Advances in Animal and Veterinary Sciences
Research Article
Activity of Isolated Staphylococcal Bacteriophage in Treatment of Experimentally Induced Chronic Osteomyelitis in Rabbits
Orooba Mohammed Saeed Ibrahim1, Sarhan Rashid Sarhan1*, Serwa Ibrahim Salih2
1Department of Physiology and Pharmacology; 2Department of Surgery, College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | The central goal of this research was to explore the use of Staphylococcus aureus specific-bacteriophage as an alternative to antibiotics in treatment of chronic osteomyelitis in rabbits by inoculation of methicillin resistant Staphylococcus aureus (MRSA) in tibia. The current study included isolation of bacteriophage from swage water by using agar overlay method. While the second experiment was evaluation of phage activity in treatment of chronic osteomyelitis in rabbits in comparison with ceftaroline. Results of our research showed that all animals of infected groups with MRSA before treatment exhibited clinical signs of osteomyelitis after 21 days of inducing infection. Group C animals that dosed with 3 × 108 pfu/ml of Staph-specific bacteriophage for two weeks intramuscularly showed faster recovery than group D that treated with 40 mg/ kg B.W of ceftaroline for two weeks intramuscularly. We came in concluded that a bacteriophage was a good choice for treatment of a multiple drug resistant pathogen such as Staphylococcus aureus in shorter time with minimal side effects.
Keywords | MRSA, Bacteriophage, Osteomyelitis, Ceftaroline
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 21, 2016; Accepted | November 01, 2016; Published | November 22, 2016
*Correspondence | Sarhan Rashid Sarhan, Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq. Email: [email protected]
Citation | Ibrahim OMS, Sarhan SR, Salih SI (2016). Activity of isolated Staphylococcal bacteriophage in treatment of experimentally induced chronic osteomyelitis in rabbits. Adv. Anim. Vet. Sci. 4(11): 593-603.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.11.593.603
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Ibrahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Osteomyelitis is illustrated as the infection of medullary tissue, periosteum, and cortical bone (Rabillard et al., 2011). It is an inflammatory process combined by bone damage and brought on by a infecting microorganism, the infection can be constrained to a single portion of the bone or can include many areas, for example, marrow, cortex, periosteum, and the encompassing soft tissue. Inflammatory responses to the attacking pathogen make an exudate that gathers locally and packs veins, prompting osteonecrosis (Zuluaga et al., 2006). Osteomyelitis is for the most part categorized as ‘acute or chronic’ and ‘haematogenous or contigouos’ based on site, source and duration of infection and patient health status and more specifically to histopathological and radiographical finding. With an incident range of 6 % of people suffering from bone diseases are associated with a death rate of 2-10%, and a danger of losing of joint capability of 40% (Mathews et al., 2010; Garcia-Arias et al., 2011).
Staphylococcus aureus is the most commonly isolated pathogen for both acute and chronic osteomyelitis in all age groups (Jorge et al., 2010; Eid and Berbari, 2012). Lately there has been an increase in methicillin-resistant S. aureus (MRSA) due to rapid development of antimicrobial resistance and expression of virulence factors, regardless of the patient´s immune status. Coagulase-negative Staphylococci are often seen in association with foreign bodies, such as prosthetic joints (Chihara and Segreti, 2010).
Animals such as dogs, cats and horses have become an important part of most families particularly in developed countries like USA and UK (Chomel and Sun, 2011). In the UK, 1.5% of MRSA were recovered from samples of infected companion animals (Rich and Roberts, 2004) and dogs are more infected / colonized with MRSA in comparison to cats (Morgan, 2008). Reports of MRSA colonization in horses with a percentage rate of 0 to 11% has been published (Loeffler et al., 2011). Most cases and outbreak of MRSA infections were reported in large stables and post-operative complications (Weese et al., 2005; Morgan, 2008).
