Advances in Animal and Veterinary Sciences
Research Article
Effects of Ethanolic Extract of the Leaves of Pongamia glabra and Gliricidia sepium against Rhipicephalus (Boophilus) annulatus
Reghu Ravindran1*, Sanis Juliet2, Sunil Athalathil Ramankutty1, Nanjundappa Sathish2, Suresh Narayanan Nair2, Karaparambu Gopalan Ajithkumar1, Leena Chandrasekhar3, Srikant Ghosh4
1Department of Veterinary Parasitology; 2Department of Veterinary Pharmacology and Toxicology; 3Department of Veterinary Anatomy, College of Veterinary and Animal Sciences, Pookode, Lakkidi, P.O. Wayanad, Kerala-673576, India; 4Division of Parasitology, Indian Veterinary Research Institute, Izatnagar, UP-243122, India.
Abstract | The effects of ethanolic extracts of the leaves of Pongamia glabra and Gliricidia sepium against Rhipicephalus (Boophilus) annulatus were studied. Different dilutions of the extracts such as 50, 60, 70, 80, 90 and 100 mg/mL were tested using adult immersion test (AIT). The per cent adult mortality, inhibition of fecundity and hatching of laid ova were studied. Both extracts caused very low per cent adult tick mortality and inhibition of fecundity. Fifty per cent blocking of the hatching of laid ova in ticks treated with ethanolic extract of leaves of P. glabra was observed.
Keywords | Pongamia, Gliricidia, Rhipicephalus annulatus, Acaricidal
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 27, 2016; Accepted | January 01, 2017; Published | January 05, 2017
*Correspondence | Reghu Ravindran, Kerala Veterinary and Animal Sciences University, Pookode, Lakkidi, P.O. Wayanad, Wayanad, Kerala, India; Email: [email protected]
Citation | Ravindran R, Juliet S, Ramankutty SA, Sathish N, Nair SN, Ajithkumar KG, Chandrasekhar L, Ghosh S (2017). Effects of ethanolic extract of the leaves of Pongamia glabra and Gliricidia sepium against Rhipicephalus (Boophilus) annulatus. Adv. Anim. Vet. Sci. 5(1): 1-6.
DOI | http://dx.doi.org/10.14737/journal.aavs/2017/5.1.1.6
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Ravindran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Tick control throughout the world is based mainly on the use of chemical acaricides. Their indiscriminate and repeated use has already resulted in problems related to environmental pollution, milk/meat contamination and the development of resistance in target species along with a subsequent increase in cost (Dipeolu and Ndungu, 1991; Jonsson and Piper, 2007). The use of extracts from various plants is being explored as an alternative to chemical acaricides because they are potentially less toxic to the animals and safer for the environment.
Pongamia glabra. Linn. (Papilionaceae) (Synonym; leaf gall, Pongamia pinnata) which may also be called as Galdupa indica, is a large tree found in tropical regions and coastal forests of India, North Australia, Southeast Asia and Malaysia (Krishnamurthy, 1969; Satyavati et al., 1987). Antiinflammatory and antidiarrhoeal (Singh and Pandey, 1996; Singh et al., 1996; Srinivasan et al., 2001), antidiarrhoeal (Shoba and Thomas, 2001; Brijesh et al., 2006), antimalarial (Misra et al., 1991), antibacterial (Patel and Trivedi, 1962; Biswal et al., 2011), wound healing (Subramanian and Nagarajan, 1988), anticancer (Arulvasu et al., 2012), anthelmintic (Nirmal et al., 2007) and analgesic (Ganesh et al., 2008) activities of the plant were previously reported. Efficacy of oil of karanj against different mange conditions in animals (Chabbra et al., 1994; Das and Sreekrishanan, 1998; Kale and Panchegaonkar, 1969; Prajapatie and Hiregoudar, 1976) was previously reported. Bisen et al. (2011) observed 70 and 66.67 per cent efficacy for the karanj seed oil and leaf extract respectively against Rhipicephalus (Boophilus) microplus after 5 days of treatment.
