The Journal of Advances in Parasitology
Research Article
The Journal of Advances in Parasitology 1 (2): 30 – 34Study of Gastro–Intestinal Parasites of Scavenging Chickens in Fako Division, Southwest Cameroon
Ndaleh Wozerou Nghonjuyi*, Helen Kuokuo Kimbi, Christian Keambou Tiambo
*Corresponding author: [email protected]
ARTICLE CITATION:
Nghonjuyi NW, Kimbi HK, Tiambo CK (2014). Study of gastro–intestinal parasites of scavenging chickens in fako division, southwest Cameroon. J. Adv. Parasitol. 1 (3): 30 – 34.
Received: 2014–04–22, Revised: 2014–05–14, Accepted: 2014–05–14
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jap/1.2.30.34)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
This research was an active surveillance of gastrointestinal parasites that parasitize rural birds in Fako Division, South West Cameroon. Eight hundred and eighty eight sterile samples of the faeces of 889 rural birds were collected randomly from 4 localities in Fako Division. These were immediately transported to the laboratory for analysis by the saturated salt/sugar solution floatation method. Viewing with x10 objective of the microscope it was discovered that out of the 889 samples examined for gastrointestinal parasites, 500 were infected with the following prevalence values: Eimeria species (301, 33.9%), Ascaridia galli (127, 14.3%), Heterakis gallinarium (87, 9.8%), Strongyloides species (76, 8.6%), Capillaria species (51, 5.7%) and Trichuris (51, 5.74%). The highest prevalence from the four locations was registered in Muyuka (220, 76.61%), followed by Tole (56, 61.54%) and the least was registered in Muea (282, 44.48%). It was concluded that there is a considerable level of coccidian and Ascaridia galli in Fako in rural birds, which could lead to high losses. We advice for workshops with these local farmers on the importance of keeping local birds, feeding, housing and how to improve on management of these fowls for optimum yield.
INTRODUCTION
Poultry industry occupies an important position in the provision of animal protein to man and generally plays a vital role in the national economy as a revenue provider. In most African countries, backyard poultry accounts for more than 60% of the total national poultry flocks accorded an asset value of more than 5.75 billion US$ (Sonaiya, 1998). It is estimated that these provide 12 kg of poultry needs per inhabitant per year whereas cattle provides 5.3 kg (Mephosa and Masika, 2010). Therefore, poultry meat is more available to the people than beef. Unfortunately, village chicken production is constrained by many extrinsic factors among which malnutrition, poor management, lack of supplement feed and the absence of biosecurity are outstanding. In backyard production system, the chickens are exposed to a higher risk of infection by a wide variety of parasites which range from lice, mites, fleas, ticks, and helminths to gnats and coccidia (Nnadi, 2009).
Parasitism causes a reduction in growth, egg production, emaciation and anaemia as well as mortality (Kaufman et al., 2007). Moreover, it has been reported that parasitic infections or their concurrent infections result in immunosuppression, especially in response to vaccines against some poultry diseases (Anne, 2006). Studies in some countries have shown that the prevalence of parasitic infestations in village chicken flocks is very high ranging from 50 to close to 100% (Irungu et al., 2004, Mungube et al., 2008, Nnadi and George, 2010), and in most cases individual birds habour more than one parasite type (Thrusfield, 2005).
Although helminthic infections do not cause significant morbidity and mortality they however, cause substantial effects such as malabsorption, diarrhoea, anaemia and other states of poor health, particularly in young birds (Ehrenberg and Ault, 2005 and Hotez et al., 2007). On the other hand, coccidiosis causes considerable economic loss in the poultry industry such as reduced growth rate, impaired feed conversion leading to poor performance and mortality especially in broiler chickens. Chickens are susceptible to at least 9 species of coccidia. The most common species are Eimeria tenella, which causes caecal coccidiosis, while E. acervulina and E. maxima cause chronic intestinal coccidiosis.
There is a paucity of information regarding the prevalence of these endoparasites in local chickens in Fako Division. Such knowledge is essential in understanding the epidemiology of the diseases as well as the design of their appropriate control measures. This work was therefore aimed at providing base–line information on the prevalence and distribution of major gastrointestinal parasites of scavenging chickens reared under traditional systems in selected localities of Fako Division, Southwest Cameroon.
