The Journal of Advances in Parasitology
Short Communication
Necator Americanus and Plasmodium Falciparum Co-Infection and Albendazole Treatment Outcome among Individuals in Rural Ghana
Benjamin Amoani1,2,3*, Bright Adu2, Margaret T. Frempong3, Precious Barnes4, Michael Cappello4, Ben gyan2, Michael D. Wilson5
1Department of Biomedical Science, College of Health Sciences, University of Cape Coast, Cape Coast, Ghana; 2Department of Immunology, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon, Ghana; 3Molecular Medicine Department, School of Medical Sciences, Kwame Nkrumah University of Science and Technology; 4Department of Physician Assistant, School of Allied Health Sciences, University of Cape Coast, Cape Coast, Ghana; 5Partnerships for Global Health, Department of Pediatrics, Yale School of Medicine, Yale University, New Haven, CT, USA; 6Parasitology Department, Noguchi Memorial Institute for Medical Research, College of Health Sciences, University of Ghana, Legon, Ghana.
Abstract | The overlapping geographical distribution of malaria and helminth infections has led to several studies investigating the effect of co-infections by these parasites on the host. We investigated the impact of Necator americanus and Plasmodium falciparum co-infection on haemoglobin level and albendazole treatment outcome among infected individuals in Ghana. Hookworm-P. falciparum co-infection was associated with significantly reduced blood haemoglobin level (p=0.0057) and an increased odd (OR=6.44, 95%CI=1.31-53.97, p=0.042) of individuals remaining hookworm infected two weeks post-albendazole treatment. P. falciparum co-infection with hookworm may increase albendazole treatment failure. The possible implications on control programs and mechanisms underlying this phenomenon warrant further studies.
Keywords | Necator americanus, Plasmodium falciparum, Albendazole, Parasitaemia, Treatment failure, Anaemia.
Editor | Muhammad Imran Rashid, Department of Parasitology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | June 26, 2019; Accepted | December 18, 2019; Published | December 24, 2019
*Correspondence | Benjamin Amoani, Department of Biomedical Sciences, School of Allied Sciences, University of Cape Coast, Ghana; Email: [email protected]
Citation | Amoani B, Adu B, Frempong MT, Barnes P, Cappello M, Gyan B, Wilson MD (2019). Necator americanus and plasmodium falciparum co-infection and albendazole treatment outcome among individuals in rural ghana . J. Adv. Parasitol. 6(4): 51-54.
DOI | http://dx.doi.org/10.17582/journal.jap/2019/6.4.51.54
Copyright © 2019 Amoani et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Current efforts to control hookworm infection have primarily focused on community-based chemotherapy with benzimidazole for high-risk populations (Albonico et al., 2004; Keiser and Utzinger, 2008; Ndyomugyenyi et al., 2008). However, repeated large-scale treatment with benzimidazole has raised concerns about the possible emergence of resistance and decreased treatment efficacy has been reported in some studies (Albonico et al., 2004; Keiser and Utzinger, 2008).
Helminth infections in poor settings in the developing world are a public health concern, especially when they overlap with malaria (Reilly et al., 2008). However, the interaction between these widely distributed parasites have not been extensively investigated. Our previous work reported on the prevalence of hookworm and malaria infection and the association between hookworm-P. falciparum infection/co-infection with risk of anaemia among hookworm infected individuals in Kintampo North Municipality (KNM) of Ghana (Humphries et al., 2011). In addition, we have previously reported association between the haemoglobin (Hb) level and albendazole treatment outcome (Humphries et al., 2017). In the current study, we investigated the association between N. americanus and P. falciparum co-infection and albendazole treatment outcome in infected individuals in KNM, Ghana.
Material and methods
The study was approved by the Institutional Review Board of Noguchi Memorial Institute for Medical Research (FWA#: 00001824) of the University of Ghana. All study participants provided written informed consent prior to their recruitment. This study was conducted in KNM located within the forest-savannah transitional ecological zone in the middle belt of Ghana. The detail information on study area and the mode of recruitment of study participants have been described in our previous study (Amoani et al., 2019). One thousand and sixty-eight (1068) individuals aged from 4-80 years who gave informed consent were interviewed and given containers to provide stool samples. Out of these, 984 individuals provided samples which were used to determine the prevalence of hookworm infection.