Most MRSA strains are frequently resistant to most common antibiotics including tetracycline, aminoglycosides, macrolides, chloramphenicol and fluoroquinolones (Lee, 2003; Middleton et al., 2005; CDC, 2006). Antibiotics used to treat serious, multiple drug resistant MRSA infections include vancomycin, as well as newer drugs such as linezolid, tigecycline, quinupristin/dalfopristin and daptomycin (Lee, 2003; Gorwitz et al., 2006; Boucher et al., 2010; Catry et al., 2010). The indiscriminate exposure of humans and animals to antibiotics created problem through acquisition and dissemination of MRSA which limit the choice of treatment. Most antibiotics used for treatment of MRSA infection has been reported to have developed resistance (Ayliffe, 1997).
During the last 30 years, no new classes of antibiotics have been found, even with the help of modern biotechnology such as genetic engineering. Pharmaceutical companies have mainly focused on the development of new products derived from the known classes of antibiotics (Sulakvelidze et al., 2001) which is a cause of major concern. Thus, exploring alternative approaches to develop antibacterial products is also a worthwhile task, and re-examining the potential of promising older methods might be of value. One of the possible replacements for antibiotics is the use of bacteriophages or simply phages as antimicrobial agents (Shasha et al., 2004; Vinodkumar et al., 2008).
Phage therapy involves the use of lytic phages for the treatment of bacterial infections, especially those caused by antibiotic resistant bacteria. In general, there are two major types of phages, lytic and lysogenic. Only the lytic phages (also known as virulent phages) are a good choice for developing therapeutic phage preparations (Sandeep, 2006; Borysowski and Gorski, 2008). The bactericidal ability of phages has been used to treat human infections for years as a complement or alternative to antibiotic therapy (Matsuzaki et al., 2005; Kysela and Turner, 2007). Phages are bactericidal, their number increased in responding to the incidence of pathogens over the course of treatment, they are in the same manner effective against antibiotic-resistant bacteria and are capable of damaging bacterial biofilms, and have low inherent toxicities. The investment of phages as a pragmatic approach in the control of pathogens has attracted considerable interest in recent years (Jassim, et al ., 2012).
Materials And Methods
Isolation and Identification of S. aureus
A total of 50 samples of pus were taken from patient suffering from post-traumatic bone infection. All patient who were suspected to had Staphylococcal infections. Culture specimens were obtained at the time of admission, after the surface of the wound had been washed vigorously by saline, and followed by debridement of superficial exudates. Pus was collected by sterile syringe. Specimens were promptly took to the laboratory and processed. Standard methods for isolation and identification of S. aureus were used (Sowmya et al., 2014).
Detection of Methicillin Resistant Staphylococcus aureus by using Cefoxitin, Methicillin and Penicillin G Disc Diffusion (D.D.) Method
The isolates of S. aureus were subjected to cefoxitin, methicillin and penicillin G Discs (Bioanalyse, Turkey), using a 30 μg, 10 μg and 10 U disc respectively. A 0.5 McFarland standard suspension of the isolate (1.5 ×108 PFU/ml) was made and lawn culture was done on Muller-Hinton agar, (Oxoid-England) plate. Plates were incubated at 37ºC for 18 hr and zone diameters were measured (Table 1) (EUCAST, 2009; CLSI, 2012).
Table 1: Antimicrobial susceptibility testing standards (EUCAST, 2009; CLSI, 2012)
|
Antibiotic Discs |
Disc concentration |
Diameter of inhibition zone (mm) Interpretive criteria |
||
|
Resistant |
Intermediate |
Sensitive |
||
|
Cefoxitin |
30 μg |
≤ 21 |
- |
≥ 22 |
|
Methicillin |
10 μg |
≤ 9 |
10-13 |
≥ 14 |
|
Penicillin G |
10 U |
≤ 28 |
- |
≥ 29 |
Isolation and Purification of S. aureus Bacteriophage
Sample Collection: The methods used adapted from (USEPA, 2001; Jaime, 2003). 45 ml raw sewage was measured into graduated cylinder, the sample centrifuged at 3500 rpm for 15 min, the supernatant is filtered through 0.22 μm Millipore filter and decanted into Erlenmeyer flask and pipette 5 ml double sterile phosphate buffer saline and 5 ml Staphylococcus aureus suspension (1.5 ×108 cfu/ml), then incubated at 37°C for 24 hours.
