Journal of Animal Health and Production
Ammonia and Fermentation Treatment of Cymbopogon nardus L. Waste as a Substitution of Grass: Effect on Nutritional Profile and Ruminal in vitro Digestibility
Elihasridas1, Mardiati Zain1*, Rusmana Wijaya Setia Ningrat1, Erpomen1, Malik Makmur2, Ezi Masdia Putri2
1Department of Animal Nutrition and Feed Technology, Faculty of Animal Science, Andalas University, Kampus Limau Manis, Padang-25163, Indonesia; 2Faculty of Animal Science, Andalas University, Kampus Limau Manis, Padang-25163, Indonesia.
Abstract | The aim of this study was to investigate the effect of ammonia and fermentation treatment on Cymbopogon nardus L. waste as a substitute for Pennisetum purpureum grass on the nutritional profile and macronutrient digestibility under in vitro conditions. Seven rations (3 replications) were formed CN0 (100% P. purpureum), ACN1 (25% ammoniated C. nardus + 75% P. purpureum), ACN2 (50% ammoniated C. nardus + 50% P. purpureum), ACN3 (100% ammoniated C. nardus), FCN1 (25% fermented C. nardus + 75% P. purpureum), FCN2 (50% fermented C. nardus + 50% P. purpureum), and FCN3 (100% fermented C. nardus). A completely randomized design was used in this study and data integration was done using analysis of variance with posthoc Tukey’s test. The results obtained show that the nutritional profiles of ACN1 and FCN1 were slightly different from CN0. In vitro digestibility (%) of dry matter (IVDMD) and organic matter (IVOMD) after 48 h of incubation showed high significant differences (P < 0.001) among the treatments. There was no significant difference (P > 0.05) between CN0, ACN1 and FCN1 on digestibility of CP (IVCPD), NDF (IVNDFD), ADF (IVADFD) and cellulose (IVCLD). In conclusion, ammonia and fermentation treatment on C. nardus waste can be utilized as a substitute for P. purpureum at a level of 25% in a ruminant ration.
Keywords | Ammonia, Cymbopogon nardus waste, Fermentation, In vitro digestibility, Nutritional profile
Received | September 04, 2020; Accepted | November 27, 2020; Published | December 01, 2020
*Correspondence | Mardiati Zain, Department of Animal Nutrition and Feed Technology, Faculty of Animal Science, Andalas University, Kampus Limau Manis, Padang-25163, Indonesia; Email: [email protected]
Citation | Elihasridas, Zain M, Ningrat RWS, Erpomen, Makmur M, Putri EM (2021). Ammonia and fermentation treatment of Cymbopogon nardus L. waste as a substitution of grass: Effect on nutritional profile and ruminal in vitro digestibility. J. Anim. Health Prod. 9(1): 27-32.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.27.32
ISSN | 2308-2801
Copyright © 2021 Elihasridas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cymbopogon nardus L. or Ceylon citronella plants are one of the primary agribusiness commodities that produce essential oils (Citronella oil) in Indonesia and in the international market. Edible parts (stems and leaves) of C. nardus are distilled to produce citronella oil which is widely used in the food and pharmaceutical industries. In 2017, Indonesia had a plantation area that produces citronella oil which was close to 20,000 ha with plant productivity of ± 3000 tons (Sulaswatty et al., 2019). The potential biomass of C. nardus waste produced by citronella oil refining industry in Indonesia reaches more than 2,500 tons/year. The proximate analysis shows that C. nardus waste contains crude protein and fibre at 5.82 and 35.02%, respectively (Sari et al., 2017). This indicates that C. nardus waste has a great opportunity as a source of fibre feed and a substitute for tropical grass to support the production of ruminants. However, the abundant crude fibre fraction in C. nardus waste is a limiting factor that must be considered. Therefore, it requires the right feed processing method to optimize the nutritional value and digestibility in fibre materials.
Chemical and biological approaches to agro-industrial waste have been shown to reduce fibre components and neutralize anti-nutrients making it is safe for consumption by animals. In vitro and in vivo evaluations of tropical agricultural waste in oil palm frond (high in lignocellulose) with ammoniation pretreatment (3-4% urea) and fermentation (Phanerochaete chrysosporium; 3 weeks incubation) showed crude fibre content that was almost similar to native grass; and improved the digestibility of macronutrients (Zain et al., 2008, 2014; Jamarun et al., 2017). Extensive studies on various forms of agricultural biomass by products with alkaline techniques and microorganisms have been shown to remove 16.7% cellulose, 50% lignin, and 32-50.1% methane production. (Kumar and Sharma, 2017; Yanti and Yayota, 2017; Kusi et al., 2018). Recently, studies that are related to Cymbopogon sp. as feed additives and sources of essential oils have provided promising evaluation results which increased rumen fermentation efficiency, reduction of total gas production, and methane inhibitors (Wanapat et al., 2013; Zulfa et al., 2017; Singh et al., 2018).
