The Journal of Advances in Parasitology
Research Article
Effect of Systemic Ivermectin on Epidermal Tunnel Size, Severe Asthetic Appearance of Skin, Haematological and Biochemical Inbalances Caused by Sarcoptes scabie var caprae in West African Dwarf Goats, in Ibadan, Nigeria
Olufarati Oludunsin Falohun*, Nurudeen Ayinde Sadiq
Department of Veterinary Microbiology and Parasitology, University of Ibadan, Nigeria.
Abstract | Systemic ivermectin efficacy was evaluated clinically and histologically in West African Dwarf (WAD) goats naturally infested with Sarcoptes scabie var caprae. A total of ten naturally infested WAD goats were bought from different villages in Ibadan and these were divided into two groups. Group A which comprised of five goats was treated with 1% ivermectin at a dosage of 0.2mg/kg, while other group also comprising of five goats was given 1ml distilled water as control. The two groups were treated at 2-weeks interval and samples including whole blood, serum and skin scrapings were collected 3 days before treatment and 3 times per treatment while skin biopsies were collected at a week interval. Presence of mites and the tunnels created in the skin was confirmed through microscopic examination of KOH treated skin scrapings and histological sectioning of skin biopsies. Mite numbers were estimated, hematology, biochemistry, histopathology and gross lesions were evaluated in each group before treatment and approximately 3-days interval during treatment. Consistent greater reduction in mite number and epidermal tunnels heights and diameter was recorded following ivermectin treatment, as goats treated with ivermectin showed quick improvement in the healing of gross lesions, re-growth of hair on the skin, hematological and biochemical parameters. There was a significant reduction (p< 0.05) in mite count from a mean pretreatment level of 49.40±17.36 to 25.50±4.17 at day 7 of first treatment while mite egg count also reduced significantly (p<0.05) from mean pretreatment level of 20.20±6.26 to 4.50±1.85 at day 11 of first treatment and all mites were totally cleared at day 11 of second treatment, whereas epidermal tunnel height reduced significantly (P<0.05) from a pretreatment mean value of 238.00±46.45 to 161.30±39.02 at week 4 post treatment while tunnel diameter also significantly reduced (p<0.05) from 158.00±74.55 pretreatment value to 92.50±40.52 also at week 4 post ivermectin treatment. The tunnel size further reduced significantly (p<0.05) with longer post treatment days as tunnel height decreased to 35.00±16.83 and tunnel diameter to 20.00±7.07 at week 10 post treatment. Haematological and biochemical parameters showed significant improvement after second treatment with ivermectin. Although two consecutive negative skin scrapings was achieved in this study following second treatment with Ivermectin, treatment of infested goats was further extended to fourth regimen at two weeks interval to achieve complete recovery and prevent reoccurrence of infestation as systemic Ivermectin is safe to use and reaches its bioavailability unchanged with minimal toxicity.
Keywords | Sarcoptes scabie var caprae, WAD goats, Ivermectin, Histopathology, Haematology and Biochemistry
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | February 20, 2015; Revised | March 21, 2015; Accepted | March 30, 2015; Published | April 08, 2015
*Correspondence | Olufarati Oludunsin Falohun, University of Ibadan, Nigeria; Email: [email protected]
Citation | Falohun OO, Sadiq NA (2015). Effect of systemic ivermectin on epidermal tunnel size, severe asthetic appearance of skin, haematological and biochemical inbalances caused by Sarcoptes scabie var caprae in West African Dwarf Goats, in Ibadan, Nigeria. J. Adv. Parasitol. 2(1): 11-18.
DOI | http://dx.doi.org/10.14737/journal.jap/2015/2.1.11.18
ISSN | 2311-4096
Copyright © 2015 Falohun and Sadiq. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Goat production in developing countries like Nigeria is beset with series of constraints like poor health, which results in spread of infections such as mange disease. Mange is a contagious disease of animal and man (Scabie) caused by mite infestation, which damages their skin and hides, thereby reducing its quality (Blood et al., 1983; Oladeji, 2006).
Mange mites are the most notorious ectoparasite of goats and are responsible for great economic losses especially in humid area of West Africa (Akomas et al., 2011).
The condition predisposes animals to other bacterial and viral infections and it is also of zoonotic importance as the infection can be transferred to humans during husbandry (Dominguez et al., 1977). The diagnosis of mange is usually based on the detection of mites in skin scrapings.
Ivermectin, a macrocyclic lactone acaricide showed remarkable improvement in goat infested with sarcoptic mange when treated with a dosage of 0.2mg/kg (Alayande et al., 2002; Akomas et al., 2011). Ivermectin treatment has also been shown to reduce the prevalence and intensity of parasitism in West African Dwarf goats (Daramola et al., 2003). Ivermectin effectively cure goats of infestation by mange mites and thereby relieve them of the adverse effect of the mites especially on blood (Akomas et al., 2011).