Phage Isolation: Isolation of phage and seeding by using agar overlay technique (Dias et al., 2013). Sewage and S. aureus culture distributed into 8 centrifuge tubes and centrifuged the sample at 2000 RPM for 5 minute. Most of the remaining cells was pelleted. The supernatant contains bacteriophage pipetted into a Millipore filter (0.22 µm) and until all the liquid is pulled into the container, then 0.1 ml of filtrate and 0.1 ml of S. aureus added to 3 ml of top agar, then mixed and poured over a plate of bottom agar, the plate allowed to be harden, invert the plate and incubate at 37°C for 24 hours and then phage plaque detected. Top and Bottom agars were prepared according to Sanders (2012).
Experimental Design
Twenty rabbits divided into 4 groups, five rabbits for each group (treatment started immediately when the infection appeared). Group A: Negative control group (not infected nor treated). Group B: Positive control group (infected with MRSA and not treated). Group C: This group infected with MRSA and treated with Staph-specific Bacteriophage intramuscularly with 3 × 108 PFU/ml once daily for 14 days. Group D: This group infected with MRSA and treated with 40 mg/KG Ceftaroline intramuscularly once daily for 14 days (Cedric et al., 2010).
Experimental Animals
Twenty healthy male rabbits (local breed), were divided equally into 4 groups, five rabbits of each. Their age extended between 10-13 weeks, animals weighing between 1.8-2 kg. Rabbits were put in standard rabbit’s cage. Experimental animals fed with a commercial lab animals pellets and tap water was available freely. Room temperature was maintained at 20-25 oC. Room air was changed constantly by utilizing ventilation vacuum. Litter of cages was changed daily.
Methicillin Resistant Staphylococcus aureus
The pathogenic isolates of Methicillin resistant Staphylococcus aureus were obtained from orthopedic department in Alkarama hospital and it was used as a challenge strain.
Preparation of Rabbits
After two weeks adaptation period, Ketamine 50 mg/kg B.W and Xylazine 4 mg/kg B.W. were given intramuscular to anesthetized rabbits (Nuh, 2004). Right hind limb are prepared surgically (clipping the hair with electrical clipper, then shaved to remove the hair completely) from the metatarsus to the stifle joint.
Induction of Chronic Osteomyelitis
About (1 - 1.5 cm) surgical skin incision was made at the Cranio-medial site of the tibia bone of rabbit, subcutaneous structures were pushed to aside to uncover tibia bone. Dental drill was used to make a hole (one mm), then 0.04 ml of MRSA suspension was inoculated intramedully. The challenge dose that induced infection was 1.5 × 108 cfu/ml of bacterial suspension after pilot study, then the hole was closed with sterile bone-wax, subcutaneous fascia closed by simple continuous using chromic catgut 2/0 USP. Simple interrupted stitches used to close skin using silk suture (2/0). The animals were followed up clinically for signs of inflammation and radiographically for any pathological changes in the bone (Alt et al., 2006; El-Kamel and Baddour, 2007; Li et al., 2009) (Figure 1). All animals were given analgesic agent daily after surgery for 5 days.