MATERIALS AND METHODS
Study Area
The study was conducted in four selected localities (Muyuka, Muea, Tole and Tiko) of Fako Division, Southwest Cameroon based on the presence of local chickens and the willingness of the farmer to be included in the study. The Division is characterized by a long rainy season from March to November and the dry season spans for the rest of the year. The Division is a typical mountainous area with some lowland settlements.
Muea and Muyuka lie along the same stretch from Buea–Kumba highway, about 20km apart. Muyuka (410m above sea level) is situated at Latitude 4.3oN and Longitude 9.4oE with mean annual rainfall of about 3950mL. This town is about 22km from the town of Buea, the regional capital of the South West Region of Cameroon and has a mean temperature of about 27.5oC. Muea is situated at latitude 4.18oN, longitude 9.30oE just about 3km from the town of Buea. It has mean temperatures of about 25oC, rainfall of about 4000mL, humidity of 89% and lie about 534m above sea level.
Tiko lies along the Limbe–Douala highway with a relatively flat surface compared to all other three localities studied. It is situated at latitude 4.1oN; longitude 9.4oE, about 32m from sea level with average temperatures of about 26oC and a humidity of about 91%.
Tole is the coldest of all the localities and located up the mountain. It is situated at latitude 4.1oN and longitude 9.22oE with an average temperature of about 24oC. It is located about 950m from sea level, with rainfall of about 2850mm and a humidity of 92%. (http:// freemeteo. com/ default. asp?pid=15&la=1&gid=2225726).
Study Subjects
Door to door collection of faecal material were made and a total of 889 local fowls aged three weeks and above and of both sexes were sampled. The sample consisted of 303 birds from Muyuka, 91 from Tole, 213 from Tiko and 281 from Muea. For any bird to be included in the sample it had to have been reared in its area of study. These birds fed around their homes and neighbouring compounds where they interacted with other birds of their kind. The same criteria of selection of birds and sampling were done in all the four localities.
Method of Sampling and Analysis
Birds were collected from each locality based on the total number of local birds available and reared in the locality and the willingness of the farmers to donate samples. Hence, the number of birds sampled varied from one locality to another. All available birds from each household were randomly sampled without age or sex considerations. A total of 889 chickens were sampled from 167 homes in the four localities of Fako Division.
Sampling was done between the months of May and November, 2013. Prior to sampling, pre–survey visits were made to each locality and to the selected households for agreements on the sampling dates and to make sure that old faecal samples were swept and their house cleaned for new faecal samples. During our interaction with the village chicken keepers, verbal questions were asked to obtain information pertaining to mortality patterns among the various age groups and their method of housing. Some pictures were taken on the type of housing and the number of birds housed per square meter (Figures 1 and 2).
Faecal Collection and Analyses for Helminth Eggs and Coccidia Oocysts
The faecal samples were randomly collected into sterile sampling bottles (screw capped) from homes within the study areas and were immediately transported to the laboratory. They were analysed immediately using saturated simple salt/sugar floatation method. Unanalysed samples were stored in the refrigerator at 4oC for analyses the following day.
Each faecal sample was put into a test tube and the saturated salt solution poured into the test–tube, while stirring to obtain a uniform mixture. The solution was added to the test tube until the test tube almost overflowed with an upper meniscus, and then a cover slip was placed on top of the test tube with contents touching the slip. The mixture was allowed to stand for 15 –30 minutes in an erect position and the worm eggs were expected to float to the top. The coverslip was carefully removed by lifting it straight up and placed on another sterile slide with Lugol’s iodine. The slide was then examined under the x10 objective of an UNICO microscope (UNICO, USA).
The eggs and oocysts per gram (EPG and OPG respectively) of faeces were quantitatively analysed to determine the intensity of infection using the modified McMaster technique and the coccidian oocysts were quantified (MAFF, 1977).
Statistical Analysis
Data was entered into Ms Excel® 2007 (Microsoft corporation, USA) and analysed using the SPSS version 20 package. Prevalence was calculated as a percentage of n/N where n is the number of animals infected and N = Total number of animals examined. A non parametric test (Kruskal Wallis test) was used to compare mean of infection from the four localities with degree of freedom of 3. The association between the independent factors and the prevalence of the various parasites were evaluated using the Chi–square test (χ2). In all the analysis, confidence level was held at 95% and P ≤ 0.05 set for significance.