Gliricidia sepium, is a leguminous tree belonging to the family Fabaceae is used as fuel wood, animal feed, green manure, shade, living fences and as a support plant (Csurhes and Edwards, 1998). Gliricidia, which literally means ‘rat poison’ originated in Central America and its plantations, has spread to many parts of the world specifically south Asia. The plant is used by the farmers to repel insects. Moreover, the antibacterial (Nazli et al., 2008) and anthelmintic (Adama et al., 2012) properties were also recorded. Ethanolic extract of leaves of G. sepium at 20 per cent concentration produced 100 per cent acaricidal effects against the mite Tetranychus cinnabarinus (Sivira et al., 2011). Soap made using G. sepium leaf extract (20 per cent) was effective in clearing mange conditions in dogs including demodicosis (Viste et al., 2013). Also, G. sepium extract produced 53.33 % mortality against Rhipicephalus (Boophilus) microplus (Rodriguez and Pulido, 2015).
R. (B.) annulatus, one of the most important bovine one host tick species (Onofree et al., 2001), serves as the vector for the agents of animal as well as some human diseases. R. (B.) annulatus is the major one host tick species of southern India (Jagannath et al., 1979; Rajamohanan, 1982; Koshy et al., 1982). Present study focuses mainly on the effect of the ethanolic extract of the leaves of P. glabra and G. sepium against adult female R. (B.) annulatus.
Material and Methods
Plant Material
The leaves of P. glabra and G. sepium were collected from Kalpetta, Wayanad district, Kerala. The plants were identified by a botanist and the voucher specimens were deposited in the herbarium of the Department of Botany, Calicut University, Kerala (Accesssion no; P. glabra- CALI 6628, G. sepium- CALI 6643).
Preparation of the Plant Extracts
The leaves were cleaned and dried in shade at room temperature. Dried plant leaves were finely pulverized using a grinder. The powdered plant material (100g) was used for ethanolic extraction in a soxhlet extraction apparatus attached with rotary vacuum evaporator (Butchi, Switzerland). Solvent was completely removed by drying at room temperature. Required quantity of the extracts was weighed and dissolved in 1 per cent Tween-20 or water for making six different dilutions at the rate of 50 mg/mL, 60 mg/mL, 70 mg/mL, 80 mg/mL, 90 mg/ mL and 100 mg/mL and were used for acaricidal bioassay.
Ticks
Fully engorged adult female R. (B.) annulatus were collected from infested animals, washed with water and dried using tissue paper. These ticks were used for adult immersion test (AIT).
Adult Immersion Test (AIT)
Various dilutions (50-100 mg/mL) of ethanolic extracts of the leaves of the plants were tested using adult immersion test (Drummond et al., 1973). A total of 336 numbers of ticks were used for the experiment. Four replicates of six ticks were used for each dilution of the extract. Group of six numbers of ticks were weighed prior to the experiment and they were immersed for 2 minutes in the respective dilution (10 mL) in a 50 mL beaker with gentle agitation. Tween 20 (0.1%) was used as control for P. glabra extract while water was used as control for G. sepium. Ticks were recovered from the solution, dried using tissue paper towels and placed in separate plastic specimen tube (25 X 50 mm). The tubes were incubated at 280 C and 80 % relative humidity in a BOD incubator.
Adult Tick Mortality
The specimen tubes were observed for the next 15 days for mortality and the per cent adult tick mortality was determined.
Inhibition of Fecundity and Hatching
The eggs laid by the ticks of each tube were collected, weighed and observed at the same condition of incubation for the next 30 days for visual estimation of hatching. Ticks under different treatments were compared with that of the controls.
The percentage inhibition of fecundity was calculated as follows (FAO, 2004):
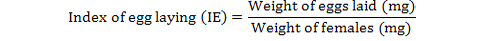

Statistical Analysis
Data were expressed as the mean + SEM. Groups were compared using one-way ANOVA for repeated measurements using SPSS software. Duncan’s test was used for post-hoc analysis. A value of P<0.05 was considered significant.
Results and Discussion
The results of adult immersion test using the ethanolic extracts of leaves of P. glabra and G. sepium are shown in Table 1 and 2. The efficacy of the extracts against adult female R. (B.) annulatus was assessed by measuring the per cent adult mortality, inhibition of fecundity and hatching rate.
