South Asian Journal of Life Sciences
Research Article
Guanophilic Fungi of Mouse-Tailed Bats
Pawan Kumar Misra, Vadamalai Elangovan*
Department of Applied Animal Sciences, School for Bioscience and Biotechnology, Babasaheb Bhimrao Ambedkar University, Vidya Vihar, Rai Bareli Road, Lucknow, Uttar Pradesh -226025, India.
Abstract | Bats utilize a wide range of structures as their roosting sites and occupy various food niches. The guanophilic fungi of insectivorous bats such as Rhinopoma hardwickii and R. microphyllum were isolated and identified morphologically using phase contrast and scanning electron microscopes. The fungal culture was carried out using potato dextrose agar (PDA) and species such as Aspergillus versicolar gr., A. flavus, A. niger, Aspergillus sp., Penicillium funiculosum, Penicillium sp., Absidia corymbifera, Cladosporium cladosporiodes, C. resinae, Chrysosporium tropicum, Paecilomyces varitii, Malbranchea sp., Trichoderma sp., Mucor sp. and yeast were isolated and characterized. Chrysosporium tropicum plays a vital role in degrading the insectivorous bat guano which has rich contents of keratin, while C. resinae decomposes the hydrocarbons available in the guano. Many other fungi isolated in this study are opportunistic and some are medically and environmentally important.
Keywords | Fungi, Guano, Insectivorous bats, Rhinopomatidae, Scanning electron microscope
Editor | Muhammad Nauman Zahid, Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | September 04, 2015; Accepted | December 28, 2015; Published | December 30, 2015
*Correspondence | Vadamalai Elangovan, Department of Applied Animal Sciences, School for Bioscience and Biotechnology, Babasaheb Bhimrao Ambedkar University, Vidya Vihar, Rai Bareli Road, Lucknow, Uttar Pradesh, India; Email: [email protected]
Citation | Misra PK, Elangovan V (2015). Guanophilic fungi of mouse-tailed bats. S. Asian J. Life Sci. 3(2): 56-62.
DOI | http://dx.doi.org/10.14737/journal.sajls/2015/3.2.56.62
ISSN | 2311–0589
Copyright © 2015 Misra and Elangovan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bats of family Rhinopomatidae are known as mouse tailed bats. They are composed of a single genus (Rhinopoma) with a large geographical range covering a part of tropical Africa and most of the southern Mediterranean, Middle East and southern Asia (Hill, 1977). The family Rhinopomatidae consists of three species of bats, namely Rhinopoma hardwickii (lesser mouse-tailed bat), R. microphyllum (greater mouse-tailed bat) and R. muscatellum (small mouse-tailed bat). They are relatively small and live in arid and semiarid habitats, where they roost in large numbers in caves and man-made structures including houses, wells, tunnels and tombs. They feed on insects, such as flies and beetles. Having such an ecologically significant position, they play an important role as pest-controller.
The excreta of wild birds and animals, including bats, contain medically significant fungi, such as Cryptococcus neoformans and C. laurentii (Garcı´a-Hermoso et al., 1997) and locations that contain large amounts of such excreta are potential sites of human infection. Bat guano acts as good substrate for fungal growth and offers optimal environmental conditions. The fungi commonly serve as saprotrophs and/or pathogens or as transient chemo heterotrophic microorganisms (Northup et al., 1997). Insectivorous bats are known to be the prime contenders as reservoirs of fungi such as Histoplasma capsulatum, Coccidioides immitis, Cryptococcus laurentii and Blastomyces dermatitidis (Yamamoto et al., 1995; Garcia Hermoso et al., 1997; Mattsson et al., 1999; Bunnell et al., 2000). Apparently, there is little information available on guanophilic fungi of Indian bats. Therefore, this study was conducted to isolate and characterize the guanophilic fungi of rhinopomatid bats and their pathogenic and ecological role in the ecosystem.