RRESULTS
General Prevalence of Gastrointestinal Parasites Infection within the four Studied Sites
Four sites were surveyed for gastrointestinal parasites of chickens and a total of 889 birds were examined. The sample consisted of 303 birds from Muyuka, 91 from Tole, 213 from Tiko and 281 from Muea. Out of the 889 birds examined, 500 were found positive with one or more parasites, giving a prevalence of 56.3%. Out of 889 chickens subjected to coprological examination 33.9% (301/889) were found to be infected by coccidia and 22.4% (199/889) by either one or more gastrointestinal nematodes. Ascaridia had a prevalence of 14.3% and Trichuris had the least (5.7%). The highest prevalence of infection (76.6%) was recorded in Muyuka while the least (44.5%) was recorded in Muea (Table 1). Overall, the highest prevalence was recorded for Eimeria (33.9%), followed in decreasing order by Ascaridia (14.3%), Heteraski (9.8%), Strongyloides (8.6%), Trichuris (5.7%) and Capillaria (5, 7%) as shown in Table 1.
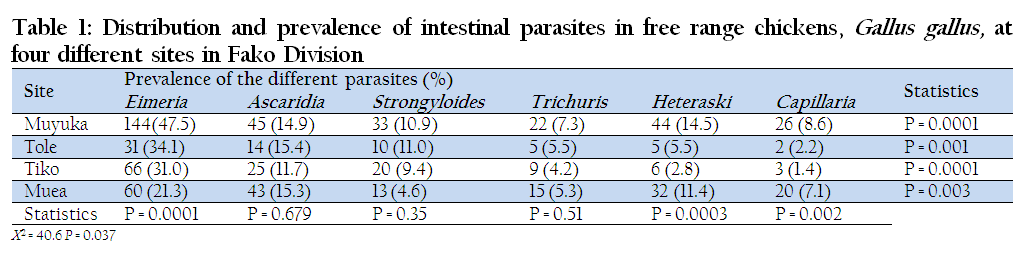
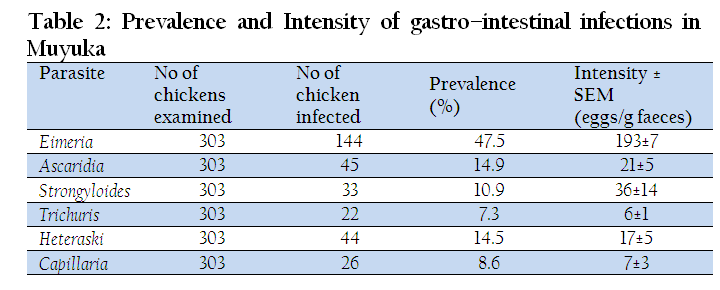
Table 1: Distribution and prevalence of intestinal parasites in free range chickens, Gallus gallus, at four different sites in Fako Division
Prevalence and Intensity of Infection of Chickens in Muyuka
A total of 303 local fowls were sampled in Muyuka and 144 of them were infected with Eimeria, giving a prevalence of 47.5%. Among helminthes, A. galli came first and second after Eimeria spp (14.9%) in terms of prevalence and the least infection was registered by Trichuris trichiura. Eimeria spp also had the highest intensity (193±7) while the least was registered by T. trichiura (6±1) as shown in Table 2.
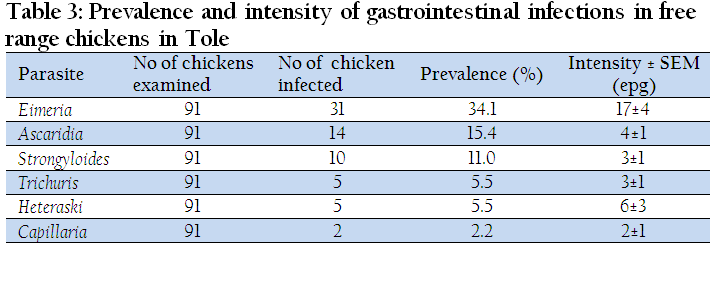
Prevalence and Intensity of Gastro–Intestinal Infections in Tole
The overall prevalence of gastrointestinal infections in Tole was 61.5%. Eimeria registered the highest prevalence of infection of 34.1% while Capillaria had the lowest prevalence of infection of 2.20%. The intensity of infection followed the same ranking as prevalence whereby Eimeria had a count of 17±4 opg and Capillaria had 2 ±1epg (Table 3).
