Advances in Animal and Veterinary Sciences
Research Article
Antibiotic Sensitivity Patterns of Methicillin-Resistant Staphylococcus Aureus Isolated from Chickens in Poultry Farms in Sokoto, Nigeria
Iliya D. Kwoji*1, Farouk M. Tambuwal2, Mikaeel B. Abubakar2, Yusuf Yakubu3, Jasini A. Musa1, Solomon Jauro1, Asinamai A. Bitrus4
1Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Maiduguri, P.M.B 1069, Borno State, Nigeria; 2Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Sokoto State, Nigeria; 3Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Sokoto State, Nigeria; 4Department of Microbiology and Pathology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor Malaysia.
Abstract | Staphylococcus aureus is an important pathogen associated with food poisoning and several forms of diseases in both man and animals. In poultry, the organism is incriminated in multiple infections and syndromes such as omphalitis, femoral head necrosis, tenosynovitis and bumble foot. The treatment of staphylococcal infections is becoming more challenging due to the emergence of antibiotic resistant strains such as methicillin resistant Staphylococcus aureus (MRSA) that are currently resistant to all β-lactam antibiotics (penicillins and cephalosporins). In order to understand the spectrum of MRSA in poultry, a total of 12 MRSA isolates positive for the presence of penicillin binding protein 2α (PBP2α) were tested for antibiotic resistance against 10 antibiotics using disc diffusion method. Isolates were found to be completely resistant (100%) against ceftazidime, followed by erythromycin and ofloxacin (91.67%), cefuroxime, cloxacillin, and cefoxitin (83.33%), gentamicin (75.00%) and vancomycin (64.29%) while the least resistance was recorded against ceftriaxone and amoxicillin/clavulanate (58.33%). The MRSA isolates also exhibited multi-drug resistance pattern with all resisting not less than four antibiotics. These data indicate that MRSA are prevalent in the poultry and precautionary measures are required to block their transmission to human.
Keywords | Antibiotics, Chickens, Methicillin resistant Staphylococcus aureus, Sokoto, Nigeria
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 31, 2017; Accepted | September 10, 2017; Published | December 09, 2017
*Correspondence | Iliya D Kwoji, Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Maiduguri, P.M.B 1069, Borno State, Nigeria; Email: [email protected]
Citation | Kwoji ID, Tambuwal FM, Abubakar MB, Yakubu Y, Musa JA, Jauro S, Bitrus AA (2018). Antibiotic sensitivity patterns of methicillin-resistant staphylococcus aureus isolated from chickens in poultry farms in sokoto, nigeria. Adv. Anim. Vet. Sci. 6(1): 8-11.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.1.8.11
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Kwoji et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In Nigeria, poultry production constitutes a very important national resource and contributes significantly to the economic growth and provision of animal protein for the populace (Oladeebo and Ambe-Lamidi, 2007). However, the development and viability of the industry is threatened by disease scourge, which could be due to poor management practices. Staphylococci are opportunistic pathogens and forms part of normal commensal flora of skin and mucous membranes of animals (Quinn and Markey, 2003). They are among the most frequent causes of clinical infections worldwide and have attracted substantial attention due to increasing mortality and morbidity associated with antimicrobial resistance (Waters et al., 2011). Staphylococci are resistant to beta-lactam antibiotics due to the production of beta-lactamases (Livermore and Brown, 2001; Quinn and Markey, 2003). The use of beta-lactamase resistant cephalosporins (such as methicillin or oxacillin) as a remedy for beta-lactam resistance in staphylococci was shortlived when these organisms also became resistant to the betalactamase-resistant penicillins and cephalosporins (Wulf and Voss, 2008). The importance of methicillin resistant S. aureus to public health is due to the ability of the organism to acquire resistance and virulence genes thus, leading to the emergence of new and highly virulent clones posing great difficulty in antibiotics chemotherapy thereby prolonging hospital admission stay (Liu, 2009). Different antimicrobial substances are used extensively at sub-therapeutic or therapeutic doses as growth promoters, routine prevention and treatment of bacterial diseases in food-animal (Gilchrist et al., 2007; Waters et al., 2011). This practice is usually common in developing countries such as Nigeria, where there are poor legislations regulating the use of antimicrobials in food-producing animals (Bitrus et al., 2015: Ugwu et al., 2015). Therefore this study was conducted to assess the antibiotic sensitivity pattern of MRSA to commonly used antibiotics in the study area.