However, comparative evaluation studies on various pretreatment of C. nardus waste have not been reported. Hence information that are related to the nutritional profile, digestibility, and level of supplementation becomes a necessity. Therefore, the objective of this study was to investigate the effect of ammonia and fermentation treatment of C. nardus waste as a substitute for tropical grass P. purpureum on nutrient composition and digestibility under ruminal in vitro conditions.
MATERIALS AND METHODS
Diet preparation and treatments
The material used in this study was P. purpureum grass (freshly cut) obtained from the UPT Teaching Farm, Andalas University. C. nardus waste was obtained from refining citronella oil in lemongrass plantations in Solok, West Sumatra. The substrate was chopped at ± 5 cm and was put in a plastic bag. The ammonia process of C. nardus waste was done using prilled urea (CH4N2O) at a dose of 4% dry matter; and was then stored in an airtight container for 3 weeks. As for the fermentation process, the probiotic starter Starbio® was used with a level of 6% DM of C. nardus waste and was then incubated for 10 days under anaerobic conditions. After the incubation process, the plastic container was opened and the substrate was dried using an oven at 60°C for 24 h and before it was milled (1 mm sieve). After these processes, the nutritional content of the substrate was ready for analysis and in vitro evaluation. The proportions in the diet mixture are presented in Table 1.
Chemical analysis
Analyses of the content of dry matter (DM), organic matter (OM), crude protein (CP = nitrogen × 6.25), and ether extract (EE) in experimental diet was done following the AOAC procedure (2005). Estimated content of crude fibre (CF) fractions (i.e., NDF, ADF, ADL, cellulose, hemicellulose, lignin, and silica) in feed was based on Goering and Van Soest (1970). Nitrogen-free extract (NFE) content using the formula NFE = 100% - (water + ash + CP + EE + CF). Determination of total digestible nutrient (TDN) using Sutardi (1980) equation on feed nutrient content at level of CF > 18%, CP <20%, TDN = 70.6 + 0.259 CP + 1.01 EE - 0.76 CF + 0.091 NFE. Ash content was determined through a sample burning in an electric furnace at a temperature of 500°C for 6 h. Non-fibre carbohydrates (NFC) that represent cell wall fraction that is rapidly digested in the rumen was calculated using the equation: NFC = 100 - CP - EE - NDF - Ash. The results of chemical analysis are presented in Table 2.
In vitro incubation and digestibility
Rumen in vitro incubation was based on Tilley and Terry Procedure (1963). A total of 2.5 g of experimental diet was put in a 250 ml fermenter tube. Each tube was added with 50 ml rumen fluid from fistulated cow and 200 ml of McDougall’s solution (pH 6.5), which per liter of distilled water was dissolved with 9.8 g NaHCO3, 9.3 g Na2HPO4, H2O.12H2O, 0.57 g KCl, 0.47 g NaCl, 0.06 g MgSO4.7H2O, and 0.04 g CaCl2.2H2O. The solution was stirred until the substrate was homogeneously mixed. The blank tube was prepared which contained only rumen liquor and McDougall’s solution without the substrate. Each tube was injected with CO2 gas for 30 seconds and sealed using a rubber cap. The fermenter tube was then placed in a shaker bath with a precondition temperature of 39°C at 100 rpm for 48 h. After the incubation process, the fermenter tube was immersed in iced water to stop the
Table 1: Composition of experimental diet (% DM).
| Ingredients | Dosage (% DM) |
Treatment** |
||||||
| CN0 | ACN1 | ACN2 | ACN3 | FCN1 | FCN2 | FCN3 | ||
| P. purpureum | Control | 100 | 75 | 50 | - | 75 | 50 | - |
|
Ammoniated C. nardus |
4% urea | - | 25 | 50 | 100 | - | - | - |
|
Fermented C. nardus |
6% probiotic starter* | - | - | - | - | 25 | 50 | 100 |
*Starbio®, ** CN0: 100% C. nardus waste; ACN1, 75% P. purpureum + 25% ammoniated C. nardus waste; ACN2, 50% P. purpureum + 50% ammoniated C. nardus waste; ACN3, 100% ammoniated C. nardus waste; FCN1, 75% P. purpureum + 25% fermented C. nardus waste; FCN2, 50% P. purpureum + 50% fermented C. nardus waste; FCN3, 100% fermented C. nardus waste.
