Research Journal for Veterinary Practitioners
Research Article
Research Journal for Veterinary Practitioners 2 (1): 9 – 12Implication of Clostridium perfringens type a in Young Calves
Mahmoud Hamouda1, Fahad Al–hizab1, Taha Fouda2, Mahmoud Fayez3
- Department of Pathology, College of Veterinary Medicine and Animal Resources, King Faisal University, Saudi Arabia
- Department of Clinical Studies, College of Veterinary Medicine and Animal Resources, King Faisal University, Saudi Arabia;
- Veterinary Serum and Vaccine Research Institute, Anaerobic Department, Cairo, Egypt
*Corresponding author: [email protected]
ARTICLE CITATION:
Hamouda M, Al–hizab F, Fouda T and Fayez M (2014). Implication of clostridium perfringens type A in young calves. Res. j. vet. pract. 2(1): 9 – 12.
Received: 2013–10–30, Revised: 2013–12–14, Accepted: 2013–12–16
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.rjvp/2014/2.1.9.12)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Clostridium perfringens produces enteric diseases in cattle, sheep and goats. The microorganism can be a normal inhabitant of the intestine of most animals and human. A total of 8 calves aged between 8 to 11 month suffering from colic, and dark clotted blood in the faeces (melena) proceeded with death. The main postmortem findings were observed as massive hemorrhage and clot formation within the small intestine as well as abomasal ulcerations. Histologically, the abomasal mucosa was sloughed and the intestinal villi appeared necrotic along with a characteristic submucosal oedema. The bacterial culture and toxin detection showed presence of Clostridium perfringens type A. There were no other potential microorganisms or aflatoxins have been identified.
INTRODUCTION
Clostridium perfringens causes food poisoning and fatal enterotoxemia (Yamagishi, 1997; Yoo, 1997). Enteric C. perfringens infections in animals and man called enterotoxemias. There are five types of C. perfringens (A, B, C, D, E), which are identified by the main types of toxins they produce (alpha, beta, iota, epsilon and theta) (Niilo, 1987; Songer, 1996). C. perfringens type A is the most common C. perfringens types. It is part of the normal gut flora in cattle. However, dietary changes or parasitism may produce a favorable growth environment, resulting in overgrowth and production of potent toxins. C. perfringens type A can rapidly produce potent toxins, primarily alpha toxin. Alpha toxin is thought to be associated with a number of potentially deadly gastrointestinal diseases (Hatheway, 1990). Some isolates of C. perfringens type A produce β2–toxin which may contribute, along with α–toxin, to the development of hemorrhagic enteritis in cattle (Jelinski et al., 1995; Bueschel et al., 2003; Abutarbush and Radostits, 2005). C. perfringens type A is also commonly isolated in calves in cases where abomasal ulcers and abomasal haemorrhage are found (Roeder et al., 1987).
History, clinical signs, and gross postmortem findings are useful in establishing a presumptive diagnosis of clostridial enterotoxemia, but confirmation requires laboratory testing. Detection of toxins in intestine is very important to establish a diagnosis. ELISAs are considered one of the most important laboratory technique for C. perfringens toxins detection (Francisco and Glenn, 2008). The purpose of this paper is to present a description of Clostridium perfringens type A in calves that died suddenly with severe intra–luminal hemorrhage in the jejunum and abomasal ulcerations.
MATERIALS AND METHODS
Animals and Samples Collection
A total of 8 calves aged between 8 to 11 month suffering from colic, and dark clotted blood in the faeces (melena) proceeded with death was admitted to the Veterinary Teaching Hospital of the college of Veterinary Medicine and Animal resources, king Faisal, Saudi Arabia. The disease was coincided with the presentation of a new total mixed ration to animals. Blood samples were collected in heparinized tubes for determination of total and differential white blood cells count (Ve.Scan 5HM–ABaxis–USA/2002). Moreover, feedstuffs were analyzed for a total aflatoxin using a slightly modified immunoaffinity method based on Association of Official Analytic Chemists method (AOAC) (Trucksess et al., 1991).
Postmortem and Histopathology
Four cadavers were available to necropsy. Impression smears prepared by scraping intestinal mucosa and the cutting surfaces of mesenteric lymph nodes were stained by Gram’s Method. Specimens of abomasums, small intestine and large intestine, and mesenteric lymph nodes were preserved in 10% neutral buffered formalin.The formalin fixed tissue samples were dehydrated through graded ethanol and embedded in paraffin blocks. Sections of 4–5 um thickness were cut and routinely stained with Haematoxylin and Eosin (HE). The selected sections were stained by Gram and Gomori methylamine silver (GMS) stains to detect bacterial or fungal organisms respectively.
