Advances in Animal and Veterinary Sciences
Research Article
In Vitro Evaluation of Isolated Staphylococcal-bacteriophage in Killing Methicillin-Resistant Staphylococcus aureus
Orooba Mohammed Saeed Ibrahim1, Sarhan Rashid Sarhan1*, Serwa Ibrahim Salih2
1Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq; 2Department of Surgery, College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | The present study was carried out to isolate and identified the Staphylococcal-specific bacteriophage from swage water by using agar overlay method and to investigate the anti-Staphylococcal activity of Isolated Bacteriophage in-vitro. Two experiments were performed in this present study; the first one was isolation and identification of Methicillin-Resistant Staphylococcus aureus (MRSA) from post-traumatic bone infection. While the second experiment included study the infectivity of bacteriophage in-vitro to MRSA as well as effect of temperature and pH on the infectivity of phage was also studied. Results of antimicrobial susceptibility test of S. aureus isolates revealed that most of isolates were resistant to Methicillin, Cefoxitin and Pencillin G. Staphylococcus aureus phage was isolated from sewage samples by agar overlay method, the phage was characterized by clear, circular plaques ranged between 2-3 mm in diameter. TTA method for determined the Phage MIC appears to be both reliable, and reproducible. This method is laborious and time consuming, and the proposed MIC method appears to be an interesting alternative.
Keywords | Methicillin-resistant, Staphylococcus aureus, Bacteriophage_Tetrazolium assay, Bone infection
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 17, 2016; Accepted | September 15, 2016; Published | October 24, 2016
*Correspondence | Sarhan Rashid Sarhan, Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq; Email: [email protected]
Citation | Ibrahim OMS, Sarhan SR, Salih SI (2016). In vitro evaluation of isolated Staphylococcal-bacteriophage in killing methicillin-resistant Staphylococcus aureus. Adv. Anim. Vet. Sci. 4(10): 550-562.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.10.550.562
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Ibrahim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
MRSA stands for Methicillin-resistant Staphylococcus aureus, a type of S. aureus that has become resistant to certain antibiotics such as methicillin, penicillin, amoxicillin, and is thus more difficult to treat. MRSA often lives on the skin or in the nose of healthy people without causing symptoms (this is called colonization). It can, however, cause skin and other infections. Most infections are minor, such as pimples and boils. Serious infections such as wound infections, pneumonia, or septicaemia (infections getting into the bloodstream) can result in life-threatening illness or, on rare occasions, death, if left untreated. Those with weakened immune systems and chronic illnesses are more susceptible to developing an infection (PICNet, 2015).
Penicillin disrupts the cross-linking of small peptide chains in peptidoglycans during the production of bacterial cell walls (Yocum et al., 1979; Anonymous, 2012). As a result, pre-existing cells are unaffected, but newly produced bacteria are unable to maintain their cell-wall rigidity and ultimately undergo lysis. Within ten years of the widespread use of penicillin, Staph. aureus had developed resistance by producing penicillinase, an enzyme which cleaves the penicillin beta-lactam ring. To combat this resistance, a semisynthetic class of penicillins (e.g. methicillin) was introduced in the 1960s. Methicillin had an additional acyl-group on the beta-lactam ring which conferred a high degree of resistance to penicillinase and produced broader antibacterial activity against some Gram-negative bacteria. Methicillin resistance developed in Staph. aureus at a slower pace than the initial resistance to penicillin (Chambers et al., 2001). Penicillin and its derivatives, including methicillin have been used for the treatments of infections caused by S. aureus (Rayner and Munckhof, 2005). Less than 90% of S. aureus strains are resistant to most penicillin derivatives (Chambers et al., 2001; Freeman-Cook and Freeman-Cook, 2006) and ordinary antimicrobial agents like drugs from the family of aminoglycosides, macrolides, chloramphenicols, tetracyclines and fluoroquinolones (Lee, 2003).