MATERIAL AND METHODOLOGY
Animals Used and Sample Collection
A total of 10 naturally infested West African Dwarf goats were bought from different villages in Ibadan which includes Ijaye and Maale areas in Akinyele Local Government, Adaba area in Ido East Local Government, and Lalupon village in Lagelu Local Government. The animals were housed separately in pens in the University of Ibadan Teaching and Research Farm. During the acclimatization period, the animals were screened for haemoparasites and dewormed with curative dose of Albendazole 5mg/kg PO and administered with oxytetracycline at 5mg/kg. They were fed with dried cassava peels, corn bran and elephant grass and were given water ad-libitum. Deep skin scrapings were collected from lesion sites for identification of the mites, deep shaved skin biopsies were also collected for histology of epidermis. Blood samples were collected from the jugular vein in EDTA bottle for hematological studies and in plain vacutainer bottle for serum biochemical studies.
Skin Scrapping
Skin scrapings were collected from different regions of the body (face, shoulder, ear, neck, limbs and abdomen) of each animal by scrapping the edges of the lesion with scalpel until capillary bleeding was seen and the scrapped material transferred to clean sample bottles and parasitological identification of the mites was conducted as described by Soulsby (1982). Skin scrapings at the area of around 2.5-cm² was treated with 20ml 10% (KOH) solution for 10min to digest the keratin. This was then centrifuged at 1500rpm for 5 min, and the supernatant was discarded and the sediment carefully mixed with saturated glucose solution. After 10 min the upper layer was collected and examined under a stereoscopic microscope to determine the presence of mites. Identification of mites was carried out with the help of morphological characteristics as described by Wall and Shearer (1997).
Skin Biopsy and Sectioning
Deep Shaved Skin Biopsy was performed as described by Olbricht (2003) in which an area of the lesion was shaved to the skin and 2% lignocaine hydrochloride subcutaneously was given, before an incision was then made at the site of the lesion with a sterile scalpel vertically into the skin then moved forward horizontally in a sawing motion turning it up towards the surface to finish the excision. The skin biopsy was then put in 70% ethanol and taken to the laboratory for histopathology. The biopsies were processed, dehydrated in increasing concentrations of ethanol, cleared in xylene and embedded in paraffin. The paraffin blocks were sectioned with a microtome at 5um thickness, and placed on slides used for Haematoxylin and Eosin stain and examined as indicated by Bancroft and Harry (1994).
Histomorphometrics
Histomorphometric studies were done using a light microscope (Bio-microscope, YJ-2005 series) connected to a laptop computer (hp, china) with TSview 1.0 and AmscopeToupView 3.2 software measuring the tunnel heights and tunnel diameter in the skin created by the mites. These figures were automatically generated with the aid of the software.
Haematology and Biochemistry
10mls of blood were collected from the jugular vein of the WAD goats into labeled sterile EDTA bottle to investigate different hematological disturbances. The Packed Cell Volume (PCV) was determined by microhaematocrit method, Haemoglobin concentration was measured by cyanmethaemoglobin method, red blood cell (RBC), total white blood cell and differential WBC were all determined by haemocytometer method as described by Schalm (1986).
5mls of serum was collected into plain vacutainer tube for serum biochemistry. Total serum protein was measured by Biuret method, Albumin values was estimated by bromocresol green, method. Serum sodium and potassium value was determined by flame photometry using electrophotometer.
Treatment Plan
The naturally infested West African Dwarf goats were grouped into two, group A comprised of five animals which were placed on 1% Ivermectin (0.2mg/kg) subcutaneously at an interval of 2 weeks until two consecutive negative skin scrapings were obtained and complete recovery is achieved and group B also consisted of five animals which were placed on placebo as control. Rectal temperature evaluation, morphological identification, haematological and biochemical analysis were done 3 days before treatment and 3 times per treatment ( day 3, day 7, day 11 of first treatment, second, third and fourth treatments) while histo-pathological analysis was done at weekly interval until two consecutive negative skin scrapping results were obtained and complete recovery is achieved.
Statistical Analysis
The data obtained were summarized as means ± standard deviations and differences between the means determined at 5% level of significance using the T- test and two-way analysis of variance (ANOVA) using Graph pad Prism 5.
RESULT
Clinical Evaluation before Treatment
Dermatological examination of the goats revealed extensive alopecia, severe erythema, hyperkeratosis and areas of adherent crust formation affecting the ventral abdomen, chest, limbs, forehead and neck region. Heavy presence of adult mites and their eggs, histopathological lesions including acanthosis, spongiosis, hyperkeratosis, mite containing epidermal tunnel, hematological and biochemical in-balances were observed and confirmed before the onset of treatment. Sarcoptes scabie var caprae was the only mite specie identified infesting all the West African Dwarf goats used for this study. These mites have oval to round, dorsally convex tortoise-like body that is covered with spines and triangular scales with only the first two pairs of legs protruding beyond the body margin. The female mites were identified bearing long bristles on the third and fourth pair of legs while the male bears long bristle on the third pair of leg alone (Figure 1).
Effect of Ivermectin on Adult Mites and Egg Count
There was no significant difference (p>0.05) in adult mite count before ivermectin treatment (49.40±17.36) and in day 3 post treatment with ivermectin (30.75±9.07), however day 7 (25.50±4.17) and day 11 (20.50±4.43) showed significant reduction (p< 0.05) in the number of adult mite when compared with the control group. Day 3 (6.75±3.30) and day 7 (0.75±0.96) with second ivermectin treatment witnessed a significant decrease (p<0.05) in the adult mite count while day 11 post second treatment showed that all mites had been eliminated (0.00±0.00), while the population of adult mite increased significantly (p< 0.05) in the control group from 89.33±10.26 before treatment to 125.30±6.51 on day 11 of third placebo injection.
