Research Journal for Veterinary Practitioners
Research Article
Gross Pathology and Histopathological Investigations of Kidney Lesions in Domestic Fowl, in Tunisia
Khaled Kaboudi1*, Abdelkader Amara2, Asma Yamoun1
1Department of Poultry Farming and Pathology, National Veterinary Medicine School of Sidi Thabet - University of Manouba, Tunisia; 2Department of Pathological Anatomy and Histology, National Veterinary Medicine School of Sidi Thabet - University of Manouba, Tunisia.
Abstract | Nephropathies in fowl are common and causing economic losses with high mortality and decrease in growth rate. Etiology of renal affections varies. Many viruses, bacteria, parasites, toxins and metabolic factors are in general incriminated. This study was carried out to evaluate the prevalence of renal lesions in 877 examined birds, received at the avian clinic of the National Veterinary Medicine School of Tunisia between 2012 and 2016. Necropsy was completed by histopathological and bacteriological analysis for a better orientation of the diagnosis. Animals were issued from commercial and free-range flocks located in the North-East of Tunisia. Global prevalence of renal lesions was 17.56% (154 animals: 79 from free-range flocks; 75 from commercial flocks), with a significant higher prevalence in >18 weeks group (20.40%), compared to young birds (<18 weeks) (15.73%) (χ2=3.158; p<0.05). Gross pathology study showed a predominance of vascular lesions (congestion, hemorrhage) (81.82%) followed by cellular lesions (hypertrophy, atrophy, steatosis, deposit urates, acute interstitial nephritis) (66.88%). Tumor infiltration was showed in 5.20% of cases. Viral diseases (47.6%) predominate suspicions, followed by metabolic disorders (29.21%) and bacterial infections (23.38%). Histopathological investigations showed nonspecific lesions, expected of tumor lymphoid infiltrations, which are compatible with Marek’s disease. Our findings in this first report showed that renal lesions occurred in Tunisian free range and commercial poultry flocks. Kidney damages can be primary or secondary to other diseases. Diagnosis of renal affections in poultry needs epidemiological, clinical, necropsy and laboratory investigations.
Keywords | Kidney, Gross pathology, Histopathology, Nephritis, Fowl, Tunisia
Received | August 01, 2019; Accepted | August 08, 2019; Published | August 26, 2019
*Correspondence | Khaled Kaboudi, Department of Poultry Farming and Pathology, National Veterinary Medicine School of Sidi Thabet - University of Manouba, Tunisia; Email: [email protected]
Citation | Kaboudi K, Amara A, Yamoun A (2019). Gross pathology and histopathological investigations of kidney lesions in domestic fowl, in tunisia. Res. J. Vet. Pract. 7(3): 67-73.
DOI | http://dx.doi.org/10.17582/journal.rjvp2019/7.3.67.73
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Kaboudi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Although nephropathies of poultry are responsible for considerable mortality and decrease in production, they are among the most neglected areas in poultry diseases. There are several reasons for this neglect. Firstly, there is economic importance of morbid phenomena causing high mortality compared to few deaths in poultry flocks due to benign renal disease were unimportant and often hardly noticed. Secondly, many poultry pathologists do not perform well in histopathology and often neglect the examination of kidneys, which are rarely sampled for laboratory analysis. The third and most important reason is that we do not adequately understand the nephropathies of poultry. Kidneys affections can be caused by different pathogens, which had renal main tropism. Nevertheless, in general renal lesions are considered as a complication of systemic diseases.
Renal lesions were of variable types: vascular, cellular, tumor and toxic. Numerous etiologies are implicated, including viruses, bacteria, toxins and metabolic factors. Among the viral diseases manifested by predominant renal disorders, infectious bronchitis, infectious bursal disease, inclusion body hepatitis (De Herdt et al., 2013; Nakamura et al., 2010; Zadravec et al., 2011), neoplastic diseases such as Marek’s disease and lymphoid leukosis (Randall and Reece, 1996; Fadly and Nair, 2008; Carvallo et al., 2011). Congestive and necrotic lesions were also attributed to Newcastle disease virus (El-Bahrawy et al., 2017) and influenza virus (Swayne and Halvorson, 2008; Suarez, 2013). However, described lesions are the consequence of septicemia. Recently, Palya et al. (2019) described outbraks of renal damage in broiler chickens in Malaysia caused by a novel virus, an Arbovirus from the genus of Orthobunyavirus. Many bacteria can induce lesions in chicken kidneys, such as Salmonella typhimurium, Escherichia coli and Enterococcus faecalis (Landman, 1999). Noninfectious etiology of renal lesions in fowl are summarized in mycotoxicosis (Kumar and Balachandran, 2009; Ul-Hassan et al., 2010), gout (Siller, 1981; Ekstrom and Degernes, 1989; Sathiyaseelan et al., 2018) and hepatorenal steatosis (Balnave et al., 1977).