Expression of an alternative penicillin-binding protein, PBP2a conferred resistance and rendered the entire antibiotic class ineffective. This is encoded on the methicillin-resistance gene mec A, which is a component of a highly mobile genetic element known as the staphylococcal cassette chromosome (SCC) (Enright et al., 2002; Wielders et al., 2002). MRSA isolates from hospital settings has been gradually increasing in the United States and other parts of the world. Depending on the study area and sample size, high rate of MRSA rates (>50%) have been reported in USA, Asia and Malta, intermediate rate (25-50%) reported in Africa, China and Europe while in some part of Europe, the prevalence rate is relatively lower than 50% (Mejìa et al., 2010).
Stafeni et al. (2012) compiled the prevalence rates of HA-MRSA in some European countries like France, Ireland and UK and reported decline in hospital cases. While in Asia particularly South Korea (77.6%), Vietnam (74.1%), Taiwan (65%) and Hong Kong (56.8%) reports on HA-MRSA infections is still high (Al-Dahbi and Al-Mathkhury, 2013), the incidence of MRSA among S. aureus was 94.3%. Babakir-Mina et al. (2012) found that among S. aureus positive cases, 88% were MRSA. Al-Hasani (2005) indicated that 41/49 (83.7%) were MRSA.
Muhammad and Al-Mathkhury (2014) found that among S. aureus positive cases, 65 % were MRSA. Another study done in Basra university, Nursing college by Al-Mussawi (2014) stated that 17 (65 %) from 26 isolates were MRSA.
Animals such as dogs, cats and horses have become an important part of most families particularly in developed countries like USA and UK (Chomel and Sun, 2011). In the UK, 1.5% of MRSA were recovered from samples of infected companion animals (Rich and Roberts, 2004) and dogs are more infected/colonized with MRSA in comparism to cats (Morgan, 2008). Reports of MRSA colonization in horses with a percentage rate of 0 to 11% has been published. Most cases and outbreak of MRSA infections were reported in large stables and post-operative complications (Weese et al., 2005; Morgan, 2008).
During the last 30 years, no new classes of antibiotics have been found, even with the help of modern biotechnology such as genetic engineering. Pharmaceutical companies have mainly focused on the development of new products derived from the known classes of antibiotics (Sulakvelidze et al., 2001) which is a cause of major concern. Thus, exploring alternative approaches to develop antibacterial products is also a worthwhile task, and re-examining the potential of promising older methods might be of value. One of the possible replacements for antibiotics is the use of bacteriophages or simply phages as antimicrobial agents (Shasha et al., 2004; Vinodkumar et al., 2008).
Phage therapy involves the use of lytic phages for the treatment of bacterial infections, especially those caused by antibiotic resistant bacteria. In general, there are two major types of phages, lytic and lysogenic. Only the lytic phages (also known as virulent phages) are a good choice for developing therapeutic phage preparations (Sandeep, 2006; Borysowski and Gorski, 2008). The bactericidal ability of phages has been used to treat human infections for years as a complement or alternative to antibiotic therapy (Alisky et al., 1998; Matsuzaki et al., 2005; Kysela and Turner, 2007).
Bacteriophages, nature’s tiniest viruses and it is estimated that there are about 1031 phages on earth making viruses the most abundant life form on earth (Ashelford et al., 2000; Hendrix, 2002; Dabrowska et al., 2005). Bacteriophages do not only help in the treatments of bacterial infections in animals and human beings but also used in birds, fishes, plants, food material and biofilm eradication (Flaherty et al., 2000; Goode et al., 2003; Leverentz et al., 2003; Park and Nakai, 2003; Curtin and Donlan, 2006).
Materials and Methods
Isolation and Identification
50 samples of pus were taken from patient suffering from post-traumatic bone infection. All patient who were suspected to had Staphylococcal infections. Culture specimens were obtained at the time of admission, after the surface of the wound had been washed vigorously by saline, and followed by debridement of superficial exudates. Pus was collected by sterile syringe. Specimens were promptly took to the laboratory and processed. Standard methods for isolation and identification of S. aureus were used (Sowmya et al., 2014) (Table 1).