The mite egg count in animals before treatment was 20.20±6.26 which reduced significantly (p<0.05) to 9.75±3.86 on day 3 post first treatment but there was significant reduction (p <0.05) on day 11 of first ivermectin treatment to 4.50±1.85. On day 7 of second treatment, no mite egg was observed (0.00±0.00) in the animals following ivermectin administration (Table 1).
Effect of Ivermectin on Epidermal Tunnel Size
The epidermal tunnel height and tunnel diameter decreased from week 1 to week 10 following ivermectin treatment, as there was significant reduction (p<0.05) in tunnel height from a pre-treatment mean value of 238.00±46.45 to 161.30±39.02 at week 4 post treatment while tunnel diameter also significantly reduced (p<0.05) from 158.00±74.55 pretreatment mean value to 92.50±40.52 also at week 4 post ivermectin treatment. The tunnel size further reduced significantly (p<0.05) with longer post treatment days as tunnel height decreased to 35.00±16.83 and tunnel diameter to 20.00±7.07 at week 10 post treatment. However, in the control group the tunnel size increased significantly (p< 0.05) from pretreatment height of 256.7±38.86 to 316.7±2.89 at week 10 and the epidermal diameter from pretreatment value of 165.0±82.61 to 238.3±55.08 at week 10 of observation (Table 2 and Figure 2).
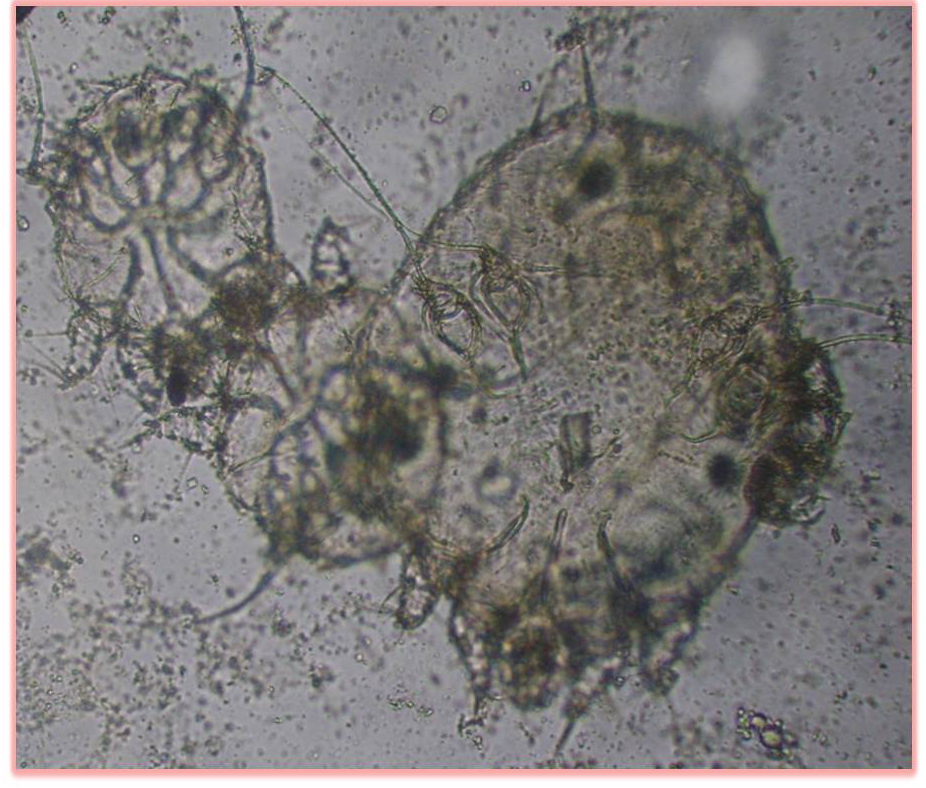
Figure 1: Heavy infestation before treatment with the female mite bearing long bristles on the third and fourth pair of legs while the male bears long bristle on the third pair of leg
Table 1: Efficacy of ivermectin on Adult mite and mite egg in goats naturally infested with mange
|
Parameters |
Adult Mite Count |
Mite Egg Count |
|||||
|
Ivermectin group |
Control group |
Ivermectin group |
Control group |
||||
|
Pretreatment |
DOSC |
49.40±17.36a |
20.20±6.26a |
43.00±18.08a |
89.33±10.26a |
||
|
First Treatment |
Day 3 |
30.75±9.07a |
9.75±3.86a |
48.33±16.01a |
89.67±9.71a |
||
|
Day 7 |
25.50±4.17b |
5.75±2.01a |
52.67±7.79b |
93.00±5.13a |
|||
|
Day 11 |
20.50±4.43b |
4.50±1.85b |
54.33±12.50c |
95.00±7.94b |
|||
|
Second treatment |
Day 3 |
6.75±3.30b |
2.00±1.63b |
62.33 ±16.44c |
97.67±5.51b |
||
|
Day 7 |
0.75±0.96c |
0.00±0.00b |
67.67 ±17.79c |
103.00±6.08c |
|||
|
Day 11 |
0.00±0.00c |
0.00±0.00c |
90.00 ±7.94d |
116.00±7.00c |
|||
|
Third Treatment |
Day 3 |
0.00±0.00c |
0.00±0.00c |
92.67 ±6.81d |
117.00±8.89c |
||
|
Day 7 |
0.00±0.00c |
0.00±0.00c |
95.33 ±4.04d |
119.70±6.81c |
|||
|
Day 11 |
0.00±0.00c |
0.00±0.00c |
98.00 ±6.25d |
125.30±6.51c |
|||
|
Fourth Treatment |
Day 3 |
0.00±0.00c |
0.00±0.00c |
103.00 ±9.17d |
127.30±4.62d |
||
|
Day 7 |
0.00±0.00c |