Trained field assistants administered a demographic and health questionnaire and distributed labeled stool-collection containers to the participants. Stool samples were collected the following day, and about 5 ml venous blood was drawn at the same time into EDTA vacutainers for haematological analysis. Malaria Rapid Diagnostic Test (CareStart™ Malaria PfHRP2/pLDH Ag RDT, Access Bio, Inc, USA) and thin and thick blood film slides were made. Blood films were stained with Giemsa and examined under the light microscope for malaria parasitaemia. In addition, P. falciparum-specific 18S rRNA gene PCR using specific primers (Snounou, 2002), was carried out to detect submicroscopic parasitaemia as described in our pervious study (Amoani et al., 2019). All malaria parasitaemia detected were asymptomatic and hence were not referred for treatment. Faecal samples were analyzed for the presence of helminth eggs on the day of collection using the Kato-Katz and formol-ether concentration methods (WHO, 1998) . Hookworm speciation was carried out for hookworm positive cases by PCR using specific primers (Amoani et al., 2019). Individuals who were positive for hookworm or other soil transmitted helminths (STHs) were treated with a single observed dose of 400 mg albendazole (Remedica, Limassol, Cyprus).
The levels of haemoglobin among the study participants were determined using haematology analyser (ABX Pentra 60C+, HORIBA Medical, Rue du Caduce’, France) by following the manufacturer’s instructions.
Data analysis was performed with R version 3.3.2 and GraphPad Prism v.5.04 (GraphPad Software Inc. La Jolla, CA, USA). Normalized variables were compared between groups using either the Welch two sample t test or one-way analysis of variance (ANOVA) where appropriate. Associations between binomial outcomes and covariates were carried out using multivariable logistic regression analysis. P values <0.05 were considered statistically significant.
Results
Out of the 984 participants who submitted baseline stool samples for parasitological analysis; 178 tested hookworm positive and 806 were hookworm negative. Altogether, only 198 out of the initial 984 participants volunteered to donate blood and stool for the albendazole treatment outcome study, of which, 40 were infected with hookworm (Na) only, 59 were infected with P. falciparum (Pf) only, and 63 were co-infected with both hookworm and P. falciparum (Na/Pf). Also, 36 uninfected subjects were randomly selected to serve as uninfected assay controls. Of those that volunteered, 51.5% (102/198) were females and 48.5% (96/198) were males.
The overall mean Hb level for males was significantly higher than that of the females [Welch Two Sample t (180) =3.6, p=0.00035). A two-way analysis of variance was performed on Hb by infection status and gender (Figure 1). There was no evidence of significant interaction between status and gender on Hb. However, there was a statistically significant main effect of infection status [F (3, 183) = 6.5, p=0.00033] and gender [F (1, 183) = 16.2, p<0.0001] respectively, on Hb levels. The post-hoc test (Tukey HSD) for all possible pairwise comparisons indicated a significant mean difference between the negative endemic control group and: (1) those infected with P. falciparum only (p=0.0014) and (2) those co-infected with hookworm - P. falciparum (p=0.0057). None of the other pairwise comparisons were significantly different. The negative endemic control group had on average 1.9 g/dl higher Hb level than those infected with P. falciparum only and 1.6 g/dl higher Hb level than those co-infected with hookworm - P. falciparum.
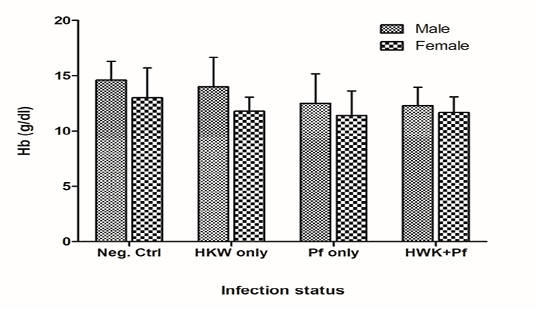
Figure 1: Mean haemoglobin (Hb) levels among the different infection status groups at baseline survey. Neg. Ctrl: Endemic negative control; HWK: Hookworm; Pf: Plasmodium falciparum. Error bars represent the standard deviation of the mean.
