The Journal of Advances in Parasitology
Research Article
The Journal of Advances in Parasitology. 1 (2): 12 – 20Human Urogenital Myiasis Caused by Psychoda Species Larvae: Report of Five Cases and Morphological Studies
Doaa A Yones1*, Hanaa Y Bakir1, Diaa A Hameed2,
- Departments of Medical Parasitology,Faculty of Medicine, Assiut University, Egypt
- Urology, Faculty of Medicine, Assiut University, Egypt
*Corresponding author: [email protected]
ARTICLE CITATION:
Yones DA, Bakir HYand Hameed DA (2014). Human urogenital Myiasis caused by psychoda species larvae: report of five cases and morphological studies. J. Adv. Parasitol. 1 (2): 12 – 20.
Received: 2014–02–27, Revised: 2014–03–02, Accepted: 2014–03–03
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.jap/2014/1.2.12.20)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Myiasis is the infestation of body tissues or organs by dipterous fly species and is often associated with poor hygiene. Although infestation by fly larvae is much more prevalent in animals, it occurs relatively frequent in humans in rural, tropical and subtropical regions of Africa and America. Urogenital myiasis is one of accidental myiasis that may be seen in humans. Urogenital myiasis is commonly associated with poor personal and environmental hygiene, low educational level and urogenital troubles. The current study presented five cases of urogenital myiasis. Patients were residing in Assuit and Qena Governorates (Upper Egypt). Some patients complained of intermittent passage of small, motile, greyish black wormiform objects in their urine and some were discovered accidentally. Larvae were collected and studied microscopically and detailed structures were described us¬ing scanning electron microscope (SEM). The larvae were morphologically identified as Psychoda spp. larvae. Special attention was given to cephalic region, vestiture, setae distribution and caudal extremity. It is worth mentioning that the sensillary necklace–like structure at the junction of the head with the first thoracic segment and the hollow appearance of setae were also clarified. It was concluded that despite the fact that urinary myiasis is very rare in humans; it should be considered in patients with urinary complaints.
INTRODUCTION
Myiasis is an infestation of human and vertebrate tissues by larvae (maggots) of more than 50 fly species (myia is Greek for fly) within the arthropod order Diptera. It was discovered by Hope in 1940 (Burgess, 2003; Musa and Wegi–Allah, 2008). Myiasis is encountered worldwide with more cases being reported from tropical, subtropical and warm temperate areas such as in Latin America, Africa, Middle East and Asia (Johnston and Dickinson, 1996). It is usually associated with poor hygienic conditions, low educational level, and is more common in children (Hall and Wall, 1995)
There are two forms of myiasis: obligate, in which the maggots feed themselves on living tissues, and facultative, where the maggots opportunistically take advantage of wounds or degenerative necrotic conditions to incubate their larvae (Burgess, 2003). Most common type of myiasis is mucocutaneous type while body cavity myiasis including urinary myiasis is less common. Accidental myiasis occurs when fly larvae occasionally find their way into the human body. Usually eggs of flies are ingested on contaminated food or come in contact with the genitourinary tract. Accidental myiasis is a self–limited infection (Zumpt, 1965; Markel et al., 1992). Urogenital myiasis is one of the rare myiasis. It occurs in sites usually protected by clothes and inaccessible for the flies. It is usually associated with poor general health and hygiene, restricted mobility, urinary obstruction and ulcerating lesions (Hyun et al., 2004). Commonly implicated dipterous families in urogenital myiasis include: Psychodidae, Muscidae, Sarcophagidae, Calliphoridae, Anisopodidae and Scinoinidae (Lane and Crosskey, 1993).
Psychoda spp. are classified under Phylum Arthropoda, Class Insecta, Order Diptera, Suborder Nematocera, Family Psychodidae. About 250 Psychoda species are described; the commonest are P. alternata and P. albipennis. Psychoda fly is commonly named drain, sewer or moth flies. They are small (less than 5mm), dark, fuzzy, moth–like insects. The body is hairy with wings held roof–like over body. Species identification is mainly by the number and shape of antennal segments carried by head of adult fly (Durden, 2002). Cases of urogenital myiasis presented with haematuria, bladder irritative symptoms, pruritus, dysuria, fever and passing larvae in urine. Severity of myiasis depends on the location of the infestation, lesions and tissue inflammation (Hyun et al., 2004). Accurate identification of fly larvae which rarely cause myiasis is sometimes difficult and requires thorough study of unfamiliar morphological details of the larvae.
Treatment of some cases of myiasis of the body cavity is sometimes difficult and frequently resistant to treatment (Wadhwa et al., 2006). Until recently, the traditional treatment of myiasis of the body cavity is mechanical removal (Gopalakrishnan et al., 2008). Surgical intervention is sometime needed, which is painful, costly and sometimes made impossible in smaller cavities (Wadhwa et al., 2006) The aim of the present study was to report five cases with urogenital myiasis caused by larvae of a dipterous fly of the genus Psychoda. We used ordinary microscopy for identification of the larvae; in addition, scanning electron microscopy (SEM) of the maggots was carried out as a supportive measure to illustrate some important ultrastructures that may constitute useful criteria in the larval identification and species differentiation.