0.000.00c |
107.30 ±10.69d |
132.30±10.69d |
|||
a, b, c, d Superscripts between groups in columns differed significantly (p<0.05). DOSC : Days of sample collection
Table 2: Efficacy of ivermectin on epidermal tunnel size created by burrowing Sarcoptes scabie var caprae mite infesting the goats
|
Days of sample collection |
Ivermectin Group |
Control Group |
||
|
Tunnel Height (µm) |
Tunnel Diameter (µm) |
Tunnel Height (µm) |
Tunnel Diameter (µm) |
|
|
Pretreatment |
238.00±46.45a |
158.00±74.55a |
256.7±38.86a |
165.0±82.61a |
|
Week 1 |
228.80±52.66a |
128.80±60.60a |
258.3±34.03a |
173.3±76.38a |
|
Week 2 |
218.80±38.38a |
121.30±54.83a |
266.7±25.17a |
180.0±78.58a |
|
Week 3 |
210.00±43.97a |
117.50±60.76a |
268.3±22.50a |
180.0±78.58a |
|
Week 4 |
161.30±39.02b |
92.50±40.52b |
273.3±22.55b |
186.7±77.67b |
|
Week 5 |
122.50±27.54b |
66.28±28.69c |
278.3±25.66b |
193.3±77.51b |
|
Week 6 |
117.50±33.04b |
61.25±27.80c |
286.7±15.28b |
206.7±67.14c |
|
Week 7 |
105.00±26.46c |
50.00±18.26c |
303.3±15.28c |
215.0±69.46c |
|
Week 8 |
71.25±28.39d |
30.00±18.26d |
306.7±11.55c |
218.3±68.25c |
|
Week 9 |
67.50±25.98d |
26.25±12.50d |
311.7±7.64d |
226.7±70.72d |
|
Week 10 |
35.00±16.83d |
20.00±7.07d |
316.7±2.89d |
238.3±55.08d |
a, b, c, d Superscripts between groups in columns differed significantly (p<0.05)
Effect of Ivermectin on Haematological Parameters
There was improvement in the PCV of the animals following ivermectin treatment. PCV significantly increased (P <0.05) from pretreatment level of 11.40 ±1.67 to 27.74±3.37 at day 7 of second treatment. Haemoglobin concentration was significantly increased (p<0.05) to 8.78±0.71 at day 7 of second treatment from a pretreatment value of 3.74±0.62 and this further increased significantly (p< 0.05) to 10.50±0.99 after the fourth treatment as compared with the control group. Red blood cell increased significantly (11.0±0.96) at day 3 of second treatment from a pretreatment value of 6.04±1.55. White blood cell significantly increased (p< 0.05) from a pretreatment value of 15.28±1.82 to a value of 13.30±0.41 at day 7 of second treatment. A further significant decrease (P< 0.05) was also observed following third treatment (8.30±0.22) and fourth treatment (7.85±0.24) whereas the White blood cell value in the untreated (control) animals remained consistently high throughout the experiment. A significant reduction (P< 0.05) in eosinophil count was seen as the value reduced from 4.40±0.89 before treatment to 0.00±0.00 at day 11 of third treatment. The untreated animals did not witness any significant decrease (P>0.05) throughout the experiment. (Table 3).