MATERIALS AND METHODS
The guano samples of R. hardwickii were collected from Jhushi fort and Khusurubagh fort at Allahabad (25.45°N, 81.85°E), Atala mosque at Jaunpur (25.73°N, 82.68°E), Diyara fort at Sultanpur (26.45°N, 82.11°E) and Thar Ganga Ghat at Varanasi (25.28°N, 82.95°E), while the guano samples of R. microphyllum were collected from a natural cave at Chitrakoot (25.00°N, 80.83°E). The population size of R. hardwickii colonies ranged from 600 -
Table 1: Colony characteristics of guanophilic fungi isolated from the guano of rhinopomatid bats
|
Fungal species |
Colony diameter (mm) |
Colony character |
Zonation |
Sporulation |
|
|
Surface colour |
Texture |
||||
|
A. versicolar gr. |
14.4 |
Antique bronze |
Velvety |
Rounded |
Moderate |
|
A. flavus |
13.84 |
White -green |
Floccose |
Rounded |
High |
|
A.niger |
50.08 |
Black with White margin |
Powdery |
Rounded |
High |
|
Aspergillus sp. |
16.5 |
Army green with white margin |
Downy |
Rounded |
Moderate |
|
Penicillium funiculosum |
32.35 |
White with brown appearance |
Cottony |
Rounded |
Moderate |
|
Penicillium sp. |
28.42 |
Dark moss green |
Velvety |
Rounded |
Moderate |
|
Penicillium sp. |
6.13 |
Cambridge blue |
Velvety |
Rounded |
Moderate |
|
Absidia corymbifera |
58.01 |
White |
Woolly |
Rounded |
Non sporulating |
|
Cladosporium cladosporiodes |
9.39 |
Bronze yellow |
Velvety |
Rounded |
Moderate |
|
Cladosporium resinae |
16.43 |
Dark brown |
Velvety |
Moderate |
|
|
Chrysosporium tropicum |
7.58 |
White cream |
Woolly |
Rounded |
Moderate |
|
Paecilomyces varitii |
66.29 |
Burly wood |
Suede-like |
Rounded |
High |
|
Malbranchea sp. |
14.62 |
Bronze |
Velvety |
Rounded |
Moderate |
|
Mucor sp. |
58.01 |
White |
Woolly |
Rounded |
Non sporulating |
|
Trichoderma sp. |
58.01 |
Acid green |
Woolly |
Ellipsoid |
Very little |
|
Yeast |
7.72 |
Pink |
Watery |
Rounded |
Non sporulating |
750 individuals, while the colony of R. microphyllum was about 1800 individuals. The relative humidity of the roost sites of R. hardwickii and R. microphyllum was 80 ± 2 % and 75 ± 15 %, respectively. A fresh polythene sheet (3 x 2 m) was spread on the floor beneath the bat roosts at wee hours and 5 g of bat guano was aseptically collected in sterile vials using forceps. Individuals of R. hardwickii and R. microphyllum were captured using mist nests, 9 m length, 2 m width and 38 mm mesh size (Avinet, Dryden, USA) which were erected at early morning closest to their roosts for species recognition and also for sample collection. The identification of bat species was carried out based on morphological measurements by following Bates and Harrison (1997). Individual bat was kept in a bat cage for 20 - 30 min for defecation and thereafter released at the site of capture.
Isolation of guanophilic fungus was carried out by suspending 1 g of guano in 9 ml of sterile water to make 10 ml stock suspension. From the stock, 1 ml was taken and tenfold serial dilution was made (Raper et al., 1949; Thom and Raper, 1945). Streptomycin sulphate and tetracycline hydrochloride (8µg/l) were mixed as antibacterial agents with the potato dextrose agar media (Hi-Media) for fungal culture. The serially diluted samples were inoculated onto the culture plates and incubated at 28°C for 7-9 days. The colonies were extirpated and purified in PDA media at 25°C for 7 days. The fungal samples were carefully collected from the inoculation plates and mounted on the aluminum stubs using double side carbon adhesive tapes and kept overnight in desiccators. The stubs were sputter coated with palladium coater (JFC-1800) and the morphology of fungus was studied under scanning electron microscope (JEOL JSM 6400 LV, Japan) between 5 and 15 kV at different magnifications.