Ethanolic extract of leaves of P. glabra produced adult tick mortality ranging from 4-16 per cent and inhibition of fecundity 4-15 per cent. Adult tick mortality was very low even at higher concentrations (80-100 mg/mL) tested. Ethanolic extract of P. glabra (50-100 mg/mL) considerably blocked the hatchability of the eggs in comparison to the control.
Table 1: Effect of ethanolic extract of leaves of P. glabra against R. (B.) annulatus
|
S No. |
Acaricide |
Mean ticks weight per replicate + SEM |
Mean % adult mortality within 15 days + SEM |
Mean egg mass per replicate + SEM |
Index of fecundity + SEM |
Percentage inhibition of fecundity (%) |
Hatching % (visual) |
|
1 |
0.1% tween20 (control) |
0.8993+0.018a |
0+0a |
0.4923+0.028a |
0.5478+0.031a |
0 |
100 |
|
2 |
50 mg/mL |
0.8890+0.051a |
0+0a |
0.4353+0.047a |
0.4862+0.028a |
11.24 |
50 |
|
3 |
60 mg/mL |
0.9430+0.043a |
0+0a |
0.4383+0.039a |
0.4620+0.021a |
15.66 |
50 |
|
4 |
70 mg/mL |
0.9073+0.006a |
0+0a |
0.4757+0.007a |
0.5243+0.005a |
4.29 |
50 |
|
5 |
80 mg/mL |
0.9643+0.030a |
4.165+4.165ab |
0.4588+0.026a |
0.4751+0.017a |
13.27 |
50 |
|
6 |
90 mg/mL |
0.9703+0.047a |
8.33+8.3ab |
0.4580+0.036a |
0.4779+0.050a |
12.76 |
50 |
|
7 |
100 mg/mL |
0.9325+0.023a |
16.66+6.80b |
0.4438+0.026a |
0.4747+0.018a |
13.34 |
50 |
n: 4; Values are Mean + SEM, a,b(P<0.05): significant difference when compared with the control
Table 2: Effect of ethanolic extract of leaves of G. sepium against R. (B.) annulatus
|
S No. |
Acaricide |
Mean ticks weight per replicate + SEM |
Mean % adult mortality within 15 days + SEM |
Mean egg mass per replicate + SEM |
Index of fecundity + SEM |
Percentage inhibition of fecundity (%) |
Hatching % (visual) |
|
1 |
Water (control) |
0.8663+0.101bc |
0+0a |
0.4433+0.072b |
0.5034+0.027a |
0 |
100 |
|
2 |
50 mg/mL |
0.8663+0.044bc |
0+0a |
0.4275+0.021b |
0.4968+0.021a |
1.31 |
100 |
|
3 |
60 mg/mL |
0.6053+0.038a |
4.165+4.16a |
0.2998+0.041a |
0.4891+0.044a |
2.84 |
100 |
|
4 |
70 mg/mL |
0.7565+0.034b |
4.165+4.16a |
0.3593+0.020ab |
0.4743+0.006a |
5.78 |
100 |
|
5 |
80 mg/mL |
0.9040+0.049bc |
12.49+7.97a |
0.4220+0.035b |
0.4649+0.020a |
7.65 |
100 |
|
6 |
90 mg/mL |
0.9523+0.023c |
12.49+4.16a |
0.4333+0.009b |
0.4565+0.020a |
9.32 |
100 |
|
7 |
100 mg/mL |
0.9455+0.019c |
12.49+4.16a |
0.4120+0.022ab |
0.4349+0.014a |
13.61 |
100 |
n: 4; Values are Mean +SEM; a, b, c(P<0.05): Significant difference when compared with the control
Ethanolic extract of G. sepium also produced adult tick mortality in the range of 4-12 per cent. The inhibition of fecundity was dose dependent ranging from 1.31 to 13.61 per cent. The extract did not affect the hatching of eggs laid by treated females.
The toxic phytochemicals that primarily constitute the secondary metabolites affect the nerve axon and synapses, muscles, respiration and behaviour of the insects (Klocke, 1989). In fact, some of these phytochemicals were used to develop commercial insecticides and could serve as models for new insect control agents (Balandrin et al., 1985).