PATIENTS AND METHODS
Clinical Data: Table (1)
Five cases of urogenital myiasis were referred from Urology Department, Assiut University Hospital to Parasitology Department, Faculty of Medicine, Assiut University for identification and proper diagnosis from February 2009 to June 2013.
History taking, clinical examination, and laboratory investigations in the form of complete urine examination, blood picture, stool examination using direct smear and formol ether concentration technique were conducted. Imaging and interventional investigations including pelvic ultrasonography, x–ray, contrast radiography and CT scanning of the urinary system, cystoscopy and bladder wash were also done.
Medical treatment in the form of antihelmenthic (two oral doses of ivermectin 200 ug/Kg to the adult patients and 100 ug/Kg for the child patient) and antibiotic treatment were prescribed. The patients were followed up two months later.
The data collection was conducted after written consent from the patients, and permission from the ethics committee of the Faculty of Medicine, Assiut University. The investigation was carried out in accordance with the declaration adopted by the 18th WMA (World Medical Association) General Assembly, Helsinki 1964, and as revised by 64th WMA General Assembly in Fortaleza, Brazil, October 2013.
Precautions took place to protect the privacy of research subjects and the confidentially of their personal information, including limiting the amount of personal information to the absolute minimum, assigning an identification number to each subject and attaching the identification number to the actual research information, removing the subject names as soon as data were analyzed and maintaining any identifying information and lists of identification numbers in a safe and locked file.
Collection and processing of larvae for entomologic study
Collected larvae were washed several times in saline. Larvae were fixed in 70% ethyl alcohol for examination and identification.
Preserved larvae were brought down to water in descending grades of alcohol 50 % and 30%, 5 minutes each. They were transferred to 10 % potassium hydroxide, after puncturing the specimens on the ventral side, overnight until soft parts were dissolved. The specimens were washed thoroughly in distilled water, dehydrated in ascending grades of ethyl alcohol 5 minutes each, cleared in clove oil for 10 minutes, mounted in Canada balsam and dried in an oven at 38°C for five days (Adams and Hall 2003).
For SEM, preserved larvae were washed thoroughly in distilled water and fixed for 2 hr in 4% glutaraldehyde and 5% paraformaldehyde in 0.1 M cacodylate buffer at pH 7.2. They were rinsed overnight in cacodylate buffer, dehydrated in graded aqueous ethanol (30%, 50%, 70%, 90%) followed by critical–point drying according to Hayat (1981), sputter–coating them with gold in the sputter coating apparatus for 6 minutes. Specimens were processed, examined and photographed in the Scanning Electron Microscope Unit, Assiut University by JEOL–JSM–5400 LV.
Following taxonomic identification keys of Zumpt (1965), Greenberg (1971), Foote (1991), the description of Rutledge and Gupta (2002) and based on biogeography the wormlike organisms were identified as larvae belonging to genus Psychoda, subfamily Psychodinae (Nematocera: Psychodidae). The mounted larvae were illustrated by photomicrographs. The measurements were considered from the average measurements of five larvae to the nearest millimeters.
RRESULTS
Four patients were adult males (age range 18–68), the 2nd case was a young girl aged 9 years. All the patients were from Assiut Governorate except for third case was from Qena Governorate. All cases were outpatients except for the fifth case that was hospitalized.
Three of the patients complained of intermittent passage of 6 to 10 living worms in every urine void. They described the worms as being small, grayish black in color, motile and slender. In the second and fifth cases, worms were discovered during routine urine examination at clinical pathology laboratory.
The 3 patients who complained of passing worms had no history of abdominal pain; bowel irregularity or any other symptoms related to either gastrointestinal or genito–urinary system; while the second and fifth cases had intermittent abdominal pain and diarrhea. General and systematic examination revealed no findings except mild pallor and non–tender hepatomegaly of 2 cm size in fifth case.
The results of the laboratory tests are shown in table (1). The imaging and cystoscopy were within normal in all patients.
The larvae were dark brown to black in colour when freshly passed except the well–developed head, dorsal plates, and the last abdominal segment at the end of larva which were darker. Larvae were immotile when presented to the laboratory, measured from 8 – 12 mm in length. They had rounded and ventrally curved anterior end and tapered dorsally curved posterior end (Plate I; Figure 1).