RESULTS
A total of 16 species of fungi belonging to ten genera were isolated from the guano samples of R. hardwickii and R. microphyllum. Majority of them belonged to ascomycota (13 species), followed by zygomycota (2 species) and a yeast species (Table 1). Fungal species such as Aspergillus versicolar gr., A. flavus, A. niger, Absidia corymbifera, Paecilomyces varitii, Cladosporium cladosporiodes, C. resinae, Chrysosporium tropicum and Penicillium funiculosum were isolated from the guano samples of R. hardwickii. In addition, two species of genus Penicillium, and one species of each of the genera Aspergillus, Malbranchea, Trichoderma and yeast were also isolated from the guano samples of R. hardwickii. The guano samples of R. microphyllum which collected from Godavari cave temple at Chitrakoot had only Absidia corymbifera and sterile mycelia.
The colony of Aspergillus versicolar was antique bronze, rounded and velvety (Figure 1A). It attained a diameter of 14.4 mm on seventh day (Table 1). The hyphae boar chains of rough conidia on terminal ends (Figure 2A). The colony of A. flavus was pale green with white margin, circular and floccose (Figure 1B). The hyphae of A. flavus were septate and dichotomously branched. Conidial heads were radiated, uni-and biseriate. The conidium was pale green and
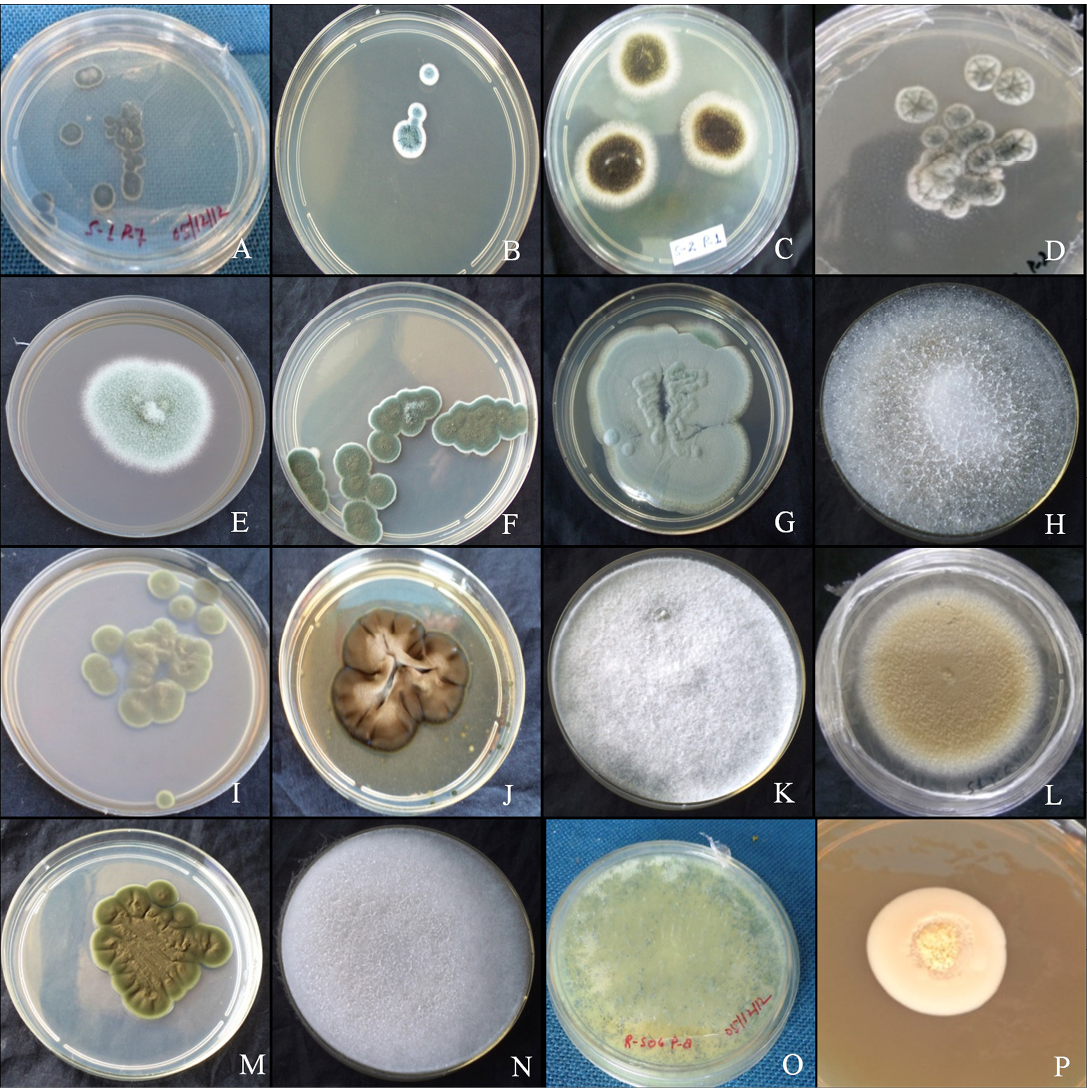