Table 3: Efficacy of ivermectin on hematological parameter of WAD goats naturally infested with mange
|
Pretreatment |
1st Treatment |
2nd Treatment |
3rd Treatment |
4th Treatment |
|||||||||||
|
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
|||||
|
PCV (%) |
11.40 ±1.67ᶜ (11.67 ±1.53a) |
14.00 ±1.71c (11.33 ±2.08ᶜ) |
14.75 ±0.82c (13.00 ±2.00ᶜ) |
14.25 ±2.06ᶜ (14.00 ±1.00ᶜ) |
14.7 ±1.71c (14.23 ±1.00ᶜ) |
27.74 ±3.37ᵇ (14.33 ±1.53ᶜ) |
29.78 ±3.86b (14.67 ±2.52c) |
30.00 ±2.16ᵇ) (14.33 ±1.53ᶜ) |
331.25 ±2.25a 1(14.33 ±2.08c) |
32.00 ±2.22a (14.33 ±2.31c) |
30.75 ±1.83b (14.00 ±1.00ᶜ) |
31.00 ±0.82a (14.67 ±0.58ᵇ) |
|||
|
HB (g/dl) |
3.74 ±0.62a (3.77 ±0.15ᵃ) |
4.40 ±0.61a (4.10± 0.20ᵃ) |
4.93 ±1.14a (4.93 ± 0.78a) |
5.30 ±1.27a (6.07± 0.85a) |
7.80 ±0.98a (5.93± 0.60a) |
8.78 ±0.71b (5.27± 0.81c) |
9.30 ±0.88b (5.37 ±1.01c) |
9.55 ±0.89b (5.13± 0.42c) |
10.23 ±0.65c (5.07± 0.93c) |
10.35 ±0.81c (5.27± 0.67c) |
10.38 ±0.99c (5.17± 0.61c) |
10.50 ±0.99d (5.57± 0.76c) |
|||
|
RBC (X106/µl) |
6.04 ±1.55c (7.20± 1.15ᶜ) |
6.95 ±0.48c (7.07± 0.67c ) |
6.45 ±0.76c (7.00 ± 0.46c ) |
6.28 ±1.13c (6.27± 0.55c ) |
11.0 ±0.96b (6.10± 0.20d) |
12.05 ±0.97b (5.27± 0.85d) |
12.73 ±0.97b (5.63 ±0.76d) |
11.82 ±1.83b (5.30 ± 0.70d) |
10.96 ±1.66b (6.37± 1.29d ) |
10.30 ±1.62b (6.30± 0.96c) |
11.15 ±1.13b (5.73± 1.11d) |
11.10 ±0.94b (5.80± 1.05d) |
|||
|
WBC (X10³ /µl) |
15.28 ±1.82a (15.0 ±1.63a) |
14.43 ±0.40a (14.80 ± 0.56a) |
14.40 ±0.37a (14.7 ± 0.40a) |
14.13 ±0.66a (14.93 ±1.01a) |
14.1 ±0.34a (15.57 ±0.87a) |
13.30 ±0.41b (16.90 ±0.62c) |
10.60 ±0.93b (15.80 ±1.11c) |
8.13 ±1.06b (17.00 ±0.76c) |
8.78 ±0.56b (15.70 ±0.56c) |
8.30 ±0.22b (15.63 ±0.35c) |
8.05 ±0.13b (16.47 ±0.70c) |
7.85 ±0.24b (15.33 ±0.81c) |
|||
|
EOS (x10³/µl) |
4.40 ±0.89a (4.67 ±1.53a) |
4.35 ±1.30ᵃ (4.00± 1.00c) |
4.25 ±1.50a (5.00± 1.00a) |
4.25 ±0.50a (5.00± 1.00a) |
2.25 ±0.50a (5.33± 0.58a) |
1.50 ±0.58a (5.00± 1.00a) |
1.25 ±0.50c (5.00 ±1.00a) |
1.00 ±0.82c (5.33± 1.16a) |
1.00 ±0.82c (5.33± 1.53a) |
0.00± 0.00c (6.00± 1.00a) |
0.00 ±0.00c (5.33± 0.58a) |
0.00 ±0.00c (4.67± 2.08a) |
|||
a, b, c, d Superscripts between groups in columns differed significantly (p<0.05); Parenthesis indicates control animals (not treated); EOS: Eosinophils, WBC: White blood cells, RBC: Red blood cell, HB: Haemoglobin, PCV: Packed cell volume.
Table 4: Efficacy of ivermectin on serum biochemistry on the goats naturally infested with mange
|
Pretre- atment |
1st Treatment |
2nd Treatment |
3rd Treatment |
4th Treatment |
|||||||||||
|
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
Day 11 |
Day 3 |
Day 7 |
|||||
|
T.P (g/ dL) |
3.44± 0.23b (3.53± 0.25b ) |
3.48± 0.29b (3.37± 0.61b ) |
3.63± 0.28b (3.73± 0.15b ) |
4.38± 0.40b (4.03± 0.42b ) |
5.23± 0.40 b (4.70± 0.44 b ) |
5.65± 0.57d (4.27± 0.45a ) |
6.75± 0.26 d (4.63 ± 0.67 a ) |
7.07± 0.14d (4.70± 0.56a) |
7.33± 0.09b (4.23± 0.31a) |
7.40± 0.08c (4.23± 0.25a ) |
7.40 ±0.0 8c (4.37 ± 0.25 a ) |
7.35 ±0.0 6c (4.17 ± 0.15 a ) |
|||
|
ALB (g/ dl) |
1.20± 0.07c (1.23± 0.16c ) |
1.20± 0.22c (1.03± 0.38c ) |
1.33± 0.25c (1.30± 0.10c ) |
1.88± 0.26c (1.60± 0.40c) |
2.30 ± 0.26 c (2.07 ± 0.21 c ) |
2.50± 0.18b (1.60± 0.10a ) |
2.55 ± 0.37 b (1.90 ± 0.46 a ) |
2.90± 0.22b (1.93± 0.31a) |
