorate considered endemic for malaria in Yemen. 148 of the donors were males (74%) and 52 were females (26%). Their age ranged from 20 to 55 years old with an average of 29.8±8.4. In donor samples examined by thin and thick blood films, analysis by light microscopy showed that 28(14%) were detected positive for malaria, 2 (1%) of them were identified as having only P. vivax and 26 (13%) as having only P. falciparum infections (Figures 1, 2 and 3), 172 (86%) were slide-negative.
There was no P. malariae or P. ovale in these specimens. The thin films showed parasitaemia ranging from 0.001% to 5%. PCR analysis for the detection of the Plasmodium genus and species determination is shown in Table 2.
Table 2: Comparisons between microscopic examination and PCR for detection of malaria in blood samples of 200 blood donors
|
Results |
Microscopic examination (n=200) |
PCR (n=200) |
||
|
No. |
% |
No. |
% |
|
|
Positive cases |
||||
|
P. Falciparum |
26 |
13 |
33 |
16.5 |
|
P. Vivax |
2 |
1 |
0 |
0 |
|
Mixed |
0 |
0 |
0 |
0 |
|
Negative cases |
172 |
86 |
167 |
83.5 |
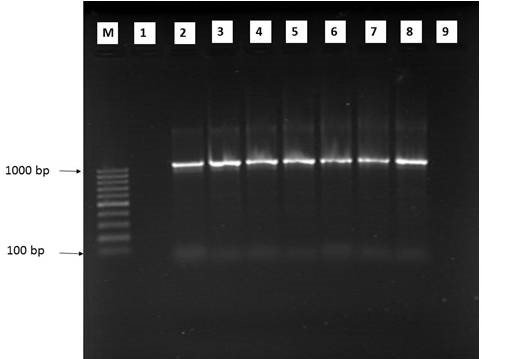
Figure 4: Agarose gel electrophoresis of 1st run nested PCR product using Plasmodium genus-specific primers
M: 100 bp ladder; PCR amplification product of 1100 bp was seen in all samples (lane 2-7); Lane 1: negative case control; Lane 8: positive case control; Lane 9: no template control
PCR detected malarial parasites in 33 samples including 5 samples that had been detected negative for malarial parasites on light microscopy. The percentages of P. vivax mono infection, P. falciparum mono infection, and mixed infections were 0 (0%), 33 (16.5%) and 0 (0%), respectively. The samples positive for P. falciparum included 7 samples from women (7/52 women) and 26 from men (26/148 men). All positive samples were within the age range of 20 to 37 years, and their average age was 26.2±3.9 (Table 3). A typical gel was shown in Figures 4 and 5. Five out of 200 specimens microscopically diagnosed as negative were positive as P. falciparum with nested PCR (Table 4). In order to ensure that the results were true and not due to technical errors such as cross-contamination, assays were all carried out with concurrent human negative controls (healthy individuals, with no history of malaria, living in non-endemic areas of the country). Sensitivity and specificity of PCR diagnosis were detected in comparison with microscopy were shown in Table 5.
Table 3: Number of samples detected by nested PCR for Plasmodium parasite according to age groups and gender
|
Age (year) |
Negative samples (167) |
Positive samples (33) |
||
|
Male (122) |
Female (45) |
Male (26) |
Female (7) |
|
|
No. % |
No. % |
No. % |
No. % |
|
|
20-28* |
68 55.7 |
26 57.8 |
22 84.6 |
5 71.4 |
|
29-37* |
24 19.7 |
11 24.4 |
4 15.4 |
2 28.6 |
|
38-46 |
19 15.6 |
6 13.3 |
0 0.0 |
0 0.0 |
|
47-55 |
11 9.0 |
2 4.5 |
0 0.0 |
0 0.0 |
*There was significant difference in the comparison between different positive age groups (p<0.01), however, there is no significant difference relating to gender p=0.268 (p>0.05).