The odds of albendazole treatment failure was significantly (OR=6.44, 95% CI=1.31-53.97, p=0.042) higher in the
Table 1: Association between infection status, parasite burden and albendazole treatment outcome
| Comparison | OR | 95% CI | p-value |
| Infection status vs treatment failure | |||
| Hookworm only | 1 | ||
|
Hookworm-P. falciparum co-infection |
6.44 | (1.31, 53.97) | 0.042 |
| Hookworm infection intensity vs treatment failure | |||
| Heavy | 1 | ||
| Moderate | 0.86 | (0.14, 4.58) | 0.86 |
| Light | 0.43 | (0.12, 1.65) | 0.2 |
|
P. falciparuminfection intensity vs treatment failure |
1.19 | (0.95, 1.51) | 0.12 |
Odds ratios (OR), 95% confidence intervals (95%CI) and p-values were calculated by separate multivariable logistic regression for each comparison adjusting for age and gender. Hookworm intensity was categorized according to WHO standards as: Light, < 2000 eggs per gram [epg] of stool; Moderate, 2000–3999 epg and heavy, >4000 epg [10]. P. falciparum intensity was transformed [natural log (x + 1)]; where x = parasite density.
Na/Pf co-infected group compared to the group infected with hookworm only. The intensity of hookworm and P. falciparum infections respectively, did not significantly affect albendazole treatment outcome (Table 1).
Discussion
Hookworm and P. falciparum infections are common in the tropics and the consequences of their co-infection on morbidity and current modes of treatment need to be better understood for more effective control measures. This study investigated the effect of hookworm and malaria parasite co-infection on haemoglobin level and efficacy of albendazole treatment among individuals living in Kintampo North Municipality (KNM) of Ghana.
While hookworm infection intensity had no association with albendazole treatment outcome, co-infection with P. falciparum was found to increase the likelihood of hookworm treatment failure. This finding is consistent with previous studies that reported higher prevalence of hookworm infection in children with concurrent P. falciparum infection in the KNM (Humphries et al., 2011; Humphries et al., 2013) and elsewhere (Midzi et al., 2008). It is plausible that mass drug administration to school children in KNM with concurrent P. falciparum infection may have had less treatment success thus further contributing to the hookworm prevalence in the area. The possible mechanism through which concurrent P. falciparum infection alters anthelmintic treatment outcome is not clearly known, however, it may be linked to reduced blood Hb level. In the current study, concurrent P. falciparum infection with hookworm was significantly associated with a decrease of 1.6 g/dl in blood Hb level and in previous studies, albendazole cure rate was found to decrease with blood Hb level (Humphries et al., 2017). The destruction of red blood cells (RBCs) by the malaria parasite and clearance of malaria-infected RBCs by the immune system coupled with the blood feeding habit of hookworm are contributory factors to the Hb reduction in the co-infected group (Price et al., 2001; Chang and Stevenson, 2004). It is not clear whether the reduction in Hb also corresponds to either a reduced dose of drug (albendazole) reaching the hookworm or an altered drug half-life and thus influencing treatment outcome. These thoughts will warrant further investigations in order to inform whether or not helminthic mass drug administration programs should precede with anti-malaria treatment in areas co-endemic with both parasites. The overlap in geographical distribution of hookworm and P. falciparum and the fact that albendazole remains central to current hookworm intervention strategies are strong incentives for further studies in larger cohorts to properly elucidate the possible mechanisms of interaction underlying treatment outcome during co-infections with these parasites.
This study shows an interaction between hookworm and P. falciparum infection that may influence both haemoglobin level and the efficacy of albendazole in the treatment of hookworm. The possible mechanisms remain to be elucidated and could potentially have implications on control strategies against hookworm parasites in areas where P. falciparum is co-endemic.
Consent for Publication
The study team accepts the terms of the journal publishing the work.
Availability of Data and Materials
The data sets generated during the current study and for this manuscript are available from the corresponding author on reasonable request.
Funding
This work was supported by National Institutes of Health [1R01AI099623 to MDW]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We thank all the study participants. We are also grateful to staff of the Immunology and Parasitology departments of the Noguchi Memorial Institute for Medical Research, with special mention of Mr. Joseph Otchere, Ramat Bint Yusif Ishmael, Nana Adwoa Pels, and Ms. Millicent Opoku, for technical assistance.
Conflict of interest
The authors declare no conflict of interest.
authors contribution
This work was carried out in collaboration among all authors. All authors read and approved the final
manuscript.
References