Phytoconstituents of P. glabra include furanoflavonols, chromenoflavones, flavones, furanodiketones and flavonoid glucosides (Pathak et al., 1983; Ahemed et al., 2004; Yin et al., 2006). Karanjin, β-sitosterol, pongamol, pongaglabrone, pongapin, kangone, glabrachromene, pongaflavone, pongol, glabrachrome-II and glabrachalcone were previously isolated from seeds of P. glabra. Kaempferol, β-sitosterol and pongaglabol were isolated from flowers while demethoxy kanugin, pongachromene were isolated from stem bark of the same plant (Nirmal et al., 2007). Furanoflavonoids (pongapinnol A–D, coumestan, pongacoumestan), flavonoids (pongamones A–E), sterols and fattly acids were also reported from P. pinnata (Shameel et al., 1996; Yadav et al., 2004; Li et al., 2006). The phytoconstituents of the G. sepium bark and leaves include flavonoids (Manners and Jurd, 1979), triterpenoid saponins (Rastrelli et al., 1999a, b), stigmastanol glucoside (Herath and de-Silva, 2000), rhamnogalactoside of kaempferol (Rangaswami and Iyer, 1966), coumarin, coumaric acid and melilotic acid (Griffiths, 1962).
Both extracts tested in the present study were previously reported for their larvicidal effects against mosquitoe vectors (Shanmugasundaram et al., 2008; Sharma et al., 1998). Deltamethrin, the common synthetic pyrethoid compound used in field conditions for cattle tick control, produced a mean adult mortality of 16.662 ±6.803, inhibition of fecundity of 57.3 per cent and hatching of 0-10 per cent (Sunil et al., 2013; Divya et al., 2014) at 30 ppm concentration. Similarly, cypermethrin at 200 ppm concentration also caused mortality of 45.83 per cent and inhibition of fecundity of 86.99 per cent, while the drug did not affect hatching of ova laid by treated ticks (Ravindran et al., 2014).
In the present study, the crude ethanolic extracts of the leaves of the P. glabra and G. sepium did not cause considerable adult mortality or inhibition of egg laying capacity of the treated ticks. The extract significantly inhibited the hatching of the laid ova by treated ticks. Our observation is contrary to that of Bisen et al. (2011) who revealed that the leaf extract of P. glabra produced a 66.67 per cent efficacy against R. (B.) microplus. However, seed oil of P. glabra was widely reported as acaricidal agent especially against mites (Chhabra et al., 1994; Das and Sreekrishanan, 1998; Kale and Panchegaonkar, 1969; Prajapatie and Hiregoudar, 1976; Ravindran and Subramanian, 2000, 2002). Inhibition of fecundity of karanj oil against R. (B.) microplus was previously recorded (Bisen et al., 2011).
Leaf extract of G. sepium was reported as acaricidal against plant mite Tetranychus cinnabarinus (Sivira et al., 2011) and demodicosis in dogs (Viste et al., 2013). Moreover, the plant extract revealed activity against Rhipicephalus (Boophilus) microplus (Rodriguez and Pulido, 2015) also. However, in the present study we could not observe any acaricidal effects for this extract. We presume that the insect repellant activity of G. sepium leaves when used freshly as paste may be due to the highly volatile compounds present in it which might have lost during shade drying and ethanolic extraction.
In the present study, it was observed that ethanolic extract of leaves of P. glabra significantly inhibited the hatching of the laid ova by treated ticks. Previously, similar hatching blocking effect was observed with two other extracts viz., Leucas aspera (Ravindran et al., 2011) and Jatropha curcas (Juliet et al., 2012). However, the phytochemicals responsible for this activity could not be ascertained.
Conclusion
Ethanolic extract of the leaves of Gliricidia sepium did not reveal any acaricidal effect useful for control of Rhipicephalus (Boophilus) annulatus. However, ethanolic extract of the leaves of P. glabra significantly inhibited the hatching of the laid ova by treated ticks.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this research paper.
Acknowledgements
Financial supports from Indian Council of Agricultural Research (NAIP/Comp-4/C2066/2007-08, NFBSFARA/BSA-4004/2013-14, 7(2)/2011 EPD) and Kerala State Council of Science and Technology and Environment (020/SRSAGR/2006/CSTE, 010-14/SARD/13/CSTE) are thankfully acknowledged.
Authors’ Contributions
All authors contributed equally.
References