Table 2: Nutritional profile of experimental diet (% DM).
| Nutritional profile | Treatment* | SEM | ||||||
| CN0 | ACN1 | ACN2 | ACN3 | FCN1 | FCN2 | FCN3 | ||
| Water content | 6.89 | 7.68 | 8.23 | 10.03 | 7.90 | 9.30 | 11.45 | 0.59 |
| Dry matter | 93.11 | 92.32 | 91.77 | 89.97 | 92.1 | 90.7 | 88.55 | 0.59 |
| Organic matter (OM) | 89.34 | 90.58 | 91.75 | 89.59 | 89.46 | 88.78 | 88.06 | 0.46 |
| Crude protein (CP) | 11.97 | 11.72 | 11.26 | 10.88 | 12.04 | 10.55 | 9.76 | 0.32 |
| Crude fibre (CF) | 25.14 | 25.77 | 26.41 | 27.67 | 27.42 | 29.7 | 34.25 | 1.17 |
| Ether extract (EE) | 0.87 | 1.16 | 1.45 | 2.03 | 1.25 | 1.63 | 2.39 | 0.20 |
| Ash | 9.93 | 8.7 | 7.57 | 9.37 | 9.71 | 10.18 | 10.57 | 0.38 |
| Nitrogen-free extract (NFE) | 45.2 | 44.97 | 45.09 | 40.02 | 41.68 | 38.65 | 31.58 | 0.38 |
| Total digestible nutrient (TDN) | 50.76 | 51.52 | 52.29 | 53.81 | 50.02 | 49.27 | 47.78 | 0.75 |
| Neutral detergent fibre (NDF) | 71.07 | 71.92 | 72.2 | 70.92 | 68.57 | 67.28 | 64.71 | 1.05 |
| Neutral fibre carbohydrate (NFC) | 6.16 | 6.5 | 7.52 | 6.8 | 8.43 | 10.36 | 12.57 | 0.89 |
| Acid detergent fibre (ADF) | 35.02 | 36.22 | 36.17 | 38.66 | 31.9 | 37.17 | 39.22 | 0.92 |
| Acid detergent lignin (ADL) | 3.36 | 3.38 | 5.22 | 5.48 | 4.5 | 6.31 | 9.08 | 0.75 |
| Silica | 0.56 | 0.51 | 0.21 | 0.66 | 0.22 | 1.37 | 1.43 | 0.19 |
| Hemicellulose | 36.05 | 35.7 | 36.03 | 32.27 | 36.67 | 30.12 | 25.49 | 1.58 |
| Cellulose | 31.66 | 32.84 | 30.95 | 33.17 | 27.4 | 30.86 | 30.14 | 0.73 |
| Lignin | 2.8 | 2.87 | 5.01 | 4.82 | 4.29 | 4.94 | 7.65 | 0.62 |
*CN0, 100% C. nardus waste; ACN1, 75% P. purpureum + 25% ammoniated C. nardus waste; ACN2, 50% P. purpureum + 50% ammoniated C. nardus waste; ACN3, 100% ammoniated C. nardus waste; FCN1, 75% P. purpureum + 25% fermented C. nardus waste; FCN2, 50% P. purpureum + 50% fermented C. nardus waste; FCN3: 100% fermented C. nardus waste; SEM, standard error of the mean.
Table 3: In vitro digestibility of experimental diet (%).
| Apparent digestibility* |
Treatment** |
SEM | P-value | ||||||
| CN0 | ACN1 | ACN2 | ACN3 | FCN1 | FCN2 | FCN3 | |||
| pH |
6.68a |
6.72a |
6.73a |
6.77b |
6.68a |
6.73a |
6.76b |
0.012 | <0.001 |
| IVDMD |
62.53a |
58.38b |
48.50d |
43.72e |
55.04c |
47.04d |
42.62e |
0.576 | <0.001 |
| IVOMD |
61.37a |
56.82b |
47.43c |
42.69d |
54.12b |
46.29c |
41.35d |
0.623 | <0.001 |
| IVCPD |
49.53a |
45.78ab |
43.40b |
40.78c |
45.74ab |
42.73bc |
39.25c |
0.828 | <0.001 |
| IVNDFD |
41.00a |
37.70ab |
34.68ab |
32.65ab |
37.78ab |
33.86ab |
30.25b |
1.807 | <0.001 |
| IVADFD |
43.32a |
40.62ab |
38.17ab |
34.18b |
37.76ab |
34.68b |
31.67b |
1.108 | <0.001 |
| IVCLD |
39.74a |
38.86a |
37.25ab |
35.25ab |
36.59ab |
32.78b |
33.03b |
0.970 | <0.001 |
*IVDMD, in vitro dry mater digestibility; IVOMD, in vitro organic mater digestibility; IVCPD, in vitro crude protein digestibility; IVNDFD, in vitro neutral detergent fibre digestibility; IVADFD, in vitro acid detergent fibre digestibility; IVCLD, in vitro cellulose digestibility; **CN0, 100% C. nardus waste; ACN1, 75% P. purpureum + 25% ammoniated C. nardus waste; ACN2, 50% P. purpureum + 50% ammoniated C. nardus waste; ACN3, 100% ammoniated C. nardus waste; FCN1, 75% P. purpureum + 25% fermented C. nardus waste; FCN2, 50% P. purpureum + 50% fermented C. nardus waste; FCN3, 100% fermented C. nardus waste; SEM, standard error of the mean. Different superscripts (a, b, c, d) in a row showed higher significance level (P <0.001).