Clinical manifestation of renal disorders are nonspecific. Commemoratives, necropsy investigations and laboratory analysis are fundamental in order to establish reasonable diagnosis. The aims of this paper were to determine the nephropathies prevalence in fowl (Gallus gallus) and to study the gross pathology and the histopathological features of renal lesions described in birds received at the avian clinic of the National Veterinary Medicine School of Tunisia.
Material and methods
Animals and Samples
This study was conducted on 877 birds (Gallus gallus) of different type of production. Animals were received in the avian clinic of the National Veterinary Medicine School of Tunisia, during the period between 2012 and 2016. Birds were issued from different regions of Tunisia and from commercial and traditional farms.
All animals were autopsied according to standard protocol. Particular attention has been paid to the examination of the urinary system and especially the kidneys. Suspected kidneys were examined on four times: shape, size, color and consistency. Thus, pathological features were noted. Gross pathology of the different viscera allowed us to establish a first clinical suspicion, which offered a better orientation for the interpretation of the histological lesions.
The samples concerned the kidneys with lesions, and sometimes-other affected tissues (liver, bursa of Fabricius). The removed organs were immersed directly, after examination, in 10% formalin solution before sending them to the laboratory of the histopathology of the National Veterinary Medicine School.
Histopathological Analysis
All organs were identified. Representative pieces of 5 mm thickness tissues specimens from each organs were collected in 10% formalin for a better fixation. After suitable fixation, tissues were trimmed to 2 mm thickness, deposited in cassettes and placed in programmable robot, where sections dehydrated, by successive alcohol increasing concentration baths, impregned with toluene and embedded with paraffin. After this step, sections were placed in molts, embedded in paraffin wax to prepare paraffin blocks (refrigeration during 24 hours). Theses blocks were cut at 5-micrometer thickness with a hand-operated microtome. Sections were passed through descending grades of alcohol, dried at 40 °C and then stained by routine hematoxylin-eosin stain. The hematoxylin stains nuclear or basophilic structures in purplish blue. However, eosin gives a red or a pink coloration to the cells cytoplasm.
Statistical Analysis
Statistical analysis was performed by using the software package SPSS version 16.0 for Windows. The differences among variables were evaluated by chi-square test. A P value <0.05 was considered statistically significant.
Results and discussion
Prevalence and Gross Pathology
The overall prevalence of nephropathies in our study was 17.56% (154 birds). Phalen et al. (1990) founded higher prevalence in their works, with 30% of 605 examined animals.
Our study showed that renal affections was observed in animals from free range flocks (79 cases; 16.40%) and animals collected from commercial flocks (75 cases; 19%) (χ2=0.012%; p>0.05).
Current investigation showed no statistically significant difference between male and female. Renal lesions were described in 20.40% and 16.75%, respectively. However, a significant higher prevalence was determined in >18 weeks group (20.40%), compared to young birds (<18 weeks) (15.73%) (χ2=3.158; p<0.05).
Prevalence of kidney lesions is influenced by the type of chickens, as described in Table 1. Significantly higher (χ2=7.839; p < 0.05) prevalence of renal lesions was observed in broilers and layer, with the same rate (20.40%), than in pullets (13%).
Different types of lesions were described in the current study as indicated in Table 2. Vascular lesions predominated (81.82%), indicating the acute evolution of the kidney damage, often observed in septicemia. The mainly vascular lesions observed was congestion (76.62%), followed by hemorrhage (5.20%).
Table 1: Prevalence of renal lesions in free-range and commercial fowl flocks according to the type of production and the age of animals
| Free range flocks (n=482) | Commercial flocks (n=395) | Young | Adult | Total | |||||
| Broiler | Pullet | Layer | Broiler | Pullet | Layer | ||||
| Total of examined animals | 68 | 285 | 129 | 128 | 53 | 214 | 534 | 343 | 877 |
|
Number of positive (%) |
14 (20.6%) | 34 (11.93%) | 31 (24.03%) | 26 (20.31%) | 10 (18.87%) | 39 (18.22%) | 84 (15.73%) | 70 (20.40%) | 154 (17.56%) |
|
Χ2 (p) |
Χ2=10.507 (p=0,001) |
X2=0.227 (p=0.5) | X2=3.158 (p=0.05) | ||||||
Table 2: Renal lesions observed at post-mortem examination
| Hypertrophy | Atrophy | Congestion | Hemorrhage | Urate deposition | Tumoral infiltration | Steatosis | Acute Interstitial nephritis | |
| Number of animals | 92 | 14 | 118 | 8 | 26 | 8 | 19 | 58 |
| (%) | 59.74% | 9.1% | 76.62% | 5.20% | 16.88% | 5.20% | 12.34% | 37.37% |
While, cellular lesions were described in 66.88% of cases. Acute interstitial nephritis represented the most frequent lesion (37.37%), followed by urate deposition (16.88%) and renal steatosis (12.34%). Acute interstitial nephritis is usually associated to enteritis, where bacterial toxins can reach the liver and the kidneys, causing degenerative lesions of the parenchyma.