Table 1: Morphological and biochemical tests to S. aureus
|
Bacterial spp. |
Morphological examination |
Biochemical tests |
|
S. aureus |
Gram stain Staph 110 agar Blood agar Motility test |
Mannitol salt agar Catalase test Coagulase test Oxidase test |
Detection of Methicillin Resistant
The isolates of S. aureus were subjected to cefoxitin, methicillin and penicillin G Discs (Bioanalyse, Turkey), using a 30 μg, 10 μg and 10 U disc respectively. A 0.5 McFarland standard suspension of the isolate (1.5 ×108 PFU/ml) was made and lawn culture was done on Muller-Hinton agar, (Oxoid-England) plate. Plates were incubated at 37 ºC for 18 hr. and zone diameters were measured (Table 2) (EUCAST, 2009; CLSI, 2012).
Isolation and Purification of S. aureus Bacteriophage
Sample Collection: The methods used adapted from (USEPA, 2001; Jaime and Tiffany, 2003). 45 ml raw sewage was measured into graduated cylinder, the sample centrifuged at 3500 rpm for 15 min, the supernatant is filtered through 0.22 μm Millipore filter and decanted into Erlenmeyer flask and pipette 5 ml double sterile phosphate buffer saline and 5 ml Staphylococcus aureus suspension (1.5 ×108 cfu/ml), then incubated at 37° C for 24 hours.
Phage Isolation: Isolation of phage and seeding by using agar overlay technique (Dias et al., 2013). Sewage and S. aureus culture distributed into 8 centrifuge tubes and centrifuged the sample at 2000 RPM for 5 minute. Most of the remaining cells was pelleted. The supernatant contains bacteriophage pipetted into a Millipore filter (0.22 µm) and until all the liquid is pulled into the container, then 0.1 ml of filtrate and 0.1 ml of S. aureus added to 3 ml of top agar, then mixed and poured over a plate of bottom agar, the plate allowed to be harden, invert the plate and incubate at 37 °C for 24 hours and then phage plaque detected. Top and Bottom agars were prepared according to (Sanders, 2012).
Preperation, Standard of Phage Suspention Stock and Storage of Bacteriophage: When plaques were identified, a pure suspension is prepared by carefully removing a portion of the phage single plaque on bottom agar plate by stabbing the center of a plaque with a sterile needle. Rinse in 10 ml sterile TSB (Tryptic Soya Broth, Hi-media-India), the broth is transferred to a sterile 25 ml centrifuge tube and centrifuging at 5000 rpm for 5 min. The supernatant filter through 0.22 micro-filter and then aseptically transferred to a sterile 15 ml tube and stored at 4 °C till use. (Dias et al., 2013; Maier et al., 2000).
Determination of Phage Typing to S. aureus by using Spot Test
The type of phage strain will determine using an isolate of S. aureus. Two microliters of concentrated phage lysate (108 PFU/ml) then dropped onto a NA (Nutrient agar, Oxoid-England), plate overlaid with S. aureus and incubate overnight. The lytic ability of phage isolates assessed using the clarity of plaques (Stenholm et al., 2008).
Measurement of Phage Titer PFU/ml by using Phage Plaque Assay
Ten-fold serial dilutions to 10-9 of phage filtrate made in PBS. 3 ml of hot, melted top agar into three 13x100 mm test tubes held in 47 oC water bath. To each tube of 47 oC top agar, 0.1 ml of S. aureus added and 0.1 ml of the 108 phage dilution quickly pipited into a tube of top agar containing host bacteria, mix well but gently to avoid bubbles and quickly pour the mixture onto the surface of bottom agar while turning the dish with the left hand, tap out the final drop, the plates rapidly tilted and distributed over the surface of the bottom layer, this procedure was repeated with others phage dilutions. The top agar allow to solidify and become firmly attached to the hard agar bottom, all the plates inverted and incubated for 24 hrs. The phage titer determined by counting the number of plaque forming units (p.f.u.) for each dilution. For determination (dilution factor) that used for all other experiments. Plate that had between 30 and 300 plaques were counted by applying the following formula (Anderson et al., 2011).