Table 4: Comparison between microscopic and PCR diagnosis in the 200 blood samples tested in the present study
|
PCR |
|||||
|
Microscopy examination |
P. vivax |
P. falciparum |
Mixed infection |
Negative results |
Total |
|
P. vivax |
0 |
2 |
0 |
0 |
2 |
|
P. Falciparum |
0 |
26 |
0 |
0 |
26 |
|
Mixed infection |
0 |
0 |
0 |
0 |
0 |
|
Negative results |
0 |
5 |
0 |
167 |
172 |
|
Total |
0 |
33 |
0 |
167 |
200 |
Table 5: Sensitivity, specificity, PPV, NPV and accuracy of PCR in relation to microscopy
|
Microscopic examination |
||||
|
+Ve |
-Ve |
|||
|
PCR |
+Ve |
28 |
5 |
PPV=84.85% |
|
-Ve |
0 |
167 |
NPV=100.0% |
|
|
Sensitivity = 100.0% |
Specificity = 97.1% |
Accuracy=97.5% CI= (93.9:99.1) |
||
PPV: Positive predictive value; NPV: Negative predictive value; CI: Confidence interval; +Ve: Positive; -Ve: Negetive
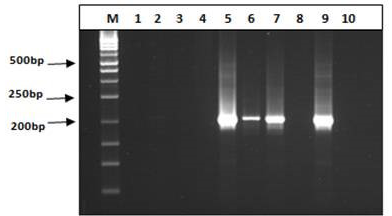
Figure 5: Agarose gel electrophoresis of nested PCR product using specific primer for P. vivax and P. falciparum
M: 50 bp ladder; The representative P. vivax were negative by using P. vivax specific primers lanes 1-4 (right panel) and the representative P. falciparum were positive at 205bp by using P. falciparum specific primers lanes 5-7 (left panel); Lane 8: negative case control; Lane 9: positive case control; Lane 10: no template control
Discussion
The transmission of malaria by blood transfusion was one of the first transfusion-transmitted infections recorded in the world. Transfusion-transmitted malaria may lead to serious problems because infection with P. falciparum may cause rapid death (Hassanpour et al., 2011).
Currently, the technique most often used in endemic areas is the gold standard of objective microscopy in thick blood smears. This technique is considered the most effective and inexpensive for the diagnosis of malaria. However, its low sensitivity and specificity in situations of low-parasite density hinder the detection of asymptomatic donors who have small amounts of parasites at the time of donation. This fact presents a transfusion risk for the recipient, and parasite detection depends on the experience of the microscopist (Singh and Sehgal, 2010; Rosanas-Urgell et al., 2010). So, there is a need for molecular methodologies based on the amplification of DNA because of its higher sensitivity for detection of picogram units of DNA.
The elevated malaria indexes found in endemic areas of Yemen increase the risk of blood transfusion transmission. Our results showed that there is great risk of this type of transmission, during checking 200 blood samples collected from participants who were randomly selected from blood donors at Almukalla Hospital, Hadramout Governorate, Yemen. Fourteen percent of the samples showed positivity for malaria by microscopic examination and 16.5% by using nested PCR. The comparison between the nested PCR and blood smear, which is considered the reference test for malaria detection, demonstrated a sensitivity of 100%. Other authors in different countries have reported lower sensitivity values of 96.6% (Boonma et al., 2007), 94.3% (Han et al., 2007), 99.41% (Khairnar et al., 2009) and 93.88% (Lima et al., 2011). The specificity of our results using nested PCR reached 97.1%, which is greater than some previously described values (89.4% (Boonma et al., 2007), 94.3% (Han et al., 2007), and 90.88% (Khairnar et al., 2009), but lower than Lima et al., (2011) who have reported a specificity of 100% (Lima et al., 2011).
A study conducted in Yemen by Saif et al. (2014) found that the total malaria cases in the blood bags in Taiz Governorate is about 8.33% by using microscopic examination and the main Plasmodium species was P. falciparum (100%), which was also the predominant species in studies conducted by Abdulsalam et al. (2010), Assabri and Muharram (2002), Azazy and Rajaa (2003), Al-Maktari et al. (2003); Bassiouni and Al-Maktari (2005), Alkadi et al. (2006) and Al-Taiar et al. (2006). More recently, Sallum et al. (2014) found that most of the subclinical infections were associated with P. falciparum. In our study we detected P. falciparum mono-infection in 13% of cases followed by mono-infection with P. vivax detected in only 1% of cases, the latter was only detected by microscopy.