Mycology and Bacteriology
The isolation of A. fumigatus was carried from the trachea, lungs, liver, kidney, brain; small and large intestine.These samples were directly streaked on sabouraud agar plates for culturing and were incubated for 7 days at 37 ° (Darise, 1987). A. fumigatus was identified according to its specific colony characteristics, slides were also prepared for identification of mycelium and hyphial arrangement with lactophenol blue staining method. Additionally, intestinal contents were cultivated in Cooked Meat Broth (CMB) and incubated anaerobically at 37°C for 48 hours. From these cultures, 0.1mL loop aliquot was streaked on 5% sheep blood agar and incubated under anaerobic conditions at 37°C for 24 hours. Colonies showing related characteristics of C. perfringens (aspect, color, and hemolysis) were submitted to Gram stain; colonies corresponding to Gram–positive bacilli were cultivated in CMB. After the incubation period, cultures were submitted to additional tests including catalase, lecithinase and gelatinase production, and glucose, lactose, and skimmed milk fermentation for species identification (Cowan, 1974). Strains identified as C. perfringens were then sub–cultivated in Triptose Yeast Extract Broth (TYB) and incubated under anaerobic conditions. Cultures were then centrifuged at 7.500 rpm for 15 min. at 4°C and cell–free culture supernatants were recovered. After that, 0.3 ml of broth culture supernatant and intestinal contents were injected into white mice (25 – 40 g) via intraperitoneal route and observed for either death or disease signs within three days.
C. Perfringens Toxins ELISA
Intestinal contents and broth cultures supernatant were tested for CPA, CPB, and ETX via a commercial capture ELISA kit (Bio–X, Diagnostics, Belgium), following the manufacturer’s instructions.
RESULTS
Affected calves were almost found dead within 24 to 36 hours after the onset of clinical signs. Alternatively, others were found recumbent and semi–conscious, or still standing. Also, there were anorexia, signs of colic and dark clotted blood in the feces (melena) (Figure 1a). Moreover, the outstanding haematological finding in most cases was an often profound neutrophilia. C. perfringens was isolated from the intestinal contents. All strains were identified as C. perfringens that was based on colonial morphology, haemolysis on blood agar, Gram stain and biochemical characterization.There was no A. fumigatus or aflatoxins have been identified.
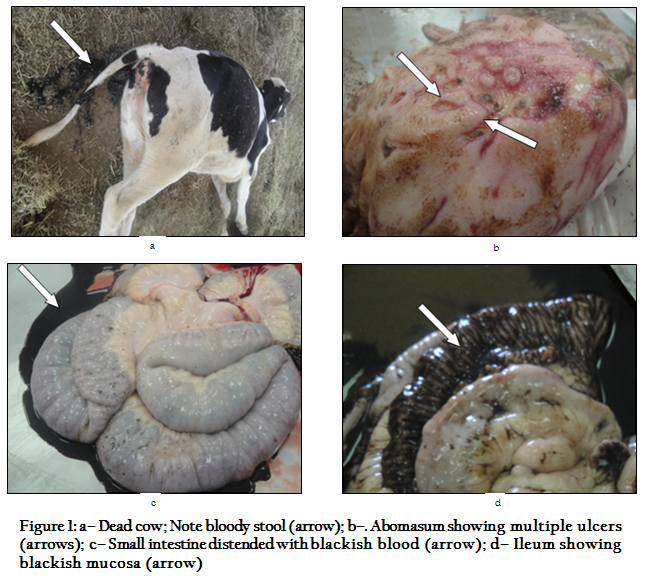
Figure 1: a– Dead cow; Note bloody stool (arrow); b–. Abomasum showing multiple ulcers (arrows); c– Small intestine distended with blackish blood (arrow); d– Ileum showing blackish mucosa (arrow)
Toxin detection
Toxin was detected in intestinal contents and broth culture supernatants, where all mice were died within 72 hours. A toxin was typed by using of an indirect ELISA assay. The results were positive for CPA and negative for CPB and ETX toxins. Hence, it was identified as C. perfringens type A.
Necropsy findings
The abomasum of most cases revealed multiple ulcers throughout the abomasal folds (Figure 1b).The small intestine contained either blackish blood or a large solid blood clot that obstructs the lumen (Figure 1c), and the mucosal surface of intestine appeared black in colour (Figure 1d). The aforementioned lesions were associated with congested and haemorrhagic mesenteric lymph nodes.
Microscopic lesions
The abomasal mucosa was denuded in more than one part and appeared as depressed areas (Figure 2a). The villi of jejnum and ileum appeared necrotic and heavy infiltrated by inflammatory cells (Figure 2b). The submucosa of ileum revealed a cluster of glands herniated into peyer's patches (colitis cystic profunda) (Figure 2c).The salient lesion in the small intestine was the presence of a characteristic submucosal oedema (Figure 2d).