Kidney hypertrophy was a predominant lesion in our study (59.74%). Several infectious and non-infectious etiologies can explain it, including infectious pathogens and metabolic disorders. While, atrophy (9.1%) was rarely described in the current investigation. It is often the consequence of the uni-lateral compression of kidney following urolithiasis or chronic salpingitis.
Tumor infiltration was showed in 5.20% of cases. These lesions were described as nodular or diffuse infiltration. Kidneys are deformed, hypertrophied with an irregular surface. The renal parenchyma was of a completely different appearance.
Lesions mentioned previously were associated to many clinical suspicions (Table 3). Viral diseases (47.6%) predominate suspicions, followed by metabolic disorders (29.21%) and bacterial infections (23.38%). Among these clinical suspicions, infectious bursal disease (20.78%), infectious bronchitis (13.64%), colibacillosis, visceral gout (16.88%) and steatosis (12.33%) are the most commonly described.
Lesions observed in kidney of animals suspected of infectious bursal disease (IBD) (20.78%) were nonspecific.
Table 3: Repartition of clinical suspicion according to the type of production
| Broiler | Pullet | Layer | Total (%) | |
| Infectious bronchitis | 6 | 3 | 12 | 21 (13.64%) |
| Newcastle disease | 3 | 4 | 5 | 12 (7.79%) |
| Infectious bursal disease | 17 | 15 | 0 | 32 (20.78%) |
| Colibacillosis | 7 | 4 | 9 | 20 (13%) |
| Salmonellosis | 6 | 1 | 9 | 16 (10.38%) |
| Marek’s disease | 0 | 2 | 6 | 8 (5.20%) |
| Visceral gout | 1 | 14 | 11 | 26 (16.88%) |
| Steatosis | 0 | 1 | 18 | 19 (12.33%) |
| Total (%) | 40 (25.97%) | 44 (28.57%) | 70 (45.46%) |
154 (100%) |
In agreement with the results of Gürel et al. (2003), nephritis with hypertrophy and congestion were the main ascertainment. Lesions that are more specific were described in bursa of Fabricius, where we noted hypertrophy, edema and hemorrhagic mucosa. Indeed, bursa of Fabricius is the principal site of virus replication. Intramuscular hemorrhage were also detected in all infected animals. Kidney damage can be explained by the accumulation of the immune complex in the endothelial cells of the renal glomeruli. The renal damage are considered as the consequence of the diarrhea in IBD.
Despite vaccination, infectious bursal disease is still suspected in Tunisian poultry flocks. The luck of immunization, especially in free-range flocks, the negligence of good vaccination practices and the emergence of hyper-virulent strains in Tunisia since 2004 can be considered as a risk factors.
In agreement with the findings of many authors (Cavanagh et Gelb, 2008; Bijanzad et al., 2013; Fellahi et al., 2014; Gola et al., 2017), renal lesions noted in birds suspected of infectious bronchitis (IB) (13.64%) were nonspecific with hypertrophy, hyperemia and urates deposition in ureters. The possible replication of certain infectious bronchitis virus strains in kidneys can explain the described lesions. Evolution of infectious bronchitis in vaccinated flocks could be explained by the emergence of variant strains, the bad quality of vaccines administration. Indeed, in little commercial poultry flocks and free-range flocks, farmers promote vaccination via drinking water, considered as ineffective way for immunizing birds against respiratory diseases from the first days of age, compared to spray vaccination.
Newcastle disease (ND) was suspected in our study in 12 birds (7.79%). Necropsy investigation showed congestive and hemorrhagic lesions in different tissues such as digestive tract, trachea and lungs. In agreement with the results of Balachandran et al. (2014), renal lesions were nonspecific with congestion and secondary hemorrhagic foci.