Figure 1: Colony morphology
A. versicolar gr (A), A. flavus (B) and A. niger (C). Aspergillus sp. (D), Penicillium funiculosum (E), Penicillium sp. (F), Penicillium sp. (G), Absidia corymbifera (H), Cladosporium cladosporiodes (I), Cladosporium resinae (J), Chrysosporium tropicum (K), Paecilomyces varitii (L), Malbranchea sp. (M), Mucor sp. (N), Trichoderma sp. (O) and yeast sp. (P)
conspicuously echinulate, smooth to very finely roughened and spherical (Figure 2B). Conidiophores were coarsely roughened and uncolored. Colonies of A. niger were isolated from the guano samples of R. hardwickii collected from a monument at Khusrubagh, Allahabad and at building roost in Sultanpur. The round and powdery colony with inner black and white margin (Figure 1C) attained 50.08 mm (Table 1). The conidium of A. niger was brown to black, very rough and globose (Figure 1C). The conidiophores bore numerous black dot-like spores at the terminal end (Figure 2C). The hyphae were translucent and septate. The conidial heads of A. niger were radiated initially and split into columns at maturity. A colony of unknown species belongs to the genus Aspergillus was isolated from the guano of R. hardwickii collected at Atala mosque, Jaunpur (Figure 2D). The rounded colony was downy; with inner army green and white margin attained 16.5 mm at maturity (Figure 1D).