Light microscopic findings are illustrated in plate I:
The body of the larvae was more or less cylindrical and segmented. The body length ranged from 8 to 12 mm and its width ranged from 0.25 to 0.75 mm (Plate I, 1). Each larva possessed a distinct triangular head, which carried two short hairy antennae and a double–toothed mandible. Mandibles were in a horizontal plane and opposed, so that their lips can be brought together (Plate I, 2). The body of the larvae consisted of 10 segments (3 thoracic and 7 abdominal). Hypopigmented saddle shaped chitinous sclerites were present on the dorso–lateral sides of each body segment, giving the larvae their banded appearance (secondary annulation). These chitinous sclerites varied in width. They were covered with long filiform setae arising from button–like basal plates on each sclerite (Plate I, 3). Body segments were covered with irregular rows of numerous minute sharp and rose thorn shaped spinules (Plate I, 3). Long filiform brown setae were irregularly distributed laterally and arising from nearly circular chitinous button–like basal plates (Plate I, 3). Two breathing tubes were seen extending along the length of the body starting from a pair of anterior spiracles at the second subdivision of the prothorax and ending at the tip of terminal segment by a pair of posterior spiracles (Plate I, 4). The caudal end of the body was obliquely truncated while the last abdominal segment was cylindrical chitinous pigmented and separated from the remaining segments by a sharp edge (Plate I, 5). It was prolonged and tapering into two distinct respiratory tubules bearing the posterior spiracles apically (Plate I, 5). The posterior spiracles were guarded by dorsal and ventral brushes of hair tufts on each tubular projection. The anal papilla was large spinose and located ventrally (Plate I, 6).
SEM findings are illustrated in plates II – V
In general, the body of the larvae was elongated, segmented with secondary annulations and slightly tapered at one end. The vestiture of thoracic and abdominal segments was scraggy coat of pointed hair–like elements; long and numerous on the dorsal surface and partially devoid from the ventral surface (plate II, 1). Randomly orientated long setae covered the whole body segments (plate II, 1). Dorso–lateral view of the head and thoracic region (Plate II, 2) revealed smooth, shiny egg– shaped head with few scattered medium sized setae. Head capsule slightly bent ventrally and the mouth filled with bunch of hair.