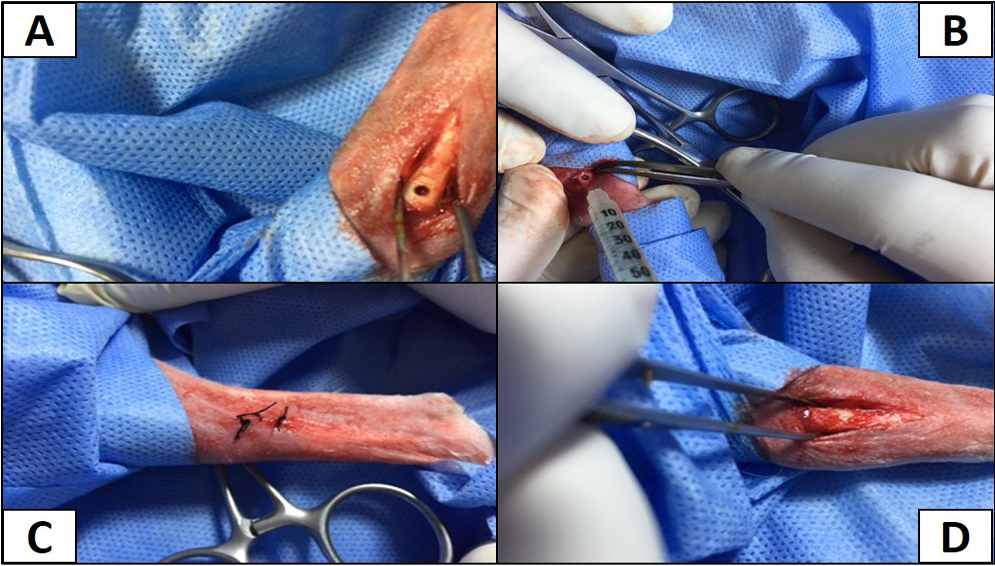
Figure 1: A) The site of operation shows the hole in right tibia; B) Inoculation of MRSA intramedullary; C) The hole filled with bone wax; D) The site of operation sutured with silk
Statistical Analysis
Data was analyzed statistically using the Microsoft Program, SAS (Statistical Analysis System - version 9.1). Statistical analysis of data was performed on the basis of Two-Way Analysis of Variance (ANOVA).
Bone Radiographical Finding
These findings was accomplished for all groups weekly, after infection and after treatment.
Results
Isolation and Identification of S. aureus
The results of isolation and culturing of bacteria including biochemical tests showed that a total of 50 pus samples which were collected from patients suffering from post-traumatic bone infection, 42 samples, showed positive results for the presence of Staphylococcus aureus (Table 2).
Table 2: Prevalence of Staphylococcus aureus from the collected samples
|
Bacteria |
No. of samples |
Percentage % |
|
Staphylococcus aureus |
42 |
84 % |
|
Other |
8 |
16 % |
|
Total |
50 |
100 % |
Detection of Methicillin Resistant S. aureus by using Disc Diffusion Method
After the identification of S. aureus, susceptibility test was done for all S. aureus isolates by disk diffusion method to detect the presence of MRSA according to National Committee for Clinical Laboratory Standards guidelines recommendation (NCCLS), as shown in Table 3. Results of antibacterial sensitivity test of Staphylococcus aureus shown that all isolates were resistance to penicillin 100%, this may explain why S. aureus is totally resistant to pencillin G. Also it was found that 92.85 % were resistant to cefoxitin and 95.23 % of S. aureus were resistant to methicillin.
Table 3: Antibiotic sensitivity test of S. aureus
|
Bacteria Antibiotic discs |
Staphylococcus aureus (n = 42) |
|||||
|
R |
I |
S |
||||
|
No |
Ration |
No |
Ration |
No |
Ration |
|
|
Cefoxitin 30 μg |
39 |
92.85 % |
- |
- |
3 |
7.15 % |
|
Methicillin 10 μg |
40 |
%95.23 |
- |
- |
2 |
4.77% |
|
Penicillin G 10 U |
42 |
100% |
- |
- |
- |
- |
Isolation and Purification of S. aureus Bacteriophage
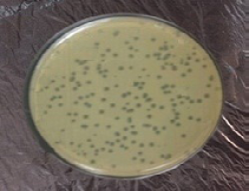
Sample Collection: Staph aureus specific- Bacteriophage associated with osteomyelitis which had been separated from sewage water near to the hospital Lab and Surgery Theatre through agar method. Phage were able to lyse bacteria and form plaques after incubation 24 hr at 37 oC. A piece of single plaque of phage on the bottom agar were picked by sterile needle. Put in broth for 3 hrs and re-infect the host to confirm obtaining phage (Figure 2).