A colony of Penicillium funiculosum was isolated from the guano sample collected from the Thar Ganga Ghat, Varanasi. The colony was cottony, white, rounded and attained
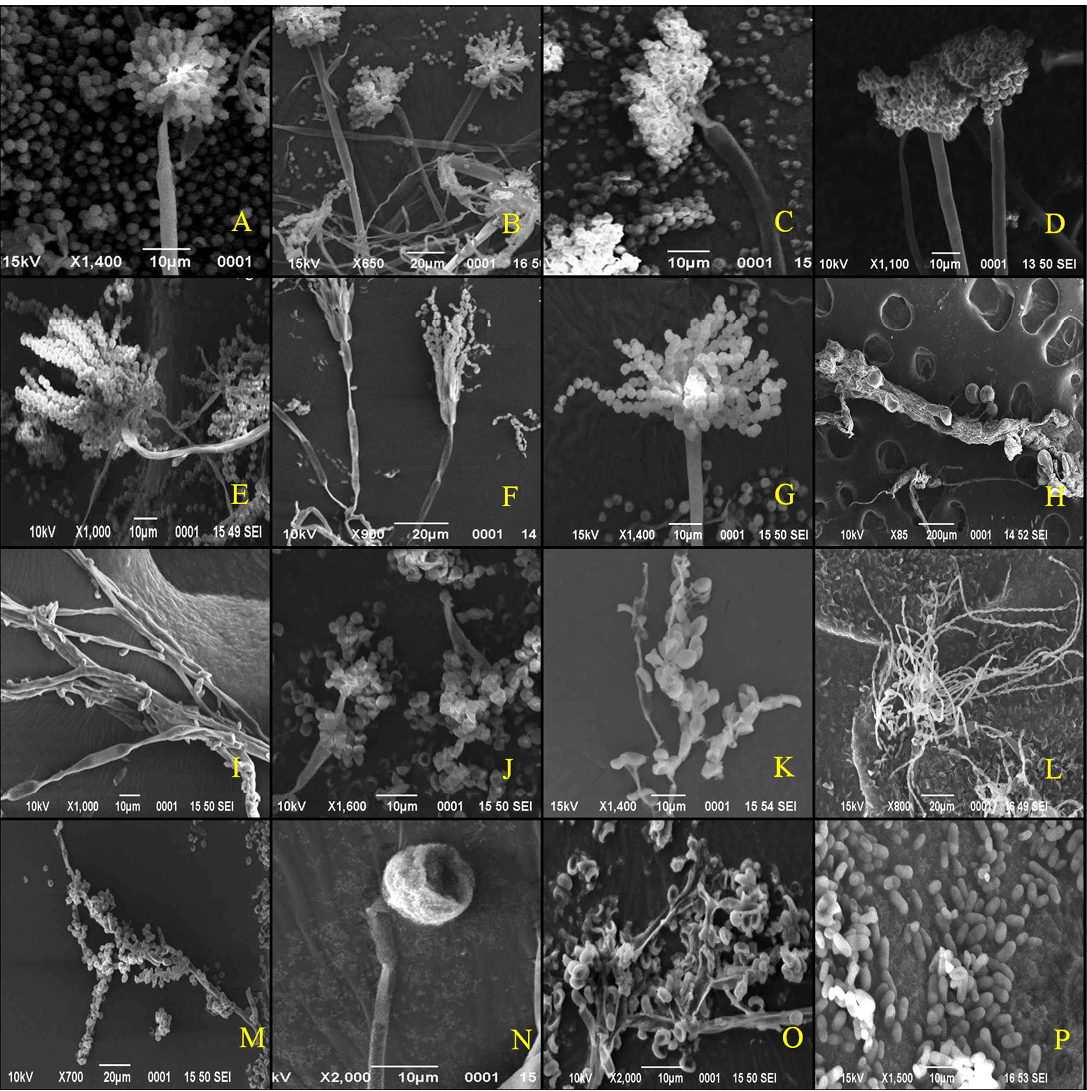
Figure 2: Scanning electron micrographs
A. versicolar gr (A), A. flavus (B) and A. niger (C). Aspergillus sp. (D), Penicillium funiculosum (E), Penicillium sp. (F), Penicillium sp. (G), Absidia corymbifera (H), Cladosporium cladosporiodes (I), Cladosporium resinae (J), Chrysosporium tropicum (K), Paecilomyces varitii (L), Malbranchea sp. (M), Mucor sp. (N), Trichoderma sp. (O) and yeast sp. (P)
32.35 mm (Figure 1E). A large number of whip-like conidial chains observed at the terminal end of conidiophore with spherical spores (Figure 2E). In addition, two isolates of Penicillium were collected from the guano of R. hardwickii. A dark moss green, velvety and round colony was isolated from the guano samples collected from Diyara, Sultanpur attained 28.42 mm (Figure 1F). The conidiophores consist long and oval spores at the terminal end (Figure 2F). Another rounded and velvety colony with Cambridge blue was isolated from the sample collected from Khusrubagh fort, Allahabad (Figure 1G). The matured colony attained 6.13 mm. The conidiophore was branched and bore chains of conidia at the terminal end (Figure 2G).
