The Journal of Advances in Parasitology
Research Article
Pathological Changes of Rainbow Trout (Oncorhynchus Mykiss Walbaum, 1792) Intestines Infected with Pomphorhynchus Laevis
Öznur Diler1, Öznur Görmez Özil1*, Halit Bayrak2, Özlem Özmen3
1Eğirdir Fisheries Faculty, Isparta Applied Sciences University, Isparta, Turkey; 2Water Institute, Suleyman Demirel University, Isparta, Turkey; 3Department of Pathology, Faculty of Veterinary Medicine, Burdur Mehmet Akif Ersoy University, Burdur, Turkey.
Abstract | The study describes the pathological changes of the acanthocephalan, Pomphorhynchus laevis (Müller, 1776) in cage-reared fish farm, infected rainbow trout, Oncorhynchus mykiss (Walbaum, 1792) in Işıklı Spring, Çivril, Turkey. In this study, a total of 3144 P. laevis individuals were detected from 84 sampled fish. 100% of these fish were infected by P. leavis, and mean intensity of infection was 37.42±0.32. At the histopathological examinations, parasites generally localised in the intestine. Also, the parasites were appeared freely in the abdominal cavity or attached visceral organs such as hepatopancreas and the serosal surface of the gastrointestinal system organs. The parasites were found encapsulated by a thin connective tissue each containing a single parasite in the internal organs of the host and the inflammatory reaction was observed around the parasite attached on abdominal organs in infected rainbow trout.
Keywords | Acanthocephala, Pomphorhynchus laevis, Pathology, Rainbow trout, Oncorhynchus mykiss
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | February 01, 2021; Accepted | February 22, 2021; Published | March 30, 2021
*Correspondence | Öznur Görmez Özil. Eğirdir Fisheries Faculty, Isparta Applied Sciences University, Isparta, Turkey; Email: [email protected]
Citation | Diler O, Ozil OG, Bayrak H, Ozmen O (2021). Pathological changes of rainbow trout (oncorhynchus mykiss walbaum, 1792) intestines infected with pomphorhynchus laevis. J. Adv. Parasitol. 8(1): 9-12.
DOI | http://dx.doi.org/10.17582/journal.jap/2021/8.1.9.12
ISSN | 2311-4096
Copyright © 2021 Ozil et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Occurrence of disease conditions particularly due to parasites has become a major constraint in aquaculture. Acanthocephalans of the genus Pomphorhynchus are intestinal, non-specific parasites of several marine and freshwater fishes (Taraschewski, 2000; Aydogdu et al., 2011; Smales et al., 2012). The proboscis and bulb of Pomphorhynchus acanthocephalans deeply penetrate the entire gut wall of the fish host and lead to extensive damage to the digestive tract. The density of the parasite burden and the depth of penetration of the acanthocephalans are two main factors for their pathogenicity (Bullock, 1963). In amphipods, P. laevis has been recorded as the most abundant larval helminth (Dezfuli et al., 1999). The histopathology of the acanthocephalan can be directly related to the depth of penetration of the host’s intestines by the presoma (Dezfuli, 1991). The pathology of P. laevis infection in the alimentary tract of the Antalya barb (Capoeta antalyensis), the chub (Leuciscus cephalus), the rainbow trout (Oncorhynchus mykiss) and the stone loach (Noemachileus barbatulus) was demonstrated in earlier studies (Aydogdu et al., 2011; Dezfuli, 1991; Wanstall et al., 1986; Wanstall et al., 2006). In Turkey, some authors have recorded P. laevis in freshwater fishes and also amphibian hosts (Yildiz et al., 2004; Cevrimel and Soylu, 2017). On the other hand, Smales et al. (2012) recorded P. tereticollis in Great Beyşehir Spined Loach (Cobitis bilseli) from Lake Beysehir, Turkey. Dusen and Oguz (2008) recovered P. laevis from marsh frog (Rana ridibunda) in Lake Işıklı. Heckmann et al. (2010) identified P. spindletruncatus in the intestine of the marsh frog Pelophylax ridibundus from Işıklı Lake. But there was limited research on the histopathology of P. laevis in cultured rainbow trout. For this reason, an examination of the histopathological lesions was aimed.