3.08± 0.09b (1.67± 0.40a ) |
3.15± 0.06b (1.77± 0.25a ) |
3.20 ±0.0 8b (1.93 ± 0.15 a ) |
3.15 ±0.0 6b (1.53 ± 0.21 a) |
|||
|
GLO (g/ dl) |
2.24± 0.23c (2.30± 0.17c ) |
2.28± 0.15c (2.33± 0.25c ) |
2.30± 0.08c (2.43± 0.06c) |
2.48± 0.33c (2.43± 0.16c ) |
2.93± 0.29 c (2.63 ± 0.35 c) |
3.15± 0.39c (2.67± 0.50c ) |
4.22 ± 0.14 a (2.73 ± 0.4 0b ) |
4.15± 0.19a (2.77± 0.25b ) |
4.25± 0.06a (2.57± 0.15b) |
4.25± 0.06a (2.47± 0.25b ) |
4.20 ±0.0 8a (2.43 ± 0.16 b) |
4.20 ±0.08 a (2.63 ± 0.25 b ) |
|||
|
K (mm ol/L) |
5.80± 0.27a (6.00 ±0.10a) |
5.82± 0.41a (6.03± 0.21a) |
5.89± 0.19a (5.93± 0.15a) |
5.72± 0.07a (5.83± 0.15a ) |
5.54 ± 0.18 a (5.97 ± 0.17 a ) |
5.41± 0.14a (5.98± 0.13a ) |
5.05 ± 0.19 a (5.90 ± 0.20 a ) |
4.88± 0.12a (24.30± 31.79a) |
4.69± 0.08a (6.20± 0.36a ) |
4.65± 0.13a (6.20± 0.20a ) |
4.68 ±0.0 9a (6.33 ± 0.40 a ) |
4.43 ±0.2 2a (6.17 ± 0.25 a ) |
|||
|
NA (mm ol/L) |
154.3± 2.31b (155.± 0.75b) |
152.5 ± 1.18b (153.8 ±1.63 b) |
153.4± 1.69b (152.7 ±0.92b ) |
151.6± 1.12b (151.7± 0.56b ) |
149.4 ± 1.00 b (152. 7 ±1.23 d) |
147.2± 0.90b (153.7 ±0.77d) |
144. 0± 1.77 a (152. 7± 1.21 d) |
144.5± 0.79a (152.4± 1.20d ) |
143.9± 1.42a (152.5± 2.11d) |
143.3± 1.59a (152.1± 0.70d) |
142.8 ±1.31 a (153. 2± 3.80 d ) |
140.4 ±1.9 5a (153 .2 ±2.2 5d) |
|||
|
CL ( mm ol/L) |
115.0± 1.87c (116.3 ±1.53c) |
114.0 ± 3.37c (113.0 ±1.00 c) |
112.5± 1.73c (112.3 ±0.58c ) |
111.3± 1.50c (112.7 ±0.58c) |
108.3 ± 1.71 c (111. 7 ±1.53 c) |
107.0± 1.41c (113.0 ±1.00c ) |
106.0 ± 1.63 c (113.0 ± 2.08 c ) |
102.0± 0.82a (112.7± 1.16b ) |
101.5± 1.29a (112.7± 2.52b ) |
101.3± 0.96a (112.7± 1.53b) |
101. 5± 1.0 0a (112. 0± 1.00 b) |
101. 5±1. 71a (112 .3 ±1.16 b) |
|||
a, b, c, d Superscripts between groups in columns differed significantly (p<0.05); Parenthesis indicates control animals (not treated); TP: Total protein, ALB: Albumin, GLO: Globulin, K: Potassium, NA: Sodium, CL: Chloride.
Effect of Ivermectin on Serum Biochemistry
There was no significant difference (p> 0.05) in the total protein level following first treatment (3.63±0.28) but a significant increase (p< 0.05) of 5.65±0.57 was observed at day 7 post second treatment and 7.40±0.08 at day 11 of third treatment from a pretreatment value of 3.44±0.23 as compared with the untreated group, Albumin value also increased at day 7 of second treatment (2.50 ±0.18) from a pretreatment value of 1.20±0.07 while globulin level also significantly increased (p< 0.05) from a pretreatment level of 2.24± 0.23 to 4.22± 0.14 at day 11 post second treatment as compared with control group in which neither albumin nor globulin level increased throughout the experiment. Potassium level of the ivermectin treated animals and that of the untreated (control) group did not change significantly (P> 0.05) throughout the experiment, while a significant decrease (P < 0.05) in sodium level at day 11 post second treatment (144.0±1.77) from a pretreatment level of 154.3±2.31 was observed and a significant decrease in chloride level (P < 0.05) following ivermectin administration at day 3 post third treatment (102.0±0.82) from a pretreatment level of 115.0±1.87 was also observed while the chloride level of the control group did not significantly show any reduction throughout the experiment (Table 4).