Absidia corymbifera was isolated from the guano samples of R. hardwickii collected at Khusrubagh fort, Allahabad and R. micorphyllum samples from Godavari cave temple, Chitrakoot. The colony of A. corymbifera was white, wooly, rounded and attained 37.93 mm at maturity (Figure 1H). The branched conidiophores bore few numbers of conidia (Figure 2H). Two species of Cladosporium were isolated from the guano samples of R. hardwickii (Table 1). Cladosporium cladosporoides was isolated from a roost at the riverbank of Ganga, Varanasi and C. resinae was isolated from a monument at Sultanpur. The colony of C. cladosporoides was bronze yellow, velvety, rounded and attained 9.39 mm (Figure 1I). The septate hyphae had spherical conidia. The conidia were sparsely attached on the hyphae (Figure 2I). The colony of C. resinae was moderately sporulated, dark brown, velvety and attained 16.43 mm (Figure 1J). The first conidium was developed in to ramoconidia with three protuberant scars and conidiophores arose laterally from vegetative hyphae (Figure 2J). Chrysosporium tropicum was isolated from the guano samples of R. hardwickii collected from Jhusi fort, Allahabad. The colony of C. tropicum was white, wooly, rounded and attained 7.58 mm at maturity (Figure 1K). The thin walled hyphae were hyaline, branched and divided by septa. The barrel-shaped conidium was solitary, terminal and stalked (Figure 2K). The colony of P. varitii was wooly, rounded, burly wood colour and attained a diameter 66.29 mm (Figure 1L). The hyphae were septate, branched and bore chains of spores (Figure 2L).
In addition, one species belongs to each of genera Malbranchea, Mucor, Trichoderma and yeast was isolated. The colonies of Malbranchea sp. and Mucor sp. were isolated from the guano samples of R. hardwickii collected from Khusrubagh and Allahabad. The colony of Malbranchea sp. was bronze colored, velvety textured and attained 14.62 mm (Figure 1M). The branched and segmented hyphae bore spores (Figure 2M). The colony of Mucor sp. was white, wooly, rounded and attained 58.01 mm (Figure 1N). The hyphae were hyaline with terminal conidia (Figure 2N). The colony of Trichoderma sp was acid green, wooly and attained 58.01 mm (Figure 1O). The hyphae were highly branched and bore spores (Figure 2O). The yeast colony was pink, wooly, rounded, non-sporulating and attained 7.72 mm with capsule-like individual yeast (Figure 1P, 2P). In addition, sterile mycelia were isolated from the guano samples of R. hardwickii collected from Atla mosque, Jaunpur, Jhunsi fort, Allahabad and Godavari cave, Chitrakoot. The colonies of sterile mycelia were white, cottony, rounded and non-sporulating.
DISCUSSION
In the present study, a total of 16 species of ecologically and medically important fungi were isolated from the guano samples of R. hardwickii and R. microphyllum. Among the guanophilic fungi, species belong to genus Aspergillus represented more than other genus. It shows that the species belong to Aspergillus distributed widely in the guano and various roost sites of rhinopomatid bats. The mycotoxin produced by A. niger causes several ailments of liver, kidney, nervous system, muscles, skin, respiratory organs, digestive tract, and genital organs in human (Durakovic et al., 1989; Rai and Mehrotra, 2005). The cosmopolitan fungus A. niger produces ochratoxin A, fumonisin B2 and aflatoxin in stored commodities (Schuster et al., 2002, Noonimabe et al., 2009; Al-Abdalall, 2009). However, A. niger has been consider as safe by the US Food and Drug Administration. Aspergillus niger produces many industrial important enzymes like amylase, amyloglucosidase, cellulases, glucoamylase, lactase, invertase, pectinase (Gautam et al., 2011). Aspergillus versicolor found in the guano of R. hardwickii was widely isolated from soil, indoor environments (Shelton et al., 2002; Engelhart et al., 2002; Amend et al., 2010; Anderson et al., 2011), various foods and hyper saline water (Kis-Papo et al. 2003; Mbata, 2008) and also associated with many health issues of humans and animals (Perri et al., 2005; Baddley et al., 2009; Edmondson et al., 2009; Moreno and Arenas, 2010). It produces sterigmatocystin, a mycotoxin that is a precursor of aflatoxin B1 (Mills and Abramson, 1986; Tuomi et al., 2000; Nielsen, 2003; Veršilovskis and Saeger, 2010). Aspergillus flavus causes chronic granulomatous sinusitis, keratitis, cutaneous aspergillosis, wound infections and osteomyelitis following trauma and inoculation. It also causes otitis, cutaneous aspergillosis, pulmonary and systemic infections in immunocompromised patients.