In the present study, histopathological changes were investigated in cage-reared rainbow trout Oncorhynchus mykiss (Walbaum, 1792) infected with Pomphorhynchus laevis.
MATERIALS AND METHODS
A routine examination of 84 O. mykiss 4.82±5 cm total length (mean±S.E.) and weighing 3.6±2 g were randomly sampled from net-cages close to Işıklı Spring, southwest of Turkey were examined in May 2019. The fish were transported to the laboratory alive, where they were weighed and measured. Fish were anaesthetised using MS-222 and their spinal cords cut with dissecting scissors. Identification of acanthocephalan speciments was made according to Moravec (2004) and Cevrimel and Soylu (2017).
Necropsy performed and lesions were grossly examined for all died fishes. For histopathological examination, tissue samples were collected from 15 fishes during the necropsy and fixed in 10% neutral formalin. Because of the fish and organs were small, the whole body was transversally cut into six pieces from head to tail. Then tissue samples were processed by an automatic tissue processing equipment (Leica ASP300S; Leica Microsystem, Nussloch, Germany). The samples were embedded in paraffin, and 5 μm sections taken by a Leica RM 2155 rotary microtome (Leica Microsystem, Nussloch, Germany). Then sections were stained with hematoxylin and eosin (HE) and microscopically examined. Microphotography was performed using the Database Manual Cell Sens Life Science Imaging Software System (Olympus Corporation, Tokyo, Japan).
RESULTS
Infected rainbow trout with P. laevis were sized 4.82±5 cm (mean±S.E.) and weighed 3.6±2 g. A total of 84 fish were randomly sampled and 3144 P. laevis individual were detected and 100% were infected. Intensity of infection was 37.42±0.32 (Figure 1).
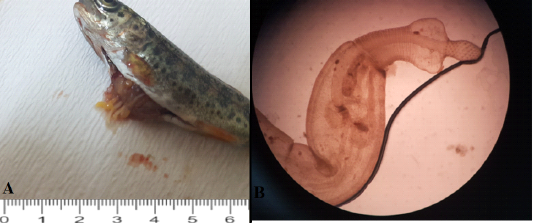
Figure 1: Morphological features analyses (A): Visceral organs cut open to show the parasite load. (B): Whole mount of the parasite, Bars=2 mm.
Identification of acanthocephalan speciments was performed on living worms. Parasites were generally localised in the distal portions of the fish’s intestine. At the histopathological examination, parasites were generally found in the intestinal lumen. Also, some of the parasites were freely in the abdominal cavity or attached visceral organs such as hepatopancreas and the serosal surface of the gastrointestinal system organs. Parasites caused no or slight inflammatory reaction when they localized intestinal lumen such as hyperemia. But the severe inflammatory reaction was observed around the parasite attached to abdominal organs. The parasites developmental stages were found encapsulated by a thin connective tissue each containing a single parasite in the internal organs of the host. In some severely affected fishes, numerous separated parasites were observed in big nodules localised same area. Generally, more than one parasites were in the intestinal lumens. The cysts contained parasites were surrounded by aggregates of macrophages and lymphocytes (Figure 2).
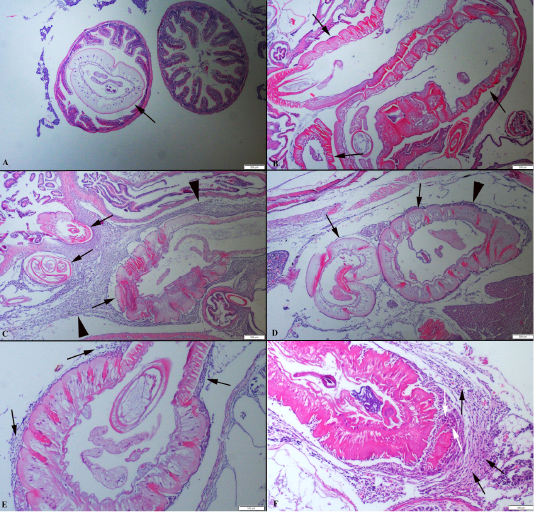
Figure 2: (A): Histopathological aspects of intestine one parasite localised in the lumen. (B): Three parasites (arrows) in an intestinal section. (C) Numerous parasites (arrows) localised abdominal cavity surrounded by severe inflammatory reaction (arrow head). (D) Two different parasites (arrows) near the hepatopancreas and moderate inflammatory reaction (arrow head), HE, Bars=200 µm. (E) Higher magnification of the inflammatory reaction around the parasite (arrows). (F) Higher magnification of the mononuclear cells (black arrows) and macrophages (white arrows), HE, Bars=100 µm.