Antero–posterior view of the cephalic region (Plate II, 3) appeared like an elephant face illustrating central projection bent ventrally with two lateral openings filled with filament like network. Two lateral small ear like structures were located lateral to both openings. A sensillary hemispherical necklace–like structure situated at the junction of the head with the first thoracic segment reaching only to the mid–lateral cephalic region (Plate II, 3).
A pair of anterior spiracles was situated laterally at the anterior edge of the second thoracic segment. Although spiracles were small, they were placed at the top of a globular bulge (Plate II, 3). Magnification of this part (Plate II, 4), the anterior spiracles appeared twice as long as wide and had a single pore in the apex with a skirt of very fine hairs at the base.
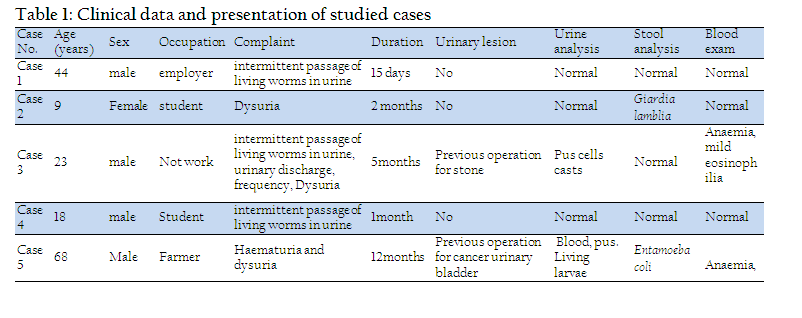
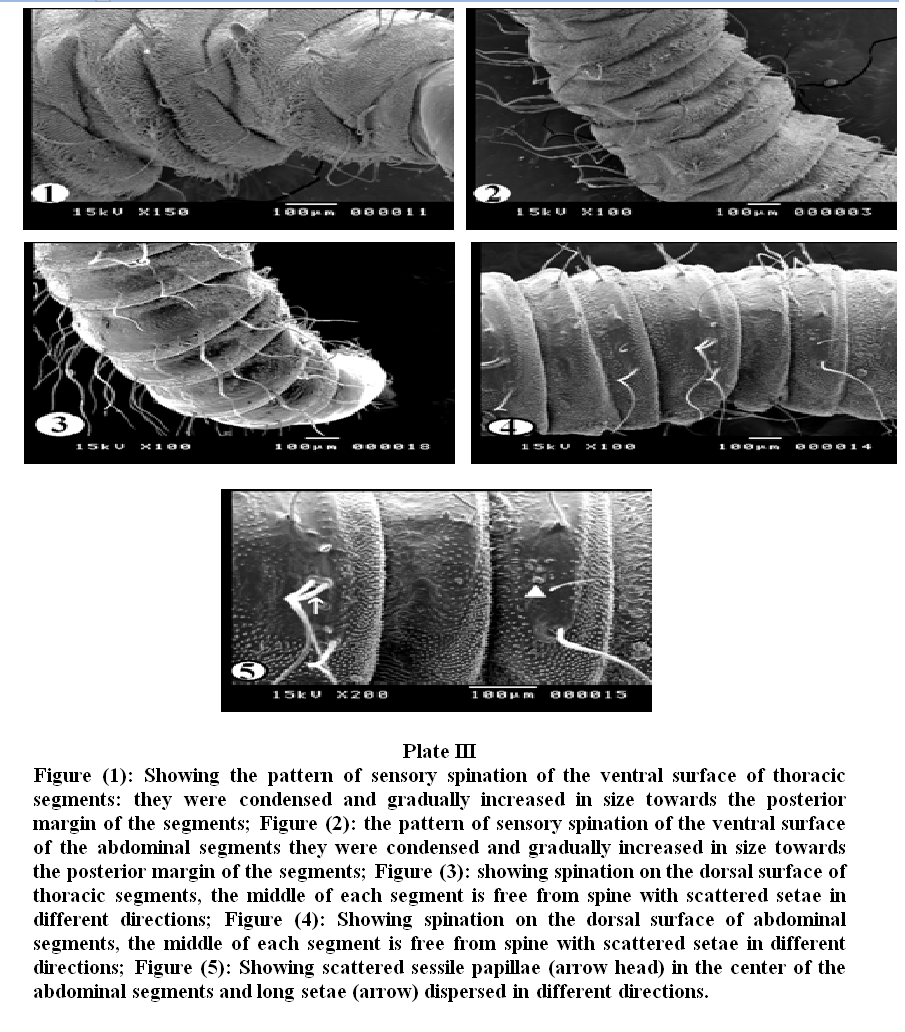
Plate III, 1 & 2 illustrated the pattern of sensory spination of the ventral surface of thoracic and abdominal segments; they were condensed and gradually increased in size towards the posterior margin of the segments. Plate III, 3 & 4 showed spination on the dorsal surface of thoracic and abdominal segments, the middle of each segment is free from spines. The dorsal surface (Plate III, 5) revealed scattered sessile papillae in the center of the abdominal segments. Long setae covered the whole segments and were dispersed in different directions. Under higher magnification (Plate IV, 1) the detailed characteristics of the spines on the ventral surface of the abdominal and thoracic segments showed irregular rows of minute sharp rose thorn shaped backwardly curved spinules at the anterior margin gradually increase in size to become long spines with sharp end towards the posterior margin of the segments. The long spines were almost covering the inter–segmental sutures. Dorsal spinations were nearly similar to those seen on the ventral surface but became more dispersed with a poor development of spines at the middle of the segments (Plate IV, 2). Plate IV, 3 illustrated that setae were long, slender and easily overlooked in the vestiture. The distal end of the setae appeared either curly or somewhat like a rat's tail. On magnification (Plate IV, 4 & 5) they were individually arising from ring like bases with raised edges (button like basal plates). The setae appeared like hollow tubes with central opening at their ends. They seemed to have a shroud of very fine filamentous hair skirting them.
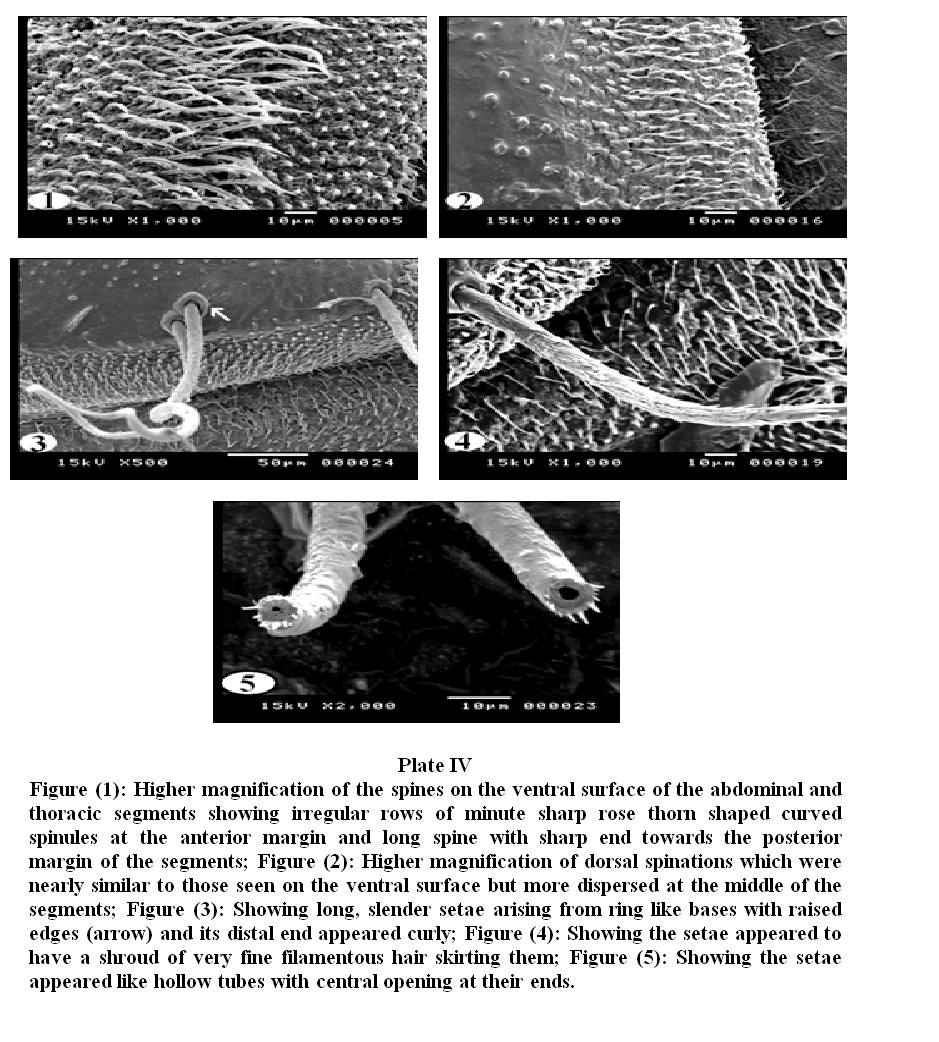
Plate V, 1 & 2 showed general appearance of the dorsal and ventral surface of the caudal extremity, the respiratory siphon looks like a cone with moderately broad base narrowing rapidly into slender tapering distal portion, few scattered setae covering its smooth surface.