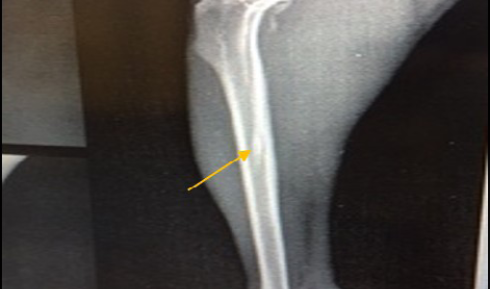
Figure 3: Site of operation, shows mediolateral view of the right tibia in non-infected group 21 days post-operatively, the hole had disappeared completely (healed, ML view)
Bone Radiographical Finding
Radiographic findings of tibia in group A exhibited normal bone anatomy throughout the experimental (no radiographic changes) and the hole had disappeared completely (healed) (Figure 3), while infected groups showed radiographic changes after 14 days post infection with MRSA such as area of bone lysis (Radiolucent spot) surrounded by bone production (Radiopaque area) and thickened cortex and severe periosteal reaction (Figure 4), while after 21 days post infection there was significant chronic osteomyelitis characterized by present Soft tissue suppuration, severe bone lysis, abscesses and Seqeustrum appeared (A segment of bone devascularized with osteonecrosis and resorption of adjacent bone tissue leaving a ‘separated’ piece. (Reservoir for infection) which is a typical sign of chronic osteomyelitis (Figure 5A and B).
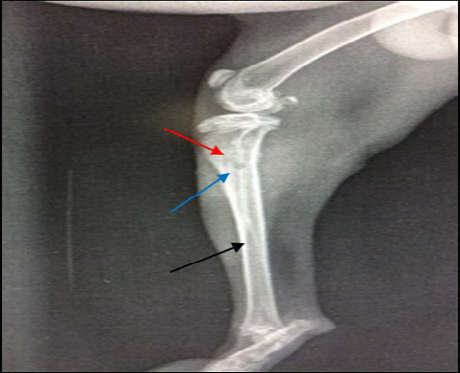
Figure 4: 14 days post infection with MRSA, Area of bone lysis (Radiolucent spot) (→) surrounded by bone production (Radiopaque area) (→).Thickened cortex and severe periosteal reaction (→) (ML view)
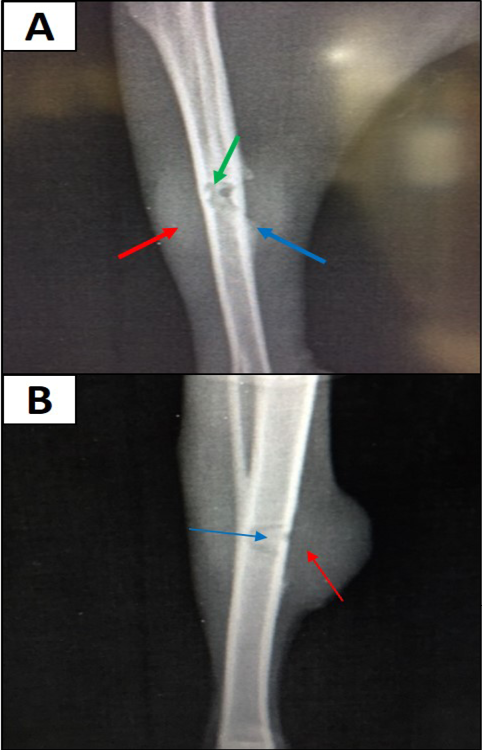
Figure 5: A) 21 days post infection with MRSA shows Sever bone lysis (→), Thickened of cortex and sever periosteal reaction (→), Soft tissue suppuration (→) (ML view); B) Seqeustrum appeared (→) and abscesses (→) (AP view)
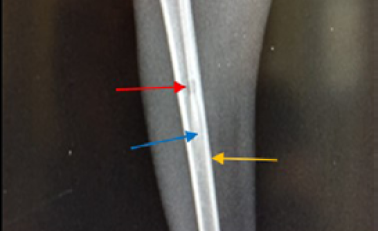
Figure 6: Site of operation, 7 days post treatment with Staph specific – bacteriophage given intramuscularly (Group C), all features of osteomyelitis disappeared, almost clear medullary canal (→), uniformed bone density and cortex returned to normal thickness (→), but the hole still exists (→) (ML View)
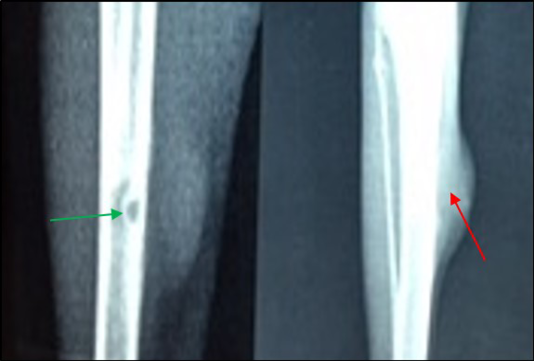