Histopathologically, the parasites were covered by a cuticle layer. The shape of the parasites was generally elliptical or round. All organs of the parasites were visible in the sections.
DISCUSSION
Oncorhynchus mykiss is the main freshwater fish species cultured in Turkey and cage farming of rainbow trout has become widespread in lakes and reservoirs during the last few decades. Turkey is the largest producer of farmed trout in Europe with an annual production of 85.250 tons (TUIK, 2019). During the study period, a total of 84 O. mykiss specimens were examined in Işıklı Spring and were found to be infected by a total of 3144 P. laevis individuals.
The Acanthocephalan infections, when parasites are attached to the epithelial mucosa, can cause serious pathological conditions resulting in extensive granuloma and subsequent fibrosis (McDonough and Gleason, 1981; Kabata, 1985). Sanil et al. (2011) observed that Tenuiproboscis sp. has considerably damaged the tissue architecture of the host’s intestine. Yildiz et al. (2004) observed a marked thickening of the lamina propria and localized inflammation in P. laevis infection in tench. In another experimental infection study, the pathological changes observed the tissue damage and mucosal epithelium at the point of attachment of the intestinal tissues, for P. laevis infection in Salmo gairdneri (Wanstall et al., 1986).
The genus of Pomphorhynchus frequently disrupted through the gut wall (Wanstall et al., 1986). The host response to P. laevis is the formation of fibrosis and encapsulation (Dezfuli et al., 2008). The presence of the inflammatory cells which is common in fish infected by acanthocephalan has been reported by previous studies (Yildiz et al., 2004; Dezfuli et al., 2011). The presence of granulocytes, a few macrophages and a small amount of collagen is used as an indicative of acute inflammation (Wanstall et al., 1986). A chronic inflammatory state is associated with the presence of a large amount of collagen, giant cells and fibroblasts. Yildiz et al. (2004) reported that fibroblasts, granulocytes, lymphocytes and macrophages in the inflammation tissue, it was indicated to acute inflammation in Tinca tinca.
In this study, the inflammatory reaction was observed around the parasite attached to the abdominal organs in infected rainbow trouts. Developmental stages of the parasites were found and encapsulated by a thin connective tissue each containing a single parasite in the internal organs of the host. In some severely affected fishes, numerous separated parasites were observed in a big nodule localized in the same area. The cysts contained parasites were surrounded by aggregates of macrophages and lymphocytes. These findings in agreement with Yildiz et al. (2004).
Dezfuli (1991) and Yildiz et al. (2004) were demonstrated that P. laevis distributed not only in the anterior part but also in the posterior part of the host alimentary canal. In this study, parasites were generally localized to the distal part of the intestine. Generally, more than one parasites were observed in intestinal lumens. In addition, numerous parasites were noticed freely in the abdominal cavity or attached visceral organs such as hepatopancreas and the serosal surface of the gastrointestinal system organs.
The pathogenicity of acanthocephalans is mainly caused by two factors, density of worms and depth of parasite penetration into the host tissues (Taraschewski, 2000). In heavy infections the amount of nutrients drained by these worms cause the damage associated with tissue reactions in the wall of intestine.
In this study, the parasites were found encapsulated by a thin connective tissue each containing a single parasite in the internal organs of the host and the inflammatory reaction was observed around the parasite attached on abdominal organs in infected rainbow trout. This study showed that P. laevis caused severe inflammatory reaction and harmfull effects in O. mykiss (Walbaum, 1792). The severity of the pathological findings related to localisation of the parasites. Present study results revealed that this parasite is a problem for growth and survival in the rainbow trout culture in Turkey.
AUTHORS CONTRIBUTION
The authors contributed equally.
CONFLICTS OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