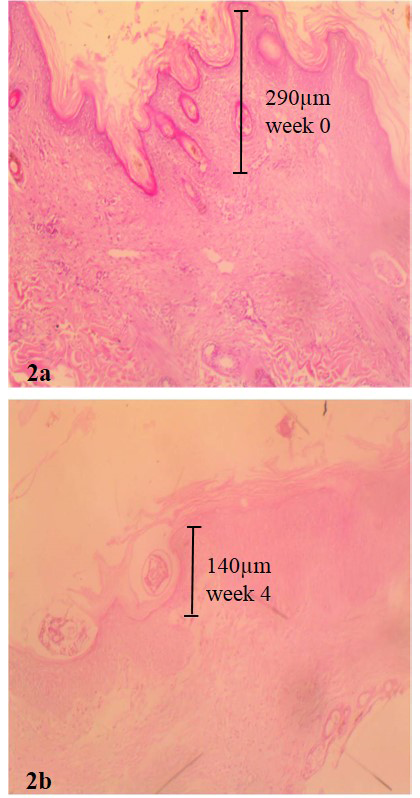
Figure 2: Pretreatment biopsy of goat skin
(a) showing massive excoriation with hyperkeratosis and epidermal tunnel height created by Sarcoptes scabie var caprae measuring 290microns Haematoxylin and eosin stain (b) showing mites in epidermis at week 4 of ivermectin treatment, epidermal tunnel height has reduced to 140microns haematoxylin and eosin stain
Effect of Ivermectin on Gross Lesion
From the improvement of lesions on skin, itching, scratching and biting ceased in the ivermectin treated goats on day 11 of first treatment. On day 3 of second treatment, breakage of crust at abdominal region was observed, skin coat thickening and alopecia has started resolving but mite wounds still present, while on day 3 of fourth treatment, Smooth and shinning skin coat with evidence of full hair growth on the body was noticed and mite lesions completely healed (Figure 3 and 4).
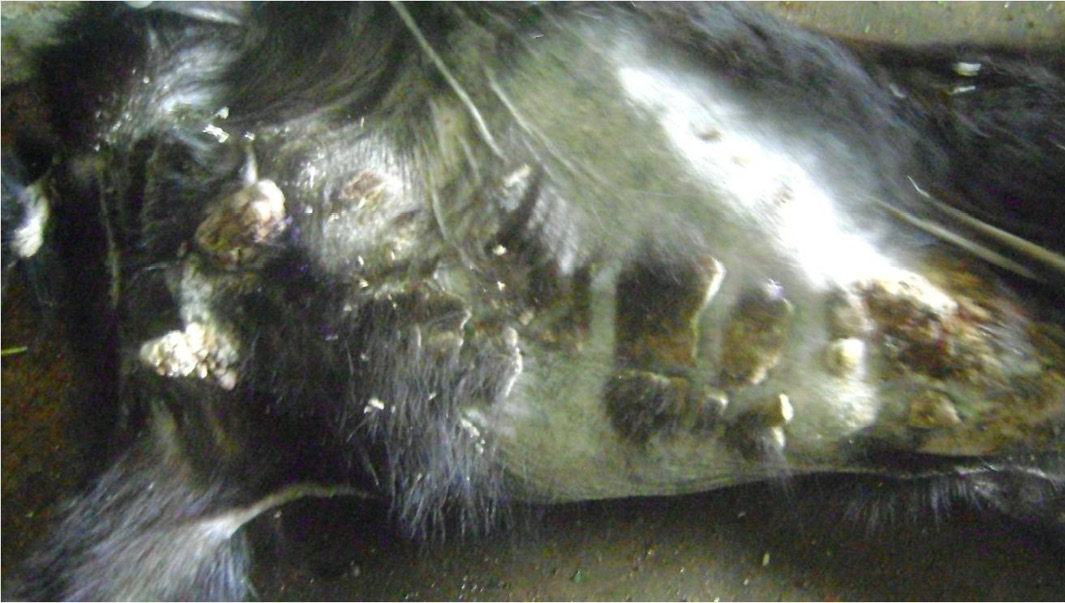
Figure 3: Breakage of heavy crust on the ventral abdomen and mammary gland region, skin thickening and wrinkling resolving after ivermectin treatment
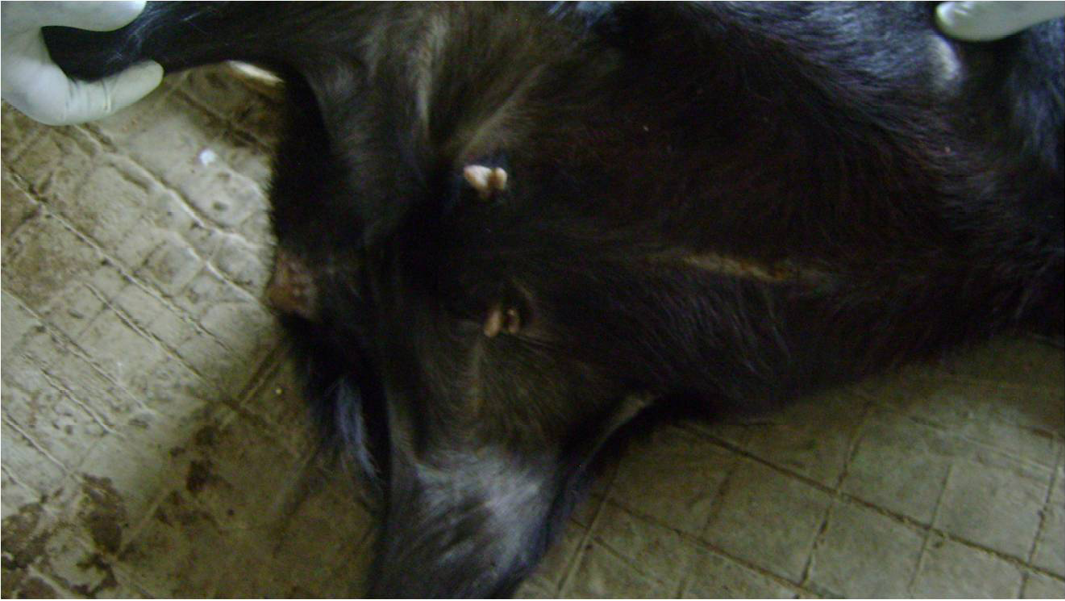
Figure 4: Day 3 of fourth treatment with ivermectin, smooth and shinning skin coat with evidence of full hair growth and mite lesions completely healed
DISCUSSION AND CONCLUSSION
Sarcoptes scabie was the only specie of mite found infesting all the goats used in this study, this is similar to the finding of Aikten (2007) who observed that sarcoptic mange caused by Sarcoptes scabiei var caprae is the most common cause of mange in goats and is found on sparsely haired parts of the body of the animals such as the face and ears.