On magnification (Plate V, 3), the posterior spiracles appeared at the end of tapering tubular siphon, with their opening between the dorsal two pairs and ventral one pair of processes. The ventral pair of terminal processes is slightly larger than the dorsal pair. The distal tip of the terminal processes revealed a fan of radiating scales. Scales were relatively long with broad base and much tapered sides. The anal papilla was large with central bulge and a fan of radiating scales (Plate V, 4).
The patients were administered two oral doses of ivermectin as suggested by the clinician. This resulted in gradual reduction in number and frequency of passing of larvae in urine till complete disappearance of the larvae within two weeks after treatment. Three patients were followed up two months later without recurrence; while the other two patients were lost for follow–up.
DISCUSSION
Family Psychodidae includes six subfamilies. Only two of them have public health significance. Phlebotominae (sand flies); are blood suckers and their larvae inhabit places where there is high organic matter; they are best known as vectors of leishmaniasis (Wagner, 1990; 1997). Psychodinae (moth flies); their mouthparts are short and not adapted for blood sucking. They breed in moist places and are often found around sewerage installations, hence are called "filter flies" or they may originate in drain pipes and emerge from sink and bath tub drains. Myiasis is endemic throughout the tropics and subtropics. It occurs more readily in warm and humid environments. In the tropics, cases present the year round, but in more temperate zones, myiasis is generally restricted to the summer months (Noutsis and Millikan, 1994). It is to be noted that in our patients, the presentation took place in summer. In our report, the cases were residing in rural areas and had low standards of living, which is the usual standard of living for urogenital myiasis patients as previously described (Markel et al., 1992). It is possible that some eggs may have been laid in or around the moist urogenital region when the patients were urinating; and after hatching, the larvae burrow in to the urogenital tract to feed giving rise to urogenital myiasis. This explanation was previously suggested by Salimi et al. (2010) and Lotfy (2011).
In a literature search by Oğuz et al. (2012), there were less than 30 case reports of urogenital myiasis. In general, it is uncommon to encounter dipterous fly larvae in human urine in Egypt. To the best of our knowledge, three cases of urinary myiasis with Psychoda spp. infestation were reported from Egypt (Sakla et al., 2003; Ezzat and Younis, 2009; Lotfy, 2011). More scattered cases of urogenital myiasis by larvae of the same genus have been reported before including; Kuwait (Hira et al., 1997), Turkey (Dinçer et al., 1995; Taylan–Ozkan et al., 2004), UK (Patton and Evans, 1929), China (Wang and Liu, 2006; Zhou et al., 2008), Argentina (Mariluis et al., 2007) and Spain (Fuentes et al., 2011). Moreover, Psychoda spp. was reported to cause bronchial myiasis in Japan (Hiroshi et al., 1963).
Urogenital myiasis by larvae of other species of flies have been reported in the world including the United States of America by Dermatobia hominis (Massey and Rodriguez,2002), India by Chryzomya bezziana (Wadhwa et al., 2006), Slovakia by Lucilia sericata (Nagy, 2012), Iran by Chryzomya bezziana (Jdalayer et al., 1978) and by Lucilia sericata and Wohlfahrtia magnifica; (Salimi et al., 2010) in Spain by Eristalis tenax (Gonzalez et al., 2009), in Nigeria by Eristalis species (Utsalo and Khalifa, 1985) and in Saudi Arabia by Megaselia scalaris (Wakid, 2008).
Identification of the species of maggots prior to treatment is important since not all types of myiasis are benign (Singh and Rana, 1989). The present work focused on an ordinary microscopy for identifi¬cation of the larvae. However, SEM of the maggots was carried out as a supportive measure to illustrate some important features that may constitute useful criteria in the larval identification and species differentiation. SEM has enabled us to visualize the ultrastructure of different parts of the maggots such as the anterior end, the respiratory spiracles, the pattern and density of sensory spination on ventral and dorsal surface of body segments and the setael distribution.