Prevalence and Intensity of Infection in Tiko
The overall prevalence of gastro–intestinal infection in Tiko was 46.48%. The highest prevalence of infection (31.0%) was registered for Eimeria spp, while the lowest prevalence of infection (1.4%) was registered for Capillaria. The highest intensity of infection was registered for Ascaridia with a mean count of 51±30 epg while the lowest intensity was recorded for Strongyloides (4±1 epg) of stool (Table 4).
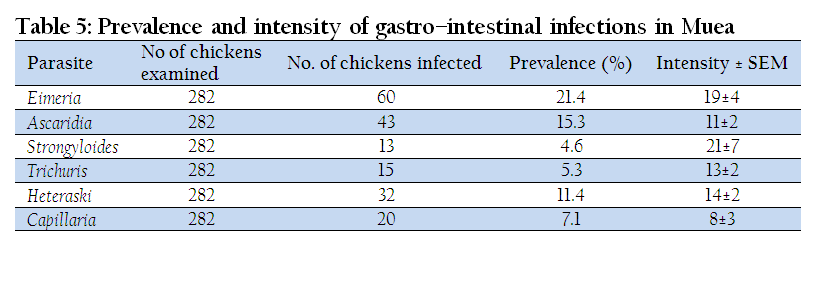
Prevalence and Intensity of Gastro–Intestinal Infection in Muea
Muea had an overall prevalence of 44.5% for gastro–intestinal parasites. The highest prevalence was registered by Eimeria (21.4%) while the least was observed in Strongyloides (4.6%). The highest intensity of 21±7epg was recorded in Strongyloides and the least mean egg count was registered in Capillaria (8±3 epg) (Table 5).
DISCUSSION
The results of this study showed a wide range of parasitic infections among village free range chickens in Fako Division, Southwest Cameroon. Overall, 56.3% of them were infected with one or more of the gastrointestinal parasites (helminth or protozoan). These results are in line with those reported by Kaufman et al., (2007) in the USA. The results are lower than those of Irungu et al., (2004), Heradine et al., (2012) and Sonune (2012) who registered prevalence values of 90.78%, 89.5% and 72 % in scavenging birds in Kenya, Morocco and India respectively. However, Nnadi and George (2010) and Vandanaa et al., (2012) registered prevalence values of 35.5% and 34.9% in South –Eastern Niger and Trinidad respectively.
The Highest prevalence of infection was registered in Muyuka (76.6%) and followed in decreasing order by Tole (61.5%), Tiko (46.5%) and Muea (44.5%). The difference between the prevalence of infection within the localities was statistically significant (p ≤ 0.037). The highest prevalence of gastrointestinal infection was in Muyuka, probably due to the flat nature of the area and the poor drainage systems. From personal observation, there were pools of stagnant water in potholes, gutters and around homes. The stagnant water and deposits from drainage usually carry oocysts and ova of coccidia and helminthes respectively. Water and moist environment also offer good conditions for the life cycle stages of these parasites. Water probably washed down faecal samples carrying life cycle stages and deposited them at some points in the area. Scavenging birds in their mode of feeding peck these life cycle stages along their feed. The appropriate internal conditions inside the birds, might have led to the development of the oocysts and ova of these parasites. The housing conditions for these birds might have contributed to the high prevalence of infection as many birds were housed in small cages in Muyuka. These clusters of birds ease parasitic transmission from one bird to the other (Bekali et al., 2009).
Eimeria species registered the highest prevalence of infection in all of the localities studied. In Muyuka, Eimeria species had a prevalence of 33.9% and this was seconded by Ascaridia galli with a prevalence of 14.30%. The difference between the prevalence of Eimeria parasites was statistically significant at p≤0.0001 in all the localities (Table 1). This might have been due to the fact that Eimeria species have very short life cycles and the cysts are more resistant than ova of nematodes. With these two characteristics, survival to the next host would have been better guaranteed and might have accounted for their high prevalence in all of the four localities. Similar studies were carried out by Bekali et al., (2009) in Dschang, West Province (region) of Cameroon where Eimeria registered a prevalence of 39.1%.