Figure 7: Site of operation, 7 days post treatment with Ceftaroline given intramuscularly (group D): A) Chronic Osteomyelitis (Seqeustrum still exists) (→) (ML View); B) Radiopaque area in soft tissue present represent accumulated pus (→) (AP View)
In group C (treated with 3 × 108 pfu/ml bacteriophage intramuscularly) showed a fast recovery at the first week of treatment and the signs of osteomyelitis had disappeared (Medullary canal is clean), bone cortex uniformed, normal bone density but the hole still present (Figure 6 and 8). Two week post treatment with Ceftaroline 40 mg/kg given intramuscularly (group D), still there is signs of osteomyelitis (Figure 7 and 9).
Susceptibility test of S. aureus isolates revealed that all isolate were resistance to penicillin 100%, this may explain why S. aureus is totally resistant to pencillin G which agreed with Al-Jundiy (2005), Zeidan (2005) and Al-Geobory (2011). The percentage of penicillin G resistant in these studies was 100%, respectively. The present study results were compatible with results of Aghazadeh et al. (2009); likewise, this results were compatible with those obtained by Brady et al. (2007) as they observed that all isolates were resistant to penicillin and other β-lactam antibiotics.
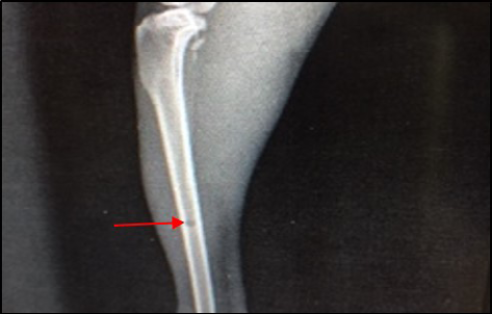
Figure 8: Site of operation, 14 days post treatment with Staph specific –bacteriophage given intramuscularly (Group C). Signs of osteomyelitis had disappeared. (Medullary canal is clear), bone cortex uniformed, normal bone density, the hole still present (ML View)
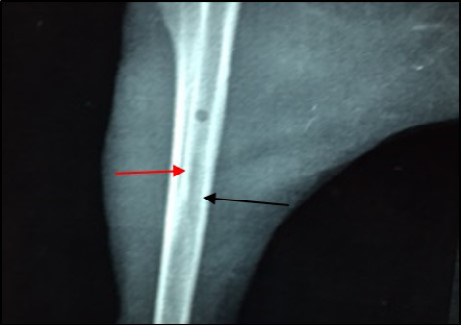
Figure 9: Site of operation, 14 days post treatment with Ceftaroline given intramuscularly (Group D). Still the medullary canal is not clear. Thickened cortex (→) and Endosteal reaction appeared clearly (→) (ML View)
It was found that 92.85 % were resistant to cefoxitin and also 95.23 % of S. aureus were resistant to methicillin. This result is close to work of Kalmus et al. (2011) and Deresse et al. (2012).