MATERIALS AND METHOD
Isolation and Identification of MRSA
This was carried out by sub-culturing pure S. aureus culture preserved on nutrient agar onto oxacillin resistant screening agar base (ORSAB) medium (Oxoid), and incubated at 370C for 20 hours. The production of deep blue colonies indicates mannitol fermentation by isolates that are resistant to Oxacillin (or methicillin) (Simor et al., 2001). The isolates where confirmed for the presence of penicillin binding protein 2α (PBP2α) using latex slide agglutination test which works based on agglutination of latex particles sensitized with monoclonal antibodies against PBP2α in accordance with the manufacturer’s protocol (Oxoid) as described by Felten et al. (2002). Clumping of latex particles by the S. aureus that showed deep–blue colonies on ORSAB confirms the presence of PBP2α.
Antibiotic Susceptibility Assessment
This was performed using Kirby Bauer’s disc diffusion method. Fresh (24 hours broth-culture) cultures of the MRSA isolates were sub-cultured using sterile swab sticks onto prepared Mueller-Hinton agar. With the aid of a sterile Thumb forceps, the antibiotic sensitivity discs (Rapid labs®, UK; Oxoid, UK.) were placed at the center of the inoculated media and allowed to stay for 30 minutes for pre-diffusion of the antibiotics. These were then incubated at 35oC for 24 hours. The diameter of inhibition zones were measured and noted using transparent plastic ruler and interpreted according to the guidelines of Clinical and Laboratory Standards Institute, (CLSI, 2013). MRSA isolates were tested for susceptibility against ceftazidime (CAZ 30 µg), cefuroxime (CRX 30 µg), gentamicin (GEN 10 µg), ceftriaxone (CTR 30 µg), erythromycin (ERY 5 µg), cloxacillin (CXC 5 µg), ofloxacin (OFL 5 µg), amoxicillin/clavulanate (AUG 30 µg), cefoxitin (FOX 30 µg) and vancomycin (Van 30 µg).
RESULTS
Figure 1 displayed the antibiotic sensitivity pattern of MRSA isolates from chickens in Sokoto and its environs. The highest resistance was recorded against ceftazidime 100% (12/12) followed by ofloxacin and erythromycin with 91.67% (11/12) each. Cefuroxime, cloxacillin and cefoxitin have the same level of resistance of 83.33% (10/12). The least resistance was recorded against amoxicillin/clavulanate and ceftriaxone of 58.33% (7/12) respectively. The highest Susceptibility among the MRSA isolates to antibiotics was recorded against amoxicillin/clavulanate and ceftriaxone of 41.67% (6/14).
The antibiotic resistant patterns of the MRSA isolates from chickens in Sokoto and its environs revealed resistance to at-least four antibiotics. Four (4) isolates resisted all the 10 antibiotics tested, while only one isolate resisted four antibiotics (Table 1).
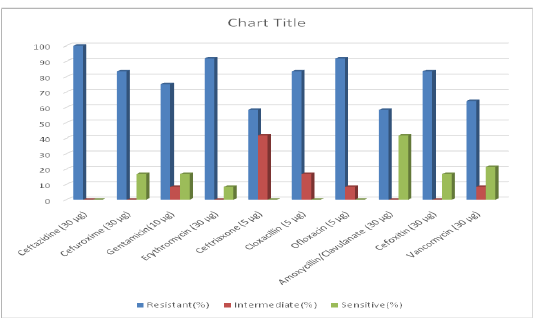
Figure 1: Chart showing antibiotic sensitivity pattern of MRSA isolated from chickens in Sokoto and environs
Table 1: Antibiotic resistant pattern for MRSA isolates from chickens in Sokoto and its environs
| Multi-drug resistant pattern | Number of isolates |
| CAZ-CRX- ERY- OFL | 1(8.33) |
| CAZ-GEN-ERY-CXC-OFL | 1(8.33) |
| CAZ- CRX- CTR- FOX- VAN | 1(8.33) |
| CAZ-GEN-ERY-OFL-CXC-FOX | 1(8.33) |
| CAZ-CRX-GEN-ERY-OFL-CXC-AUG | 1(8.33) |
| CAZ-CRX-GEN-CTR-ERY-OFL-CXC-VAN | 1(8.33) |
| CAZ-CRX-ERY-OFL-CXC-AUG-FOX-VAN | 1(8.33) |
| CAZ-CRX-GEN-CTR-ERY-OFL-CXC-AUG-VAN | 1(8.33) |
| CAZ-CRX-GEN-CTR-ERY-OFL-CXC-AUG-FOX-VAN | 4(33.33) |
Key: CAZ- Ceftazidime, CRX-Cefuroxime, GEN-Gentamicin, CTR-Ceftriaxone, CXC-Cloxacillin, OFL-Ofloxacin, AUG-Amoxycillin/Clavulanate, FOX-Cefoxitin, VAN-Vancomycin.