Tole came second in terms of prevalence of gastrointestinal parasites (61.5%). These results are lower than those reported by Offiong et al., (2013) in Akwa Ibom, Nigeria. This probably might have been because houses are built in clusters and rubbish thrown in some particular spots where birds cluster and feed together. In the course of feeding these birds (duck, turkey and wild birds) defaecate on this rubbish and fowls may pick up Ascaris eggs alongside their feeds and they develop in their gastrointestinal tract. Also, one of the probable reasons for the high infection in Tole might have been due to its location around the mountain top where climatic conditions are relatively cold. Under such conditions, oocysts and ova can remain quiescent until the next or appropriate host is met without getting decayed (Offiong et al, 2013). Intensities of infection followed almost the same trend as prevalence except in Muea.
In Muyuka, Tole, Tiko and Muea, Eimeria had the following prevalence values; 47.5%, 34.1%, 31.0% and 21.3% respectively. The difference in the distribution of this parasite was statistically significant at p≤0.0001 (Table 1). The result is similar to the range reported by Diriba et al., (2012) for Eimeriosis (20.57%) in Ambo, Western Ethiopia. Moreover, these results agree with that of Sharma et al., (2013) who had a prevalence of 53.61% for five species of coccidia (Eimeria tenella, Eimeria necatrix, Eimeria maxima, Eimeria acervulina and Eimeria mitis) in the Jammu Region of India.
In this present study, the prevalence of eimeriosis in backyard chickens is high probably due to poor managemental practices, malnutrition and non–use of coccidiostats as preventive measures. Also, many of these farmers lack proper knowledge on fowl keeping and do that just as a tradition from their foreparents. The success of Eimeria is often linked to the resistant nature of the oocyst walls and their ability to multiply faster and develop into the next stage of its life cycle (shorter life cycle compared to many helminths) (Anna, 2006).
Ascaridia galli had the second highest infection after Eimeria in terms of prevalence where Tole, Muea, Muyuka and Tiko registered 15.4%, 15.3%, 14.9% and 11.7% respectively. The difference in the results was not statistically significant across the four study sites (P≥0.679). This is similar to results obtained by Ogbaje and Ajanusi (2012) in Makurdi Township where Ascaridia showed a prevalence of 27.7%. The highest prevalence of Ascaridia galli observed in Tole, when compared to the other locations could probably be due to the location of Tole on the mountain top and its relatively cold weather that could help to preserve the eggs of this worm from desiccation. Ascaridia is also known to have a thick outer membrane that is resistant to desiccation and thereby promoting its transmission by giving it a higher chance of meeting the next host while still viable for survival (Michael et al., 2005). Ascaridia eggs are also very sensitive to their hosts environment and will quickly develop once the required conditions for growth are met and that has also resulted to high survival and distribution of Ascaridia within the population. Moreover, Ascaridia has many hosts where they can develop and proliferate such as in humans and other domestic animals like goat and sheep; zoonotic and so can be transmitted between many animals feeding together (Michael et al., 2005).
Muyuka, Tole, Tiko and Muea had respective prevalence of Strongyloides avium of 10.9%, 11.0%, 9.4% and 4.6%. The least prevalence of infection of all was registered by Capillaria plica in Tiko which had a prevalence of 1.4%. This may be due to the fact that Tiko is very hot and humid and many of the ova are destroyed before they have the chance of reaching their host.
Generally, young chickens are susceptible to these gastrointestinal parasites than older fowls because clinical signs are often witnessed with a low intensity of infection. Research has shown that older fowls can harbour high burdens of parasites without clinical signs, but act as reservoir of these parasites to the young ones as they feed together (Dahl et al., 2002).
CONCLUSION
The study clearly indicates that coccidiosis and ascaridiasis are highly prevalent in all the four studied sites in Fako Division and that these local fowls harbour very high number of these parasites with little clinical signs or symptoms. They may be termed reservoir hosts and can be detrimental to any farmer who intends opening a broiler farm because broilers are not as resistant to these parasites as local fowls. So, transmission by flies to broiler farms may lead to high economic losses to broiler farmers.
It should also be noted that though these fowls don’t show clinical signs, their sizes and reproduction rates are markedly reduced.
ACKNOWLEDGEMENTS
This work was supported by funds from the EMBRAPA–Brazil, provided for the execution of the ID 207 Africa Brazil Agricultural Innovation Marketplace project. The authors are also grateful to the Chiefs, Quarter heads and farmers of Muyuka, Tole, Muea and Tiko for their cooperation during this survey.