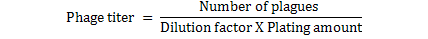
Effect of Temperature and pH on the Infectivity of Phage
pH values of 2, 7.4 and 8 were used to determine whether the bacteriophages are affected by exposure to different pH values. The values were chosen in accordance with the range of pH (2-8) obtained in Rabbit GIT. Triplicate tubes are prepare with 9.9 ml of different buffer solution; buffer A pH=2 (50 ml of 0.2M KCL + 13 ml of 0.2M HCL), buffer B pH=7.4 (100 ml of 0.1M KH2PO4 + 78.2 ml of 0.1M NaOH) and buffer C pH=8 (100 ml of 0.1M KH2PO4 + 93.4 ml of 0.1M NaOH) (BDH, England) will add to a total volume of 10 ml (0.1 ml of 3 × 108 phage). Then, 0.1 ml samples were taken with regular intervals (sampling begins at the first 1 h, 6h, and 12h) and added S. aureus culture. Plaque assays were performed for each sample to register the change in abundance of infectious phages over time.
Table 2: Antimicrobial susceptibility testing standards (EUCAST, 2009; CLSI, 2012)
|
Antibiotic discs |
Disc concentration |
Diameter of inhibition zone (mm) Interpretive criteria |
||
|
Resistant (R) |
Intermediate (I) |
Sensitive (S) |
||
|
Cefoxitin |
30 μg |
≤ 21 |
- |
≥ 22 |
|
Methicillin |
10 μg |
≤ 9 |
10-13 |
≥ 14 |
|
Penicillin G |
10 U |
≤ 28 |
- |
≥ 29 |
Optimal phage dilution prepares using saline and placed in a 40 oC, 50 oC and 60 oC water bath and 37 oC as a control. 0.l ml of phage dilution then remove after 10, 20, 30, 40, 50, and 60 min had elapsed and added to sloppy agar previously inoculated with Staph aureus culture then plated on NA and incubated overnight at each temperature the number of p.f.u. were determined for each time interval (Al-khafaji, 2012; Madsen et al., 2013).
Minimum Inhibitory Concentration of Bacteriophage against MRSA
The MICs of phages was determined by using the tetrazolium salt in the tube dilution assay and this method was modified from Vipra et al. (2013), standard bacterial suspension (1.5×108 cfu/ml) adjusted to 0.5 McFarland tube was added to the 9 tubes containing 6 ml nutrient broth. The phage preparations were diluted to contain different concentration of phages, (Ranging from 10-1 to 10-9) pfu/ml, and 1 ml of phage dilutions were added to all tubes containing bacterial cells. Corresponding cell control, phage control and media control were maintained. After 24 h incubation 35 ˚C without shaking to visually differentiate between the live and dead cells, 200 µL of TTA reagent was added to each tube and observed for colour development after 2 hr. The lowest concentration of phage at which no colour was seen was regarded as the MIC.
Statistical Analysis
Data were analyzed statistically using the Microsoft Program, SAS (Statistical Analysis System - version 9.1). Statistical analysis of data was performed on the basis of Two-Way Analysis of Variance (ANOVA) using a significant level of (P<0.05). Post hoc test was performed to assess significant difference among means.
Results
Isolation and Identification
The results of isolation and culturing of bacteria including biochemical tests showed that a total of 50 pus samples which were collected from patients suffering from post-traumatic bone infection, 42 samples, showed positive results for the presence of Staphylococcus aureus (Table 3).
Table 3: Prevalence of Staphylococcus aureus from the collected samples
|
Bacteria |
No. of samples |
Percentage % |
|
S. aureus |
42 |
84 % |
|
Other |
8 |
16 % |
|
Total |
50 |
100 % |
Morphological Examination and Culture Characteristics
The suspected bacteria was cultured on Blood agar, Mannitol salt agar and Staph 110 and after purification of bacteria, biochemical tests (Catalase, Coagulase, and Oxidase tests) were done as well as gram staining was performed, 42 samples showed positive results for the presence of Staphylococcus aureus. Results shown in Table 4, 5 and Figure 1.