microbial fermentation. Then, a rubber cap was opened to measure the pH of the rumen liquor using a digital thermometer. Furthermore, rumen liquid was rotated at 10,000 rpm for 10 min to separate the supernatant from feed suspension. The suspension was added to 20 ml of pepsin with a concentration of 0.2%. Stage two of in vitro digestion ran for 24 h under aerobic conditions. The feed residual was filtered with Whatman paper no. 41. In vitro digestibility of DM (IVDMD), OM (IVOMD), CP (IVCPD), NDF (IVNDFD), ADF (IVADFD) and Cellulose (IVCLD) were calculated using the following equations:
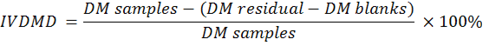


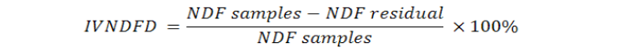
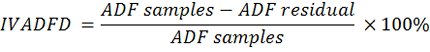
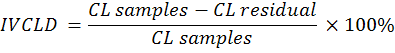
Where:
DM: dry matter (%); OM: organic matter (%); CP: crude protein (% DM); NDF: neutral detergent fibre (% DM); ADF: acid detergent fibre (% DM); CL: cellulose (% DM).
Data analysis
Data integration, analysis and hypothesis testing were done according to the analysis of variance procedure using JASP software version 0.13 (Goss-Sampson, 2019). The study consisted of seven treatments with three replications. If there is a significant difference in mean (P <0.05), it is followed by posthoc Tukey-HSD test. The linear model applied the following:
Yij = µ + τi + εij
Where:
Yij= nutrient digestibility (%); µ= average mean; τi= additives effect of treatment i; εij = experiment error.
Results and Discussion
Chemical composition
The composition of DM in a mixture of ammoniated C. nardus and P. purpureum (Table 2) ranged from 89.97-92.32% (ACN3 and ACN1), while the fermentation treatment had a closely similar value that ranged from 88.55-92.10% (FCN3 and FCN1). The compositions of OM, CP, EE, and ash of citronella waste were almost comparable in both treatments. The highest CF content was FCN3 (34.25%) and the lowest was CN0 (25.14%). For TDN, NDF, and hemicellulose, the addition of ammoniated citronella waste (25, 50, and 100%) had average values that ranged from 7-12%, higher than the CN0 and fermentation treatments. The increased composition of fermented citronella waste at various levels contributed to differences in the content of NFC, ADF, ADL, silica, and lignin among treatments.
In vitro rumen digestibility
Table 3 shows that pretreatment of C. nardus waste with different composition had a higher significant difference (P <0.001) on in vitro nutrient digestibility. In general, the pH of the rumen fluid was quite stable in the range of 6.68 - 6.76. The IVDMD parameter, CN0 gave the highest yield (62.53%) followed by ACN1 (58.38%) and the lowest was FCN3 (42.62%). A similar pattern occurred in IVOMD with the highest in CN0 (61.37%) and the lowest in FCN3 (41.35%). The digestibility of CP (IVCPD), NDF (IVNDFD), ADF (IVADFD), and cellulose (IVCLD) were not significantly different (P > 0.05) between CN0, ACN1, and FCN1. Among the ammoniation and fermentation treatments, the best IVCPD was reached by ACN1 (45.78%) and FCN1 (45.74%). Considerable levels of IVNDFD and IVADFD respectively, were CN0, ACN1, and FCN1. The IVCLD regressively decreased along with the increasing ratio of C. nardus waste.