Several groups of investigators have reported that the results of cefoxitin disk diffusion (DD) tests correlate better with the presence of mecA than do the results of disk diffusion tests using oxacillin (Swenson et al., 2005). Using cefoxitin and CLSI criteria for disc diffusion, the sensitivity and specificity for recognizing methicillin resistance were both 100 % (Fernande et al., 2005).
Cefoxitin and moxalactam have been reported as surrogate markers for the detection of methicillin resistance (Felten et al., 2002; Skov et al., 2003). The NCCLS has recently reported cefoxitin zone diameter interpretive criteria for the prediction of mecA-mediated resistance (NCCLS, 2000).
The common gold standard method for enumerating and differentiating phages is the double agar overlay assay. Therefore this current study are in agreement with Mansura et al. (2015) in possibility of isolation of phage from sewage by using double agar overlay assay. Oot et al. (2007) emphasized the ubiquitous nature of phage populations, although bacteriophage counts can only rise after an increase in host bacteria population. In studies involving dairy livestock, milk and udders were primary sources for samples from which phages were isolated (Brüssow and Desiere 2001; Garcia et al., 2007, 2009; Shi et al., 2010; Kwiatek et al., 2012). Alternate sources from which phages could be isolated include sewage or wastewater (Yoon et al., 2010), and feces (Oot et al., 2007; Niu et al., 2009; Santos et al., 2011).
Plaques were formed as clear circular with a size ranged between 2-3 mm in diameter when incubated at 37 Co for 24 hrs. This result agreed with Aidan et al. (2009) and Longping et al. (2014) due to lyses of the bacterial host (MRSA). Sewage water was observed to be the best environmental source to get lytic phages with aggressive infective qualities; this might be attributed to the fact that phages from sewage tolerate hard conditions in the sewage; thus, these phages show high degree of lysis with high tolerance to harsh physical environment. Therefore, lytic phages from sewage showed larger plaques, higher titers, and clearer plaques than others.
This is in agreement with Marwa and Ahmed (2014), they reported that the different sources of phages’ isolation and the finding that each phage showed unique profile of size, shape, clarity, and margin cut of plaques provided preliminary evidence that isolated phages are unique and no phages are identical to each other.
The radiograph is an image made up of shadows of different opacities, increased opacity (radiopacity) denoted more white shadow on the radiograph, while decreased opacity (radiolucency) denoted a darker shadow on the radiograph (Elliot and Skerritt, 2010). It can be concluded that the diagnosis of osteomyelitis is more often based on radiographic evidence of bone lysis, production and necrosis. Our results were in an agreement with Chandan et al. (2016). There are numerous reports in the literature using animal models of osteomyelitis for investigating pathogenesis, diagnosis, and treatment of bone infections. When designing an animal model of osteomyelitis, it is important to learn the currently available models as well as their advantages and disadvantages. For use as an animal model, rabbit tibia is a suitable size and has only a limited amount of surrounding soft tissues allowing easier manipulation and accessibility. The rabbit tibial osteomyelitis model was the first attempted animal model of osteomyelitis (El-kamel and Baddour, 2007; Sener et al., 2010) by injecting into the tibial medullary cavity, using an 18 gauge needle, 0.1 mL 5% sodium morrhuate, followed by 0.1 mL S. aureus suspension inoculated bacteria into the medullary canal. Detailed radiographic study of the infection demonstrated this to be a progressive chronic osteomyelitis with typical bone destruction, periosteal reaction, sequestra formation, and new bone formation.
The osteomyelitis radiologic signs are loss of trabecular pattern, which may be the first sign, lysis or destruction of bone appeared as an area of radiolucency within the bone, there was periosteal reaction and this may appear in 14 to 21 day after the infection and the periosteum often becomes elevated and there was sub-periosteal new bone formation and a sequestrum may be seen as fragment of cortical bone of normal opacity or it may appear more opaque than normal because it contrasted with the surrounding lucent zone. An involucrum was seen as an area of decreased bone opacity surrounding the sequestrum and also there was cortical destruction (The cortex became thin) (Kealy et al., 2011).