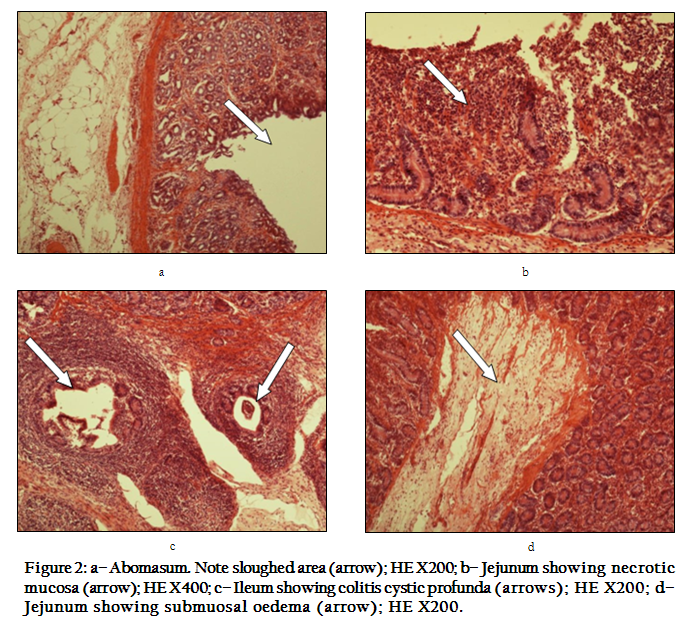
Figure 2: a– Abomasum. Note sloughed area (arrow); HE X200; b– Jejunum showing necrotic mucosa (arrow); HE X400; c– Ileum showing colitis cystic profunda (arrows); HE X200; d– Jejunum showing submuosal oedema (arrow); HE X200.
DISCUSSION
The present study describes a disease characterized by sudden death in calves. Typical gross lesions at necropsy and bacteriology along with toxin detection confirmed that Colstridium perfringens type A was incriminated in such condition. A final diagnosis was not only based solely on toxin detection, but also accompanied by pathological as well as microbiological findings (Francisco and Glenn, 2008). From pathological point of view, it is worth to find out colitis cystic profunda. This lesion may be a sequel to local damage to the muscularis mucosa (Jubb et al., 1993).
Colstridium perfringens type A is ubiquitous in the digestive tract of cattle and a hypothesis for the aetiology of C. perfringens overgrowth, is the overflow of finely ground carbohydrates from forestomach (Ewoldt and Anderson, 2005). This situation arises in association with the same factors which lead to sub–acute ruminal acidosis due to feeding on excess amounts of rapidly fermented carbohydrates or insufficient effective fiber (Gooden, 2003). Another explanation for the aetiology is sudden change in diet, C perfringens proliferates and produces potent toxins that act locally or are absorbed systemically (Niilo, 1980; Manteca 2002).
Clostridium perfringens type A produces CPA and can also produce several of the non–typing toxins, including CPE and CPB2 (Ceci et al., 2006; Brown et al., 2007). Information about pathogenesis of type A enteric infections in ruminants is minimal and often contradictory, but it is generally assumed that most clinical signs and lesions are due to the effects of CPA which is hemolytic, necrotizing, and potently lethal (Songer, 1996). C. perfringens type A also produces b2 toxin, which has a synergistic role with α toxin in the development of hemorrhagic lesions in the small intestinein cases of bovine enterotoxemia (Manteca et al., 2002) and in sheep and goats (Gkiourtzidis et al., 2001; Bueschel et al., 2003; Dray 2004). In the present cases, the presence of b2 was not investigated. More studies are warranted to understand the role of b2 toxin in enterotoxemia cases caused by C. perfringens type A. In general, it is always possible to isolate C. perfringens type A from intestinal contents and therefore the detection of lethal toxins in intestinal contents is important for the diagnosis of enterotoxemia (Hakan et al., 2007).
REFERENCES
Abutarbush SM, Carmalt JL and Wilson DG et al., (2004). Jejunal hemorrhage syndrome in 2 Canadian beef cows. Can. Vet. J. 45:48–50.
PMid:14992254 PMCid:PMC539227
Abutarbush SM and Radostits OM (2005). Jejunal hemorrhage syndrome in dairy and beef cattle: 11 cases (2001 to 2003). Can.Vet. J. 46: 711–715.
PMid:16187715 PMCid:PMC1180421
Brown CC, Baker DC and Barker IK (2007). Alimentary system In: Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 5th edn, Saunders Elsevier, St. Louis, MO, 1–296 pp.
Bueschel DM, Jost BH and Billington SJ (2003). Prevalence of cpb 2, encoding beta2 toxin, in Clostridium perfringens field isolates: Correlation of genotype with phenotype. Vet. Microbiol. 94: 121–129.
http://dx.doi.org/10.1016/S0378-1135(03)00081-6
Ceci L, Paradies P and Sasanelli M et al., (2006). Haemorrhagic bowel syndrome in dairy cattle: possible role of Clostridium perfringens type A in the disease complex. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 53: 518–523.
http://dx.doi.org/10.1111/j.1439-0442.2006.00884.x
PMid:17105573
Cowan ST (1974). Cown and Steel's Manual for the Identification of Medical Bacteria. 2nd edn. Cambridge University Press, Great Britain, 238 p.