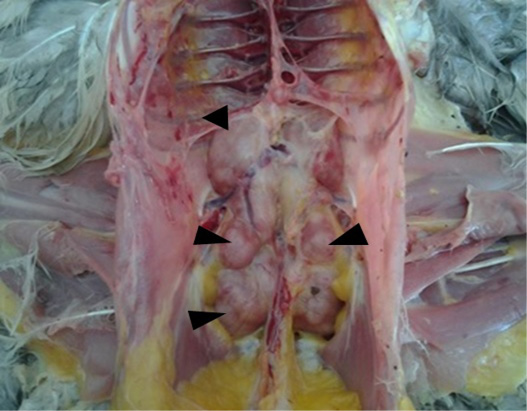
The tumor infiltrations illustrated in the current study were associated to Marek’s disease (MD) (5.20%), as the only neoplasia disease suspected in our study. Gross pathology exam showed enlarged and pale kidneys with multiple white nodules of different size deforming the surface of the organ (Figure 1). The same lesions were noted in other viscera, as the liver, the proventriculus, the lungs, the heart and the spleen. Our results are in line with Souissi (2003) and Mkadem (2018) in Tunisia, and Zeghdoudi et al. (2013) in Algeria and Poonam et al. (2017).
In bacterial infections, such as colibacillosis and salmonellosis, renal lesions were usually the consequence of septicemia. However, during a respiratory or a genital colibacillosis, the kidneys can be affected by contiguity of the air sacs, particularly the posterior sacs, or the genital tract, respectively.
Principal modifications observed during these infections were predominated by an acute interstitial nephritis and congestion with sometimes an urates deposition in the ureters, consequence of lack of watering. Our findings are in agreement with Srinivasan et al. (2014). Bacterial analysis showed the presence of Escherichia coli and Salmonella spp in all samples of kidneys and livers collected from birds suspected of colibacillosis and salmonellosis, respectively. E. coli was the most isolated bacteria from kidneys of poultry in the work of Mohamed and Shehata (2009) (75% of 80 chickens) and Ameen et al. (2015) (89.69% of 97 broilers). Many other bacteria was detected by the previously authors in kidney of affected birds, such as Staphylococcus aureus, Klebsiella pneumoniae, Proteus mirabilis, Corynebacterium spp, Clostridium perfringens and Enterococcus faecalis.
Metabolic disorders were diagnosed in our study in 29.21% of animals. The urate deposition was observed in visceral gout and urolithiasis. It is the consequence of increased uricaemia. Several predisposing factors contribute to the development of these conditions affections in poultry. High level of diet proteins, water deprivation, low drinking water quality, large difference in ambient temperature and nephritis caused by many infectious agents (infectious bronchitis virus, infectious bursal disease virus, ...etc) are incriminated in the urate deposition. Moreover, the existence of a particular portal renal system in birds and the luck of uricase are considered as important predisposing factors to the gout in this animal species. Nephropathy was characterized by unilateral to bilateral enlargement and moderate to severe congestion of kidneys. The kidney lobes of the contralateral side were atrophied, especially the caudal lobes. Kidneys were recovered by urates, which in agreement with the results of Randall et al. (1977), Batchy (1992), Lakkawar et al. (2018) and Sathiyaseelan et al., 2018) (Figure 2).
Renal steatosis is the consequence of fatty acid accumulation. It is usually associated to liver steatosis. This affection was described in good health animals. Steatosis of kidney was associated in our study to hemorrhage in four layer hens. Steatosis is favored by several factors, related to high energy rate and errors of rationing, high temperature and hormonal imbalance in hens.
Histopathology Study
Histologic analysis allow a better diagnosis orientation of some affections. The current investigations showed nonspecific lesions in kidneys associated to infectious disease,
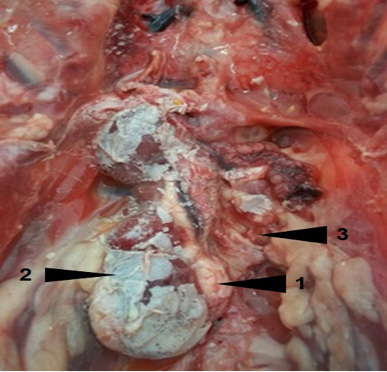
Figure 2: Visceral gout and urolithiasis (1) in a layer. Note hypertrophy (2) of the right kidney and the spectacular atrophy of the left kidney (3)
excepted of MD, as tumor pathology.
Acute and subacute interstitial nephritis, associated sometimes to necrosis foci and lymphocytic infiltration were described in IBD, IB (Figure 3), colibacillosis and salmonellosis (Figure 4). Our findings are in line with several researchers (Fakhrul Islam et al., 2006; de Dutta et al. 2013; Abalaka et al., 2017).