Staphylococcus aureus is the commonest aetiological agent implicated in acute and chronic osteomyelitis (Krogstad, 2009). S. aureus has an extraordinary capacity to adapt the hostile environment with a proven ability to develop resistance against antibiotics. Non-antimicrobial approaches to prevent and treat the infection have also been tried. Use of lysostaphin (Dajcs et al., 2001), antimicrobial peptides (Lawton et al., 2007), some natural products (tea, tree, oil) (Stapleton et al., 2007), anti-staphylococcal vaccines (Bubeck and Schneewind, 2008), and bacteriophages (Sulakvelidze and Kutter, 2005; Górski et al., 2007) are some of the alternative modalities to deal with S. aureus including methicillin resistant (MRSA) and vancomycin resistant (VRSA).
Previous in vitro studies have investigated the interaction between S. aureus and the cells responsible for bone remodeling, osteoblasts and osteoclasts. S. aureus is capable of invading and persisting within osteoblasts (Shi and Zhang, 2012). The interaction of S. aureus and osteoblasts leads to physiologic alterations that favor bone resorption by two primary mechanisms. First, S. aureus-infected osteoblasts incite osteoclastogenesis through increased expression of pro-inflammatory cytokines and the critical osteoclast-activating molecule receptor activator of NF-κB ligand (RANK-L) (Marriott et al., 2004; Somayaji et al., 2008; Claro et al., 2013). Second, S. aureus invasion of osteoblasts results in cell death (Alexander et al., 2003; Young et al., 2011). However, the specific staphylococcal factors that trigger osteoblast cell death or induce osteoclastogenesis are unknown. Furthermore, it has yet to be determined if these in vitro phenomena are responsible for pathogen-mediated bone destruction in vivo.
In the beginning of antibiotic era when antibiotic resistance was not reported, osteomyelitis caused by S. aureus could not be cured in all the (100%) cases. The possible reasons for the failure may be biofilm formation; low antibiotic levels achieved in bone tissue and reduced activity of antibiotics in bone and its marrow (Stengel et al., 2001; Lazzarini et al., 2005). In contrast to antibiotics, phages are known to penetrate the biofilm as they propagate in their bacterial host.
Many phages produce depolymerases that hydrolyze biofilm extracellular polymers and they can penetrate the inner layers of the biofilm by degrading components of the biofilm exopolymeric matrix. There is a report suggesting that the phages can enter the macrophages to kill the intracellular form (Broxmeyer et al., 2002). It is known that there is a decrease in lysis of the bacterial cells when they are in dormant state (Matsuzaki et al., 2005; Heilmann et al., 2012). This might be the reason for isolation of S. aureus till the third dose of the phage, i.e. up to 6th day of the therapy. However, single dose of the phage cocktail has been found to be effective in acute bacterial infection, e.g. septicaemia in mouse burn model caused by Pseudomonas aeruginosa (our unpublished data) as bacteria actively multiply in blood circulation.
Conclusions
S. aureus was the most frequent isolated bacteria from bone infection in all age patients. Sewage water was observed to be the best environmental source to get lytic phages with aggressive infective qualities and the isolated phages show high degree of lysis.
In vivo study showed that treatment with bacteriophage 3 × 108 PFU/ml intramuscularly was highly effective and infected rabbit cured with the first period of treatment in comparison with ceftaroline 40 mg/kg. All infected animals treated with phage showed no toxicological effect, and no other complications like anorexia or body weight loss when compared with Ceftaroline.
Radiographic finding showed that bone changes remained even when treatment course with antibiotic ended. All radiographical features of osteomyelitis had disappeared completely in case of treatment with specific phage intramuscularly.
Acknowledgement
Authors acknowledge to the Department of Physiology and Pharmacology and Surgery, College of Veterinary Medicine, Baghdad University, Baghdad, Iraq.
Conflict of Interests
There exists no conflict of interest.
Authors’ Contribution
All the authors have contributed equally in terms of giving their technical knowledge to frame the article.
References