Results of the present study showed the wide distribution of filamentous fungus Penicillium in the guano of rhinopomatid bats. Among three species of Penicillium, P. funiculosum has industrial applications as it involves in cellulose production (Roberto et al., 2013). Penicillium funiculosum is able to secrete a balanced cellulasic system (Rao et al., 1988; Castro et al., 2010). The existence of P. funiculosum in the plant parts and the insects around the plants was well established (Lim and Rohrbach, 1980). The occurrence of P. funiculosum in bat guano attributes that the insectivorous bat R. hardwickii consumes insects or insect pests which are reservoirs of P. funiculosum. Absidia corymbifera isolated from R. hardwickii and R. microphyllum is opportunistic mycoses. It causes zygomycosis in immunocompetent hosts (Hagensee et al., 1994; Ribes et al., 2000). Absidia corymbifera is most commonly reported as an animal pathogen and causes mycotic abortion in cows (Knudtson and Kirkbride, 1992). Cladosporium cladosporioides listed as occasional agent of phaeohyphomycosis was isolated from the guano of R. hardwickii. The infections of C. cladosporioides are extremely rare (Matsumoto et al., 1994). Cladosporium resinae found in guano of R. hardwickii was widely observed in soil and actively decomposes hydrocarbons (Ahearn and Meyers, 1972).
Chrysosporium tropicum is a potent keratinophilic fungus isolated from the guano of R. hardwickii. It decomposes the most abundant and highly stable animal protein keratin (Avasan et al., 2011). As R. hardwickii feeds on orthopteran, dictyopteran, lepidopteran, hymanopteran, coleopteran and dipteran insects, the occurrence of C. tropicum in its guano is ecologically important for decomposing the insect keratin. Paecilomyces varitii observed in the guano of R. hardwickii was reported in earlier studies in the substrate including pasteurized food, soil, indoor air and wood (Samson 1974; Pitt et al., 2009). Paecilomyces is listed among the emerging causative agents of opportunistic mycoses in immune compromised hosts, cutaneous or catheter related associated with almost any organ or system of the human body (Salle et al., 2005) and it causes hyalohyphomycosis (Ajello, 1986). The occurrence of Malbranchea sp. in the bat guano was apparently due to contamination. Mucor isolated from the guano of R. hardwickii causes opportunistic infections known as zygomycosis (Larone et al., 1995; Stewart et al., 1999), which includes infections in mucous membranes, nasal passages and sinuses, eyes, lungs, skin, and brain, as well as renal and pulmonary infections and septic arthritis. Trichoderma sp. is most promising and an effective biocontrol agent for vegetable diseases and an antagonist controlling wide range of microbes and their mechanism of mycoparasitism involves nutrient competition, hyperparasitism and antibiosis (Weinding, 1934). Thus, the results of current study reveal the diversity of guanophilic fungi of two insectivorous bats and their active role on ecosystem and human health.
authors’ contribution
Both authors contributed equally.
CONFLICT OF INTEREST
The authors declare no conflicts of interest. All the experiments undertaken in this study comply with the current laws of India.
ACKNOWLEDGMENTS
We thank the University Science Instrumentation Centre for extending scanning electron microscope facility and the Archaeological Survey of India for permitting us in the monuments to collect guano samples. The financial assistance of Science and Engineering Research Board, Department of Science and Technology, New Delhi through a research project (No. SB/EMEQ-009/2013) to VE is acknowledged. PKM is a UGC-RGN fellowship holder.
REFERENCES