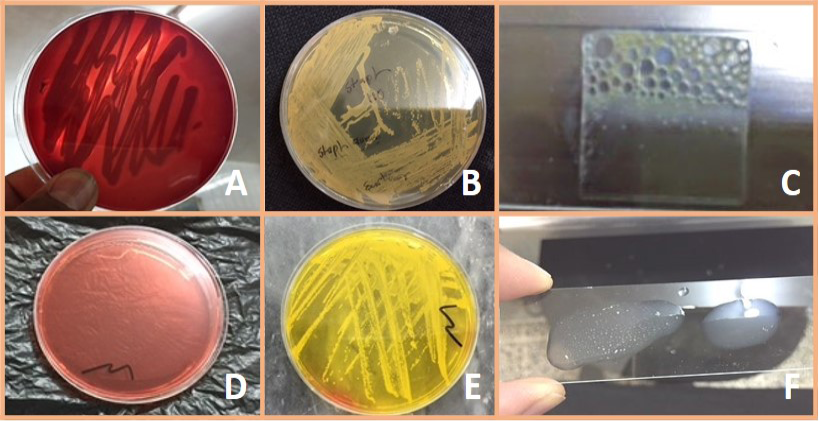
Figure 1: A) Staph aureus on blood agar (β – Hemolysis); B) Staph. aureus on Staph 110 agar (Creamy to yellow or orange); C) Positive catalase test; D) Mannitol salt agar without S. aureus culture; E) Fermentation of mannitol salt agar by S. aureus. (Formation of yellow color colony); F) Positive coagulase test
Table 4: Morphological tests used to identify Staphylococcus aureus
|
Morphological tests |
Results |
|
Gram stain |
G +ve cocci |
|
Staph 110 agar |
Creamy to yellow or orange colonies |
|
Blood agar |
Golden yellow, round, smooth colonies. β-Hemolysis |
Table 5: Biochemical tests used to identify Staphylococcus aureus
|
Biochemical tests |
Results |
|
Mannitol salt agar |
Conversion of medium from rosy to yellow |
|
Catalase test |
Formation of gas bubble |
|
Coagulase Test (Slide method) |
Clumping appeared |
|
Oxidase test |
(no purple colour appeared ), negative result |
Detection of Methicillin Resistant S. aureus by using Disc Diffusion Method
After the identification of S. aureus, susceptibility test was done for all S. aureus isolates by disk diffusion method to detect the presence of MRSA (Figure 2), according to National Committee for Clinical Laboratory Standards guidelines recommendation (NCCLS) (Table 6).
Table 6: Antibiotic sensitivity test of S. aureus
|
Bacteria |
Staphylococcus aureus (n= 42) |
|||||
|
Antibiotic discs |
R |
I |
S |
|||
|
No |
% |
No |
% |
No |
% |
|
|
Cefoxitin 30 μg |
39 |
92.85 |
- |
- |
3 |
7.15 |
|
Methicillin 10 μg |
40 |
95.23 |
- |
- |
2 |
4.77 |
|
Penicillin G 10 U |
42 |
100 |
- |
- |
- |
- |
Results of antibacterial sensitivity test of Staphylococcus aureus shown that all isolates were resistance to penicillin 100%, this may explain why S. aureus is totally resistant to pencillin G. Also it was found that 92.85 % were resistant to cefoxitin and 95.23 % of S. aureus were resistant to methicillin.
Isolation and Purification of S. aureus Bacteriophage
Sample Collection: Staph aureus specific- Bacteriophage associated with osteomyelitis which had been separated from sewage water near to the hospital Lab and Surgery Theatre through agar method. Phage were able to lyse bacteria and form plaques after incubation 24 hr at 37oC. A piece of single plaque of phage on the bottom agar were picked by sterile needle put in broth for 3 hrs and re-infect the host to confirm obtaining phage (Figure 3).
Plaque Morphology: Plaques were formed as clear circular with a size ranged between 2-3 mm in diameter when incubated at 37 oC for 24 hrs,
Preparation of Phage Stock and Titration
Overlay technique was used to prepare of Staph aureus phage stock solution and a titer between 101 to 109 PFU/ml was made. Calculation of phage titer by using Series of dilutions were used to calculation of phage titer and the p.f.u. for each dilution are (5.8×104, 5.4×105, 4.3×106, 4.0×107, 3.0×108, 2.5×109, 2.1×1010, 1.8×1011and 1.2×1012), respectively. Dilution factor that gave the best countable number of plaques is (105), this dilution factor was then used for all other experiments and the pfu/ml was (3×108), (Table 7). In addition the optical density of all dilutions were recorded at the same time.