As reported by previous authors Whitten (1955) and Abonnenc (1972), the larvae of all Psychodidae are amphipneustic, having a pair of thoracic and abdominal spiracles. The oligopneustic (which comprises the amphipneustic type) and apneustic larvae, with 0– 4 pairs of functional spiracles may be used for subsequent adaptation to a subemerged life in a fluid or semifluid medium (Fausto et al., 1998). Among Psychodidae, the aquatic larvae of Psychodinae have the post–abdominal spiracles opening at the end of a respiratory siphon as an adaptation to the aquatic mode of life in a good agreement with our description. In the current study, the anterior spiracles emerge on top of globular structures and project from the surface of the larval body. This localization probably favors the contact between the spiracles and the air.
The pattern of setae distribution is used as the main character that distinguishes the larvae of the subfamily Phlebotominae from other subfamilies of Psychodidae (Sther, 1991). According to Killick–Kendrick et al. (1989) the function of these setae is obscure. The distinctive back–and forth movement of the long setae of larvae and their fine hair suggest the presence of sensillae. In another study, Pessoa et al. (2001) proposed that the setae of all species examined bear several pores scattered along their structure, further suggesting that they may have a chemosensitive function. This suggestion is probably confirmed by the hollow structure of the setae in our description.
In this work much attention was given to the spination through which we could use them in differentiation. Spine structure of larval segment should be taken into account to determine specific patterns since the shape, density and orientation of spines varies along the length of body and also between dorsal and ventral surfaces (Colwell and O’Connor, 2000). In the current work, the spinules appeared different in size and shape, almost all of them having single points, some with sharp pointed and curved tips and others with blunt terminations. The differences in spine pattern and size together with non–sucking mouthparts could explain the variability of the clinical manifestation of this parasitism (i.e. some cases presented with urogenital problems, others only passed larvae).
However, given the difficulty in identifying the larvae at specific level, they were reported in the present study as Psychoda sp., although only P. albipennis has been related with urogenital myiasis (Oğuz et al., 2012). Accurate identification of fly larvae which rarely cause myiasis is sometimes difficult and requires thorough study of unfamiliar morphological details of the larvae. The detected larvae in the present work were mostly forth stage larvae because of their colour, size and the morphological characters of the respiratory system. Another more conservative explanation is that mature fly larvae leave the human body when they are ready to pupate; this is in agreement with the explanation given by Ezzat and Younis (2009) and Oğuz et al. (2012). Draber–Mońko et al. (2009) stated that: “It seems early to identify Psychoda spp. larvae on SEM bases only and we should resort to DNA findings to provide a thorough documentation of adult and larval stages”.
Ivermectin is a semisynthetic macrocyclic lactone that has been demonstrated to have activity against Strongyloide stercoralis, Ancylostoma braziliense, Cochliomyia hominivorax, Dermatobia hominis, Filaria bancrofti and Loa loa, as well as ectoparasites, such as Sarcoptes scabies, Pediculus humanus and Demodex folliculorum. Ivermectin has, therefore, become an important alternative for the treatment of patients with different forms of ectoparasite infestations, such as scabies, head lice, demodicidosis, and myiasis (De Tarso et al., 2004; Osorio et al., 2006). In agreement to the previous studies; the patients responded to oral dose of Ivermectin without any recurrence in the followed patients in the present work.
According to the available literature, this study reported and described cases of urinary myiasis caused by Psychoda spp. Under SEM observation, it seems that the sensillary necklace–like structure at the junction of the head with the first thoracic segment, the scattered sessile papillae on the dorsal aspects of the body segments, the shape and pattern of spine distribution and the hollow structure of the setae have not been described before.
ACKNOWLEDGEMENT
The authors would like to thank Prof. Dr. Refaat M. A. Khalifa, Professor of Medical Parasitology, Faculty of Medicine, Assuit University for valuable comments and corrections of the manuscript.
CONFLICT OF INTEREST
No conflict of interest.
REFERENCES
Abonnenc E (1972). Les Phlébotomes de la region éthiopienne (Diptera, Psychodidae). Mem Orstom; 55: 1–28. (English abstract).
Adams ZO and Hall MR (2003). Methods used for killing and preservation of blowfly larvae, and their effect on post–mortem larval length. Forensic Science International 138: 50–61.
http://dx.doi.org/10.1016/j.forsciint.2003.08.010
PMid:14642719
Burgess IF (2003). Myiasis: Maggots infestation. Nurse Times; 99:51–3.
PMid:12715561
Colwell DD and O'Connor M (2000). Scanning electron microscopy of sarcophagid (Diptera) larvae recovered from a case of human cutaneous myiasis. J Med Entomol; 37, 854–9.
http://dx.doi.org/10.1603/0022-2585-37.6.854
De Tarso P, Perre–Filho P, Minguini N, Pierre LM and Pierre AM (2004). Use of ivermectin in the treatment of orbital myiasis caused by Cochliomyia hominivorax. Scand J Infect Dis; 36:503–5.
http://dx.doi.org/10.1080/00365540410020136
PMid:15307583
Dinçer Ş, Tanyüksel M and Küçük T (1995). Two case–reports of myiasis caused by Psychoda spp. (Diptera: Nematocera) and Sarcophaga spp. (Diptera: Cyclorrhapha). Turkiye Parazitol Derg; 19:402–8.