Darise HL (1987). Medically important Fungi. A guide to identification. P. 14–15
Dray T (2004). Clostridium perfringens type A and beta2 toxin associated with enterotoxemia in a 5–week–old goat. Can.Vet. J. 45: 251–253
PMid:15072200 PMCid:PMC548614
Ewoldt JM and Anderson DE (2005). Determination of the effect of single abomasal or jejunal inoculation of Clostridium perfringens type A in dairy cows. Can. Vet. J. 46: 821–824.
PMid:16231652 PMCid:PMC1187792
Francisco AUzal and Glenn Songer J (2008). Diagnosis of Clostridium perfringens intestinal infections in sheep and goats. J.Vet. Diagn. Invest. 20: 253–265.
http://dx.doi.org/10.1177/104063870802000301
PMid:18460610
Gkiourtzidis K, Frey J and Bourtzi–Hatzopoulou E et al., (2001). PCR detection and prevalence of alpha, beta, beta 2, epsilon, iota and enterotoxin genes in Clostridium perfringens isolated from labs with clostridial dysentery. Vet. Microbiol. 82: 39–43.
http://dx.doi.org/10.1016/S0378-1135(01)00327-3
Gooden S (2003). Jejunal hemorrhage syndrome in adult dairy cattle. In: 6th western dairy management conference proc Reno, Nevada; P: 179.
Hakan Kalender, Ayşe Kiliç and Eray Atil (2007). Enterotoxemia in a cow due to clostridium perfringens type A. Turk. J.Vet. Anim. Sci. 31(1):83–84
Hatheway CL (1990). Toxigenic clostridia. Clin. Microbiol. Rev. 366–98.
Jelinski MD, Ribble CS and Chirino–Trejo M et al., (1995). The relationship between the presence of Helicobacter pylori, Clostridium perfringens type A, Campylobacter spp., or fungi and fatal abomasal ulcers in un weaned beef calves. Can. Vet. J. 36: 379–382.
PMid:7648542 PMCid:PMC1686945
Jubb KVF, Kennedy PC and Palmer N (1993). Pathology of Domestic Animals. 4thedn. Academic Press, San Diego.
PMCid:PMC513948
Manteca C, Daube G, Jauniaux T, Linden A, Pirson V, Detilleux J, Ginter A, Coppe, P, Kaeckenbeeck A and Mainil JG (2002). A role for the Clostridium perfringens b2 toxin in bovine enterotoxaemia. Vet. Microbiol. 86:191–202.
http://dx.doi.org/10.1016/S0378-1135(02)00008-1
Niilo L (1980). Clostridium perfringens in animal disease: a review of current knowledge. Can. Vet. J. 21:141–148.
PMid:6253040 PMCid:PMC1789702
Niilo L (1987). Toxigenic characteristics of Clostridium perfringens type C in enterotoxemia of domestic animals. Can. J. Vet. Res. 51:224–228.
PMid:2886206 PMCid:PMC1255307
Roeder BL, Chengappa MM and Nagaraja TG et al., (1987). Isolation of Clostridium perfringens from neonatal calves with ruminal and abomasal tympany, abomasitis, and abomasal ulceration. J. Am. Vet. Med. Assoc. 190:1550–1555.
PMid:2886483
Roeder B., Chengappa MM and Nagaraja TG et al., (1988). Experimental induction of abdominal tympany, abomasitis, and abomasal ulceration by intraruminal inoculation of Clostridium perfringens type A in neonatal calves. Am. J. Vet. Res. 49: 201–207.
PMid:2894790
Songer JG (1996). Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9: 216–234.
PMid:8964036 PMCid:PMC172891
Trucksess MW, Stack ME, Nesheim S, Page SW, Albert RH and Hansen TJ et al., (1991). Immunoaffinity column coupled with solution fluorometry or liquid chromatography post–column derivatization for determination of aflatoxins in corn, peanuts, peanut butter: collaborative study. J. Assoc. Off. Anal. Chem. 74 (1):81.
PMid:2026580
Yamagishi T, Sugitani K, Tanishima K and Nakamura S (1997). Polymerase chain reaction test for differentiation of five toxin types of Clostridium perfringens. Microbiol. Immunol. 41:295–299.
http://dx.doi.org/10.1111/j.1348-0421.1997.tb01204.x
PMid:9159402
Yoo HS, Lee SU, Park KY and Park YH (1997). Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 35: 228–232.
PMid:8968913 PMCid:PMC229544