Mite infestation caused by Sarcoptic scabie var caprae is a major constraint in goat production, which is highly debilitating and spreads rapidly within a herd by direct contact thereby causing severe damage to the skin by creating several epidermal burrows, leading to low productivity and death of animals (Morris et al., 1996; Radostits et al., 2007).
Systemic ivermectin treatment has been shown to be effective in the management of sarcoptic mange infestation in goats as these greatly reduce the intensity of parasitism in the infested animals (Manurung et al., 1990; Daramola et al., 2003).
The pretreatment investigation of this study revealed severe gross and histopathological lesions in the epidermis of the Sarcoptes scabie naturally infested goats, which lead to serious adverse aesthetic and physical effect on the skin quality of the animals as healing of these lesions was by scar formation which was visible on the skin, as this is in agreement with the observation of Gbolagade et al. (2009) and Nektarios et al. (2011).
The numerous epidermal tunnels with varying heights and diameter which was observed in the goats prior to commencement of treatment is possibly due to the burrowing ability of these mites into the skin as they feed on tissue fluids and continuously lay eggs in these tunnels which resultantly cause irritation and consequent scratching leading to inflammation and exudation that forms on the skin as this also was observed by Morris et al. (1996), Aikten (2007), Nektarios et al. (2011) and Falohun et al. (2015).
However, goats treated with ivermectin showed significant improvements in the reduction of adult mite and egg count, and in the appearance of skin coat post treatment, as there was marked reduction in the epidermal tunnel height and diameter after 4 weeks of treatment , as ivermectin has cleared all mite stages from the skin and gross lesions started resolving at day 11 of second treatment, this agrees with the work of Leon-Vizcaıno et al. (2001) in wild goat and Alayande et al. (2002) in West African dwarf goats who noted that there was marked decrease in the mean number of adult mites at 7 days post treatment with ivermectin in goats and at day 14 post treatment, almost all goats were cleared of infestation. This implies that ivermectin being systemic is fast in its mode of action and effectively rid the skin of the goats of infestation of mites and also neutralizing the histamine released by destroyed cells due to these burrowing mites thereby relieving the treated goats.
Furthermore, the marked reduction in hematological and biochemical parameters of all the infested goats which shows decrease Packed cell volume, erythrocyte count and hemoglobin level before the onset of treatment is indicative of anemia due to the blood consumption ability of mange mite and decrease cellular content in the blood after infestation (Sharma et al., 1990; Hafeez et al., 2007; Falohun et al., 2015) while the leukocytosis observed is likely due to excoriation of the skin of the animal which allows penetration of invading microorganisms which elicit the production of leucocytes in the goats and the decrease in serum protein, albumin and globulin level observed is probably due to the fact that sarcopte scabie continuously seep and suck fluid from mange infested animals as reported by Adejinmi et al. (2000).
However packed cell volume, red cell count and hemoglobin concentration increased while white blood cell level decreased in the treated animals, with these parameters falling within the range of values obtained for normal healthy WAD goats after recovery.
This implies that treatment with systemic ivermectin effectively restore the blood quality of the animals, as RBC with hemoglobin function to convey oxygen to body tissues which provide energy to the animals thereby allowing them to feed effectively and gain weight. The lower level of WBC observed in the treatment group as compared to the control depicts a reduction in mange burden. Higher immune response following recovery from mange infestation has been reported and were thought to be associated with massive release of antigen due to synchronous death of these mites (Akomas et al., 2011).
Although two consecutive negative skin scrapings was achieved in this study following second treatment with Ivermectin, treatment of infested goats was further extended to fourth regimen at two weeks interval to achieve complete recovery and prevent reoccurrence of infestation as systemic Ivermectin is safe to use and reaches its bioavailability unchanged with minimal toxicity.
AKNOWLEDGEMENT
We sincerely thank the management of Teaching and Research farm of the Faculty of Veterinary Medicine, University of Ibadan, Nigeria for giving us approval to make use of the small ruminant ward during the period of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCE