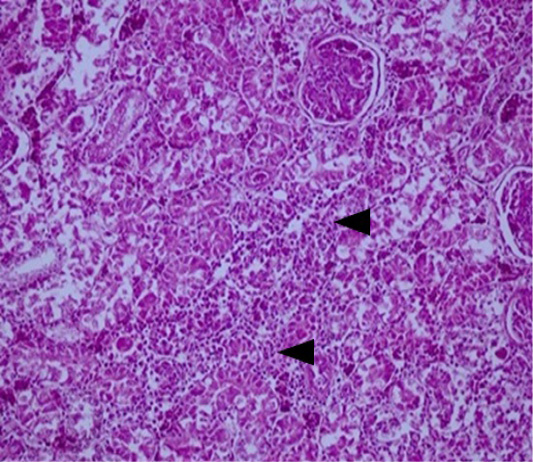
Figure 3: Kidney: acute interstitial nephritis in a layer suspected of IB. Note the marked infiltration by mononuclear cells in the interstitial space of renal tubules (arrows) (HEX200).
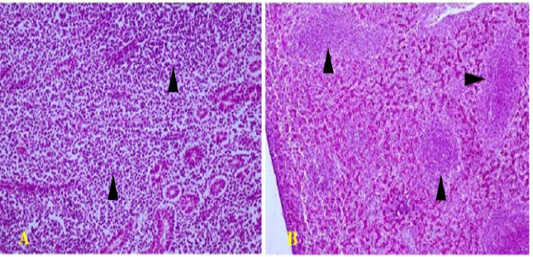
Figure 4: Polymorphic lympho-plasmocytic tumor infiltration in the kidney (arrows) (A; HEX200) and the liver (arrows) (B; HEX100)
Lesions showed in kidneys of Newcastle disease suspected animals were congestion, hemorrhagic and inflammatory cells infiltration. Renal necrosis lesions are also described by several authors (El Bahrawy et al., 2017; El-Mandrawy and Ismail, 2017; Etriwati et al., 2017).
Microscopic lesions were more specific in IBD, particularly in bursa of Fabricius. The type and the severity of lesions could be useful to determine the evolution form of the disease. The most important lesions observed in the bursa were a lymphoid depletion, vacuolation, necrosis and heterophilic and hemorrhagic infiltration. While, renal lesions were nonspecific. In this way, acute interstitial nephritis and necrosis foci were the main modifications, as reported by Okoye (1985) and Gürel et al. (2003).
Histopathological analysis was very important in the diagnosis of MD. Kidneys showed a polymorphic lymphoid and plasmocytic infiltrates (Figure 4A). The same lesions were also noted in other organs, as the liver (Figure 4B), the spleen and the proventiculus. Tumor infiltrates are very frequent in kidneys during visceral form of MD, which is in perfect agreement with the findings of several authors (Fujimoto et al., 1971; Poonam et al., 2017; Mkadem, 2018).
In bacterial infections (colibacillosis, salmonellosis), histopathological study showed the same microscopic renal lesions mentioned previously. Degenerative lesions, lymphocytic infiltration and necrosis of the kidneys was described in birds infected especially with nephrogenic bacterial strains (Figure 5). Our findings are in agreement with their of several researchers (Fakhrul Islam et al., 2006; Chousalkar et al., 2007; Bijanzad et al., 2013; Dutta et al., 2013; Abalak et al., 2017; Gola et al., 2017). However, confirmation of bacterial infection needs bacteriological investigations.
Conclusion
In conclusion, our findings in this first report showed that renal lesions occurred in free range and commercial poultry, with vascular and cellular lesions. Kidney damages in poultry can be primary or secondary to other diseases, which can lead to death of the animal.
Necropsy examination revealed several aspects with congestion, acute interstitial nephritis, urates deposition and tumor infiltration. Gross pathology study was in general sufficient to the diagnosis of metabolic disorders (visceral gout, urolithiasis and steatosis) and Marek’s disease, where lesions are evocative. In another hand, necropsy examination showed nonspecific lesions, especially in infectious diseases.
Histopathological study promote more diagnosis orientation. Nevertheless, histology did not allow confirmation of clinical suspicions because of nonspecific lesions observed in the kidneys. However, microscopic features of other organs provided better guidance, particularly in the case of IBD and MD. Similarly, the use of bacteriological tests is a considerable contribution to the diagnosis of bacterial infections.
Conflict of interest
Authors declare no conflicts of interests.
authors contribution
Khaled Kaboudi: sampling, necropsy, interpretation of results, statistical study, manuscript writing.
Abdelkader Amara: histopathological study, interpretation of results.
Asma Yamoun: sampling, necropsy, interpretation of results.
References