Draber–Mońko A, Malewski T, Pomorski J, Łoš M and Ślipiński P (2009). On the morphology and mitochondrial DNA barcoding of the flesh fly Sarcophaga (Liopygia) argyrostoma (Robineau–Desvoidy, 1830) (Diptera: Sarcophagidae) – an important species in forensic entomology. Ann Zoolog; 59(4):465–93.
http://dx.doi.org/10.3161/000345409X484865
Durden LA (2002). Medical and Veterinary Entomology; Edition: 3, Academic Press. USA: 100–49.
http://dx.doi.org/10.1016/B978-012510451-7/50009-8
http://dx.doi.org/10.1016/B978-012510451-7/50006-2
http://dx.doi.org/10.1603/0022-2585-39.2.398
Ezzat HM. and Younis AI (2009). Case report: Urinary myiasis caused by larvae of Psychoda spp. Proceedings of the 28th Annual Scientific Conference of the Egyptian society of Basic Medical sciences, March 5th, Faculty of Medicine; Cairo University, 61 – 79.
Fausto AM, Feliciangeli MD, Maroli M and Mazzini M (1998). Morphological study of the larval spiracular system in eight Lutzomyia species (Diptera: Psychodidae). Mem Inst Oswaldo Cruz, Rio de Janeiro. 93(1): 71–9.
Foote BA (1991). Order Diptra in Stehr, F.W. (ed). Immature insects Vol (2). Kendall/Hunt, Dubuque, Lowa: 690–915.
Fuentes MV, Sàez–Duràn S and Galàn–Puchades MT (2011). Potential urogenital myiasis caused by Psychoda sp. (Nematocera: Psychodidae). First case reported in Spain. Rev. Ibero–Latinoam. Parasitol; 70 (2): 212–15.
Gonzalez M, Maurice CM, Greissy MP, Javiera DV and Marcelo MC (2009). Accidental genital myiasis by Eristalis tenax. Rev chil infectol; 26(3): 270–2.
Gopalakrishnan S, Srinivasan R, Saxena SK and Shanmugapriya, J (2008). Myiasis in different types of carcinoma cases in southern India. Indian J Med Microbiol; 26:189–92.
http://dx.doi.org/10.4103/0255-0857.40542
PMid:18445964
Greenberg B (1971). Flies and Disease, Vol. (1) Ecology, classification and biotic association. Princepton University Press, Princepton N.J. : 111–21.
PMid:5565612
Hall M and Wall R (1995). Myiasis of humans and domestic animals. Adv Parasitol; 35: 257–334.
http://dx.doi.org/10.1016/S0065-308X(08)60073-1
Hira PR, Hall MJ, Hajj B, Al–Ali F, Farooq R and Muzairai IA (1997). Human myiasis in Kuwait due to Oestrus ovis, Psychoda species and Megaselia species. Med Prin Pract; 6: 129–36.
Hiroshi T, Shinpei M, Shuichi K, Hujitsugu M and Toshio, K (1963). A case report on Psychodid–fly myiasis associated with bronchiectasis. Med Entomol Zool; 14:216–9.
Hyun DY, Cain MP, Blue–Hidey DE and Conway JH (2004). Urinary myiasis associated with ureteral stent placements. Pediatr Infect Dis J; 23:179–81.
http://dx.doi.org/10.1097/01.inf.0000109957.01170.4c
PMid:14872191
Jdalayer T, Maleki M and Moghtader M (1978). Human urogenital myiasis caused by Chrysomyia bezziana. Iranian J Publ Health.7 (3): 116–18.
Johnston M and Dickinson G (1996). An unexpected surprise in a common boil. J Emerg Med; 14:779–81.
http://dx.doi.org/10.1016/S0736-4679(96)00192-8
Killick–Kendrick R, Killick–Kendrick M, Leger N, Pesson B, Madulo–Leblond G and Page AM (1989). Absence of outer caudal setae on all larval instars of Phlebotomus tobii from the Ionian Greek islands. Med Vet Entomol 3: 131–35.
http://dx.doi.org/10.1111/j.1365-2915.1989.tb00487.x
PMid:2519655
Lane RP and Crosskey RW (1993). Medical Insects and Arachnids. (3rd eds) London: Chapman & Hall. http://dx.doi.org/10.1007/978-94-011-1554-4
Lotfy WM (2011). Gastrointestinal and urogenital myiasis caused by psychodid fly (Diptera:Nematocera) in Egypt: Case Report. PUJ; 4(1):115–6.
Mariluis JC, Mulieri PR, Patitucci LD and Oliva A (2007). Cystomyiasis by larvae of Psychoda sp. (Diptera: Psychodidae): First case for Argentina. Can. Soc. Forensic Sci. J; 40(4): 187–8.
http://dx.doi.org/10.1080/00085030.2007.10757159
Markel EK, Voge M and John DT (1992). Medical Parasitology, 7th ed. Philadelphia, USA. W.B. Saunders.: 353–8.
Massey RL and Rodriguez G (2002). Human scrotal myiasis: botfly infestation. Urol Nurs; 22(5): 315–17.