Traditionally, P. falciparum has been thought to inhibit the parasitaemia of P. vivax (Albadr et al., 2011). Albadr et al. (2011) found that when P. vivax parasitaemia rose and no drug was administered, asexual P. falciparum parasitaemia fell to submicroscopic levels. This is somewhat surprising as previous experience showed that mixed infections tend to be interpreted as P. vivax rather than P. falciparum, obviously because of its characteristic morphology (Wang et al., 2014). The reason why microscopy failed to detect mixed infections could be due to the presence of higher numbers of parasites of one species relative to the others (Mekonnen et al., 2014). In our study no vivax malaria was detected by PCR, although there were 2 cases of vivax malaria detected by microscopy. This may be due to the fact that PCR test was not able to detect mixed infections. This could probably be due to the tendency for one species to be dominant over other species (Kimura et al., 1995; Albadr et al., 2011). This result does not agree with Jill et al. (1999) who stated that PCR could diagnose mixed infection more than microscopy. Also the recurrence of P. vivax infections cannot be theoretically predicted by PCR assays because PCR cannot detect relapses of the P. vivax hypnozoite liver stage (Kimura et al., 1995; Albadr et al., 2011). That is why the WHO recommends microscopic examination as the gold standard for the diagnosis of vivax malaria (Kim et al., 2014).
Although malaria affects all ages, the present study showed that all positive cases were among donors between the ages of 20 and 37. This result confirmed the greater risk of an exposure to malaria infection in adults. Also the study conducted by Abdulsalam et al. (2010) confirmed that malaria in Yemen is age dependent, identifying the younger age as a high risk group (Abdulsalam et al., 2010). This finding is concordant with previous results from Yemen and other countries (Sintasath et al., 2005; Al-Taiar et al., 2006; Bin Mohanna et al., 2007). This pattern may be the result of professional activities that foster individual exposure to the mosquito vector (Batista-dos-Santos et al., 2012). Also the bias of malaria infection to younger age group could be explained by the fact that protection against malaria may be acquired with age due to repeated exposure in endemic areas (Carneiro et al., 2010).
Our study also showed that malaria positive cases are gender biased, with males being more infected, although no significant difference existed between them. Similar results have been reported in Yemen (Al-Taiar et al., 2006; Abdulsalam et al., 2010), Saudi Arabia (Malik et al., 1998) and many African countries (Oduro et al., 2007).
Our results indicated that nested PCR is a highly sensitive method that can be helpful for the confirmation of malaria infection in different units of blood transfusion organizations especially in malaria-endemic areas where the majority of donors may be potentially infected with malaria parasites. Many authors used nested PCR as a reference standard for calculating the sensitivity, specificity, and agreement of other PCR-based assays (Thongdee et al., 2014; Wang et al., 2014). Although nested PCR has been reported as a specific and sensitive diagnostic method to detect malaria, the assay is inadequate for use in a clinical setting because the turnaround time is incompatible with the urgency required to initiate treatment. However it can be a valuable tool when malaria cases with low parasitemia need to be identified as in screening blood donors in endemic areas or even monitoring malaria treatment (Kim et al., 2014).
This study concluded that nested PCR enabled the identification of P. falciparum in a high proportion of clinically healthy donors, highlighting the potential risk for transfusion-transmitted malaria. Additionally, this molecular diagnostic tool can be adopted as a new laboratory screening method in haemotherapy centres, especially in malaria-endemic areas. Although, till now, the usage of PCR in Yemen is limited as a confirmatory tool for donor screening in blood banks due to cost and technical inadequacy, the test proved to be useful in screening blood donors in endemic areas which is of higher importance because of the scarce number of blood donors and the increased risk of disease transmission.
Authors’ contributions
All the authors participated in drafting and designing the manuscript. Ragaa Ali Othman, Hanan Elsayed Eldeek, Amal Mohammed Almatary and Asmaa Alsakaf performed parasitological examination while Hanan Elsayed Eldeek and Ayat Abdelrahman Sayed analysed data. All authors approved final form and additional revisions.
CONFLICT OF INTEREST
There exisit no conflict of interest.
References

 http://dx.doi.org/10.1590/S0074-02762011000600008
http://dx.doi.org/10.1590/S0074-02762011000600008