DISCUSSION
The high usage of antibiotics in poultry industries in Nigeria has facilitated the acquisition of resistance genes by S. aureus, thereby making treatment of infections caused by these organisms more difficult (Otalu et al., 2011; Suleiman et al., 2013). The MRSA isolates from chickens used in this study showed varying patterns of susceptibilities to antibiotics. The isolates showed highest resistance to ceftazidine (100%) followed by erythromycin (91.67%), ofloxacin (91.67%), cefoxitin (83.33%), cefuroxime (83.33%), cloxacillin (83.33%) and gentamicin (75%). The high resistance against erythromycin (91.67%) recorded in this study is in agreement with that of Suleiman et al. (2013) and Geidam et al. (2012b) who reported 100% and 85%, respectively. However, this is in contrast to that of Nworie et al. (2013) who reported 65% resistance of MRSA against erythromycin. The high resistance to erythromycin (91.67%) in this work could be linked to high usage of the antibiotic by farmers in the study area for the treatment of respiratory tract infections in chickens. This study also reveal a high resistance against vancomycin of 64.29% which disagrees with the findings of Nworie et al. (2013), Fridkin et al. (2005) and Anupurba et al. (2003) who reported 0% resistance against vancomycin. However, since vancomycin is not a commonly used antibiotic for routine chemotherapy in veterinary practice, the variations may not be due to its abuse in poultry. Therefore, the high vancomycin resistance recorded might be as a result of emergence of vancomycin resistance strains or the transmission of these bacteria by infected personnel to chickens in the study area which calls for a serious concern since vancomycin is currently one of the drugs of choice for the treatment of unresponsive infections caused by S. aureus. A high resistance against ofloxacin (91.67%) and gentamicin (75%) were also recorded in this study which was higher than the reports of Suleiman et al. (2013) who reported 57.4% and 0% resistance against ofloxacin and gentamicin respectively, in Maiduguri. According to Suleiman et al. (2013), sub-therapeutic administration of antibiotics as prophylactics, growth promoters or inaccurate dosages given to sick flocks by unqualified personnel may likely result in plasma concentrations that are inconsistent with the desired objectives which may result to resistance development as is the case in the present study.
All of the MRSA isolates were resistant to at least four of the antibiotics tested. This finding may be a picture of the extent of poor drug control and legislations concerning sales and administration of antimicrobials in Nigeria as posited by Geidam et al. (2012a). Also, MRSA with multi-drug resistance has been reported in poultry farms in China with prevalence that varied from farm to farm in relation to severity of application of antibiotics (Liu et al., 2009). The antibiotic susceptibility pattern of MRSA as revealed by the present study indicates serious challenge for choice of antibiotics for treatment of infections that may be due to these pathogens since all the isolates are resistance to majority of the antibiotics in high proportions. Therefore, this work recommends on the need for strong legislation and enforcement of laws that will regulate the prescription, dispensation and administration of drugs to food producing animals especially in poultry production.
ACKNOWLEDGEMENT
The authors duly acknowledge the effort of all the member of staff of Veterinary microbiology laboratory, Usmanu Danfodiyo University Sokoto for their contribution towards the success of this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTIONS
The design and execution of this research study is a collective effort of all the authors. All authors were also involved in the critical analysis and review of the manuscript.
REFERENCES