The comparison of macronutrient compounds in the diet mixtures showed that ammonia and fermentation treatments of C. nardus waste have comparable profiles. The study by Manurung et al. (2015) using a substrate of Cymbopogon winterianus waste reported contents of CP, CF and NFE to be 9.72, 22.40 and 54.11%, respectively. In addition, Nurhayu and Warda (2018) revealed a proximate analysis of C. nardus waste (non-pretreatment) from an essential oil refinery, at DM (93.05%), CP (6.92%), EE (2.83%), and crude fibre (33.71%). Meanwhile, Al-Kindi et al. (2020) reported the contents of CP and CF to be 7.52 and 29.63% respectively, in fermentation approach. But superior results achieved by ammoniation application progressively increased CP concentration. This is affected by the ammonia process using urea as nitrogen source that is able to stretch lignocellulose bonds and silica deposits and increase nitrogen accumulation through NH3 hydrolysis. Decrease of CF content was lesser in C. nardus waste, especially in the fermentation treatment due to the shorter incubation time. This reduced the opportunity for microorganisms to produce more extracellular enzymes to break down structural carbohydrates in high fibre feed (Desnamrina et al., 2019).
Fractions of NDF and ADF in the mixture of ammoniated C. nardus and P. purpureum had lower yields when compared to other tropical agriculture by-products. Jayanegara et al. (2017) reported that ammoniated rice straw (1% urea; 4 weeks incubation) processed under temperature 121°C and 1.4 atm for 60 min had NDF (71.8%) and ADF (53.1%). The fermented oil palm frond as basal diet (60% DM) showed NDF, ADF and cellulose components of 66.10, 51.23, and 36.20%, respectively (Pazla et al., 2018); relatively equal when compared to fibre composition of FCN2. Utilization of other agricultural waste such as rambutan (Nephelium lappaceum) peel indicated that fibre fraction profile is more preferable in NDF (31.3%) and ADF (26.8%) but had lower protein concentration (Gunun et al., 2018). The feed preservation technique such as ensiled P. purpureum resulted in higher levels (g/kg DM) of NDF at 730 and ADF at 490 (Monção et al., 2020). This indicates that pretreatment of C. nardus waste still shows potential as a substitute for grass in ruminants ration.
In vitro evaluation of DM and OM digestibility of C. nardus waste with preservation treatment showed potential as a source of fibre feed for ruminants. However, an increasing percentage of C. nardus waste to more than 50% level can suppress nutrients digestibility. This is due to the fact that higher fibre fraction can act as an inhibitor in ruminal biodegradation (Frei, 2013). Comparative in sacco study conducted by Sari et al. (2017) on C. nardus (fresh leaves and stem) and C. nardus waste that had been incubated in buffalo rumen for 48 h revealed different levels of degradation of DM (44.1% versus 49.9%) and OM (45.7% versus 52.1%). The widely used agricultural waste as alternative fibre sources in tropical areas, such as fermented cocoa pods (3% molasses + Phanerochaete chrysosporium), has IVDMD and IVOMD (<60%) which is almost no different from the value of ACN1 and FCN1 (Laconi and Jayanegara, 2015). However, IVCPD levels in this study were lower than other tropical agricultural waste based diet. In vitro evaluation of ammoniated rice straw based feed (40% DM) showed CP digestibility in a range of 57 - 71% (Zain et al., 2019). Besides, the digestibility of NDF, ADF and cellulose in total mixed ratio of ammoniated rice straw basis were higher than lemongrass (Ningrat et al., 2019). This can be explained as due to a higher content of plant cell wall in the roughage that implicated limiting access of nitrogen and soluble carbohydrate to rumen microbes for fulfilling growth requirements. Sugiharto et al. (2018) confirmed that some tropical agroindustry waste still contains bioactive agents that influence the activity and the population of microbes. Therefore, utilization of pretreatment C. nardus waste is recommended at 25% level as a fibre source replacer.
Conclusion and Recommendations
Ammonia and fermentation treatment of C. nardus waste did not improve nutrition profile, in vitro dry matter and organic matter digestibility. Nevertheless, partial substitution (25% DM) of P. purpureum to C. nardus waste presented a comparable degradation level on crude protein and fibre compound.
Acknowledgments
This study was supported by Professor Research Cluster Grant by BOPTN Andalas University Contract No. T/9/UN-16.17/PP. Pangan-KRPIGB/LPPM/2020. This research was carried out together with doctoral students and laboratory staff of Ruminant Nutrition, Faculty of Animal Science, Andalas University.
Author’s Contribution
Elihasridas, Mardiati Zain, Rusmana Wijaya Setia Ningrat, and Erpomen formulated the experimental design and did experimental work. Malik Makmur and Ezi Masdia Putri analyzing the data and drafted the manuscript. All the authors approved the final version of the manuscript.
References