PMid:12432716
Musa HA and Wegi–Allah EM (2008). Cutaneous myiasis caused by Cordylobia Anthropophaga: Description of a case from Gazira State – Sudan. Sud J Publ Health; 3(2): 91–3.
Nagy V (2012). Unusual presentation of the urogenital myiasis caused by Lucilia sericata (Diptera: Calliphoridae). Ann Agricul Environ Med; 19(4): 802–4.
PMid:23311811
Noutsis C. and Millikan L. (1994): Myiasis. Dematol Clinic; 12:729–36.
PMid:7805302
Oguz U, Reşorlu B, Çizmeci Z and Ünsal A (2012). A rare urogenital myiasis caused by Psychoda albipennis: a case report of Psychoda albipennis. Turkish J Urol; 38(3):168–9.
http://dx.doi.org/10.5152/tud.2012.035
Osorio J, Moncada L, Molano A, Valderrama S, Gualtero S and Franco–Paredes C (2006). Role of ivermectin in the treatment of severe orbital myiasis due to Cochliomyia hominivorax. Clin Inf Dis; 43: 57–9.
http://dx.doi.org/10.1086/507038
PMid:16912935
Pessoa FA, Guerra de Queiroz R and Ward RD (2001). External morphology of sensory structures of fourth instar larvae of neotropical species of phlebotomine sand flies (Diptera: Psychodidae) under Scanning Electron Microscopy. Mem Inst Oswaldo Cruz, Rio de Janeiro; 96 (8): 1103–8.
Rutledge LC and Gupta RK (2002). Moth flies and sand flies (Psychodidae). In: Medical and Veterinary Entomology (Gary Mullen, Lance Durden, Eds.). Academic Press. San Diego. USA. 147–61.
Sakla AA, El–Hady HA and El–Nadi NA (2003). Psychoda albipennis maggots (Diptera: Psychodidae) as a cause of urinary myiasis in an Upper Egyptian male, the second world case. El–Minia Med Bull; 13:220–4.
Salimi M, Goodarzi D, Karimfar MH and Edalat H (2010). Human urogenital myiasis caused by Lucilia sericata (Diptera: Calliphoridae) and Wohlfahrtia magnifica (Diptera: Sarcophagidae) in Markazi Province of Iran. Iranian J Arthro–Borne Dis; 4(1): 72–6.
PMid:22808392
PMCid:PMC3385545
Singh TS and Rana D (1989). Urogenital myiasis caused by Megaselia scalaris (Diptera: Phoridae): a case report. J Med Entomol; 26: 228–9.
PMid:2724321
Sther FW. (1991): Immature Insects, Knedall/Hunt Publishing Co., Iowa, Vol 2: pp. 930.
Taylan–Ozkan A, Babur C, Kilic S, Nalbantoglu S, Dalkilic I and Mumcuoglu KY (2004). Urogenital myiasis caused by Psychoda albipennis (Diptera: Nematocera) in Turkey. Int J Dermatol; 43:904–5.
http://dx.doi.org/10.1111/j.1365-4632.2004.02051.x
PMid:15569013
Utsalo SJ and Khalifa RM (1985). Urinary myiasis presenting as urinary tract infection in a Nigerian adult male. Assiut Med J; 9 (2): 43– 53.
Wadhwa P, Kharbanda S and Rai B (2006). Urogenital myiasis due to Chrysomyia bezziana. Indian J Med Microbiol; 24(1):70–1.
http://dx.doi.org/10.4103/0255-0857.19903
PMid:16505564
Wagner R (1990). Family Psychodidae, In: Soós Á, Papp L (Eds): Catalogue of Palaearctic Diptera; Vol. 2, Akadémiai Kiadó, Budapest; pp. 11–65.
Wagner R (1997). Family Psychodidae, In: Papp L, Darvas B (Eds): Contributions to a manual of Palaearctic Diptera with special reference to flies of economic importance; Vol. 2 of Nematocera and Lower Brachycera, Science Herald, Budapest; pp. 205–25.
Wakid MH (2008). A laboratory–based study for first documented case of urinary myiasis caused by larvae of Megaselia scalaris (Diptera: Phoridae) in Saudi Arabia. Korean J Parasitol; 46(1):33–6.
http://dx.doi.org/10.3347/kjp.2008.46.1.33
PMid:18344675 PMCid:PMC2526290
Wang XC and Liu RF (2006). Psychoda larvae in urine of a woman. Chinese J Parasitol Parasit Dis; 24: 256.
Whitten JM (1955). A comparative morphological study of the tracheal system in larval diptera. Part I. Q J Microsc Sci; 96: 257–78. .
Zacharuck R and Shield SV (1991). Sensilla of immature insects. Ann Rev Entomol; 36: 331–54.
http://dx.doi.org/10.1146/annurev.en.36.010191.001555.
Zhou BJ, Yang LJ and Xiang Z (2008). Two cases infected by Psychoda larvae in Yunnan province. Chinese J Parasitol Parasit Dis 26: 1.
Zumpt F (1965). Myiasis in Man and Animals in the Old World. London Butterworths; 1–247.