Table 7: Determination of S. aureus phage titer
|
Plate No. |
Plaque per plate |
TTA* |
Titer Plaque forming unit |
|
1 |
580 |
580×101 /0.1 |
5.8×104 |
|
2 |
540 |
540×102/0.1 |
5.4×105 |
|
3 |
430 |
430×103/0.1 |
4.3×106 |
|
4 |
400 |
400×104/0.1 |
4.0×107 |
|
5 |
300 |
300×105/0.1 |
3.0×108 |
|
6 |
250 |
250×106/0.1 |
2.5×109 |
|
7 |
210 |
210×107/0.1 |
2.1×1010 |
|
8 |
180 |
180×108/0.1 |
1.8×1011 |
|
9 |
120 |
120×109/0.1 |
1.2×1012 |
*: Titer calculation = Plaque×DF / Volume of phage plated (ml)
Determination of Host Range (Phage Typing)
Specifity of bacteriophage to MRSA was determined by using Spot lysis technique, MRSA are efficiently lysed by specific bacteriophage. Clear lyses zone was formed and bacterial growth inhibited at the point of phage dropped Approving these Staph. aureus isolates have particular receptor on its cell wall for phage attachment (Figure 4).
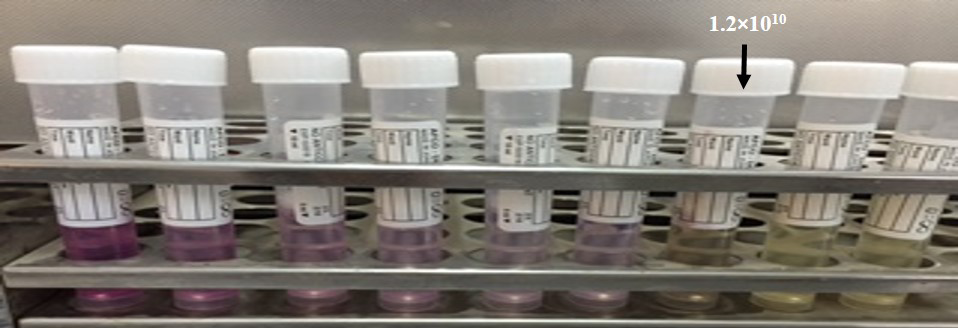
Minimum Inhibitory Concentration of Bacteriophage against MRSA
The MIC of phage was the dilution 10-7 (1.2 ×1010) (Figure 5). TTAs basically depend on reduction of tetrazolium to a reduced substance called formazan in mammalian cells and this test have been used to differentiate between the live and dead bacterial cell because only live bacteria convert the tetrazolium to purple formazan. We adopted this method to estimate the MIC of phages.
Effect of Temperature and pH on the Infectivity of Phage
pH values of 2, 7.4 and 8 used to estimate if the bacteriophages are influenced by exposure to various pH values. These different values were selected according to the range of pH (2-8) found in Rabbit GIT (Table 8). PFU were measured for every specimen to record the change in the infectivity phages over time in different pH.