REFERENCES
Anna F (2006). Parasite Management for Natural and Organic Poultry: Coccidiosis. 1–800–346–9140. www.attra.ncat.org.
Bekali J, Tume C, Achenjang F, Zoli P (2009). Disease pressure on the chickens. We cannot ignore the impact on livestock production and productivity.
https://gustavus.edu/chemistry/seminar/Aschenjang.abstract.pdf.
Dahl C, Permin A, Christensen JP, Bisgaard M, Muhaiwa AP, Petersen KMD, Poulsen JSP, Jensen AL (2002). The effect of Concurrent Infection with Pasteurella multocida and A. galli in Free Range Chicken. Journal of Veterinary Microbiology. 89: pp313–324.
http://dx.doi.org/10.1016/S0378-1135(02)00015-9
Ehrenberg JP, Ault SK (2005). Neglected diseases of neglected populations: Thinking to reshape the determinants of health in Latin America and the Caribbean. Biomedical Central Public Health 5:119.
http://dx.doi.org/10.1186/1471-2458-5-119
PMid:16283932 PMCid:PMC1318484
Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L (2007). Control of neglected tropical diseases. New English Journal of Medicine. 357:1018–1027.
http://dx.doi.org/10.1371/journal.pntd.0000118
http://dx.doi.org/10.1371/journal.pntd.0000077
http://dx.doi.org/10.1371/journal.pntd.0000149
PMid:18160982 PMCid:PMC2154393
Irungu LW, Kimani RN, Kisia SM (2004). "Helminth parasites in the intestinal tract of indigenous poultry in parts of Kenya," Journal of the South African Veterinary Association. 75(1):58–59.
http://dx.doi.org/10.4102/jsava.v75i1.452
PMid:15214699
Kaufman PE, Koehler PG, Butler JF (2007). External Parasites of Poultry, University of Florida institute of Food And Agricultural Sciences, Gainesville, Fla, USA.
Maphosa V, Masika PJ (2010). Ethnoveterinary uses of medicinal plants: a survey of plants used in the ethnoveterinary control of gastro–intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharmaceutical Biology. 48(6):697–702.
http://dx.doi.org/10.3109/13880200903260879
PMid:20645744
Mungube EO, Bauni SM, Tenhagen BA, Wamae LW, Nzioka SM, Muhammed L, Nginyi JM (2008). Prevalence of parasites of the local scavenging chicken in a selected semi–arid zone of Eastern Kenya. Tropical Animal Health and Production. 40: 101–109.
http://dx.doi.org/10.1007/s11250-007-9068-3
PMid:18422252
Nnadi O (2009). "Patterns of disease outbreak among village chicken population in Imilike Community. (Personal Communication).
Nnadi PA, George SO (2010). A Cross–Sectional Survey on Parasites of Chickens in Selected Villages in the Subhumid Zones of South–Eastern Nigeria. Journal of Parasitology Research. 10: 1418–1426.
Offiong EEA, Obioku OE, Umoh JU, Essien CA, Idiong NB (2013). A survey of Gastrointestinal Helminthes of local chickens in Abak Local Government Area of Akwa I Ibom State. International Journal of Science: Basic and Applied Research 9:pp1–4.
Ogbaje EO, Ajanusi OJ (2012). Prevalence of Ascaridia galli, Heterakis gallinarum and Tapeworm Infections in Birds Slaughtered in Makurdi Township. International Journal of Poultry Science. 11(2): 103–107.
http://dx.doi.org/10.3923/ijps.2012.103.107
Sonaiya EB (1998). Towards Sustainable Poultry Production in Africa, FAO Expert Consultation on strategies for sustainable animal agriculture in developing countries, Rome, Italy.
Sonune MB (2012). Analysis of Gastrointestinal Parasites of Poultry Birds Around Chikli, Buldana MS India. Science Research Reporter. 2(3): 274–276.
Thrusfield M (2005). Veterinary Epidemiology. 2nd Edn., Blackwell Science Ltd., London, UK., P: 479.
Vandanaa B, Vijaya S, Lana G, Gabriel B, Nkechi VO, Abiodun AA, Asoke KB (2012). The Prevalence of Intestinal Helminths in Broiler Chickens in Trinidad. Veterinary archive. 82(6):591–591.