Table 8: Change in infectivity of the Bacteriophage (mean Log10 of phage concentration PFU/ml) over time at different pH values
|
Time (hr) |
Dilution factor |
Titer: plaque forming unit (mean Log10 PFU/ml) at different pH values |
||
|
pH 2 |
pH 7.4 |
pH 8 |
||
|
1 |
105 |
6.77±0.07 B a |
8.25±0.04 A b |
8.17±0.06 A b |
|
6 |
105 |
6.30±0.03 B b |
8.36±0.02 A b |
8.44±0.07 A a |
|
12 |
105 |
0.0±0.0 C c |
8.5±0.05 A a |
8.0±0.02 B c |
Values represent mean ±S.E; LSD: 0.1359; Different capital letters mean significant (P<0.05) results between different pH values; Different small letters mean significant (P< 0.05) results between different hours
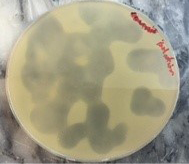
Figure 4: Spot lysis test after 24 hrs incubation
Table 9: Sensitivity determination of staph aurous phage titer (mean Log10 of phage concentration PFU/ml) in relation to different degrees of temperature
|
Time (min) |
Dilution factor |
Titer: plaque forming unit (mean Log10 PFU/ml) |
|||
|
37 oC |
40 oC |
50 oC |
60 oC |
||
|
10 |
105 |
8.37±0.08 A a |
8.29±0.12 A a |
8.11±0.13 A a |
0.0±0.0 B a |
|
20 |
105 |
8.44±0.11 A a |
8.39±0.15 A a |
7.69±0.12 B b |
0.0±0.0 C a |
|
30 |
105 |
8.50±0.09 A a |
8.44±0.06 A a |
0.0±0.0 B c |
0.0±0.0 B a |
|
40 |
105 |
8.57±0.11 A a |
8.44±0.08 A a |
0.0±0.0 B c |
0.0±0.0 B a |
|
50 |
105 |
8.68±0.18 A a |
8.60±0.05 A a |
0.0±0.0 B c |
0.0±0.0 B a |
|
60 |
105 |
8.68±0.13 A a |
8.62±0.04 A a |
0.0±0.0 B c |
0.0±0.0 B a |
Values represent mean ±S.E; LSD: 0.3412; Different capital letters mean significant (P<0.05) results between different temperature; Different small letters mean significant (P< 0.05) results between minutes
The aim of this experiment was to test the staph aureus specific-bacteriophage degradation and survival in different pH environment that influenced the decay rates of phage and the infectivity of the bacteriophage. There was no significant decrease in bacteriophage infectivity to lyse the bacteria during the 6 hr incubation at pH 7.5 and 8, and the pfu/ml was 2.3×108, mean Log 10 (8.36), 2.8×108 (8.44), respectively. This is approved that there are no significant differences (P<0.05) between these two pH values during the first 6 hr., but there are a significant differences (P<0.05) between the phage titer at pH 7 and pH8 in comparison with phage titer at pH 2. While the pfu at pH 8 was decrease slightly to 1.0 × 108 after 12 hr. bacteriophage lost its infectivity when incubated at pH 2 between (1 hr - 6 hr) incubation from 0.6×108 (6.77 mean Log10) to 0.2×108 (6.30 mean Log10) and the pfu became 0.0 after 12 hr incubation at pH 2.
Ideal phage dilution prepared with normal saline and put in graded temperatures 40oC, 50oC and 60 ºC and 37ºC as a control. 0.l ml of phage sample were taken after 10 min, 20 min, 30 min, 40 min, 50 min, and 60 min incubation in these temperatures and then overnight culture of staph aureus added. PFU were measured using double layer technique for each time. And phage titer pfu/ml and mean log10 during 10, 20, 30, 40, 50, and 60 minutes was 2.3×108 (8.37), 2.8×108 (8.44), 3.2×108 (8.5), 3.8×108 (8.57), 4.3×108 (8.68) and 4.8×108 (8.68), respectively in the temperature 37 C° and phage titer mean log10 was 1.9×108 (8.29), 2.5×108 (8.39), 2.8×108 (8.44), 2.8×108 (8.44), 3.0×108 (8.60) and 3.3×108 (8.62), respectively in the temperature 40 °C. The phage titer and mean log10 was (1.3×108 (8.11), 0.8×108 (7.69), 0.0, 0.0, 0.0 and 0.0), respectively in the temperature 50 °C. While the phage titer and mean log10 was (0.0, 0.0, 0.0, 0.0, 0.0 and 0.0), respectively in the temperature 60 °C (Table 9).
The statistical analysis results showed significant increase (P˂0.05) in phage titer in temperature 37°C and 40oC comparing with phage titer in temperature 50°C and 60°C.
Most of the bacteriophage were sensitive to temperatures above 50°C and inactivated instantly at 60°C and there are no significant differences (P˂0.05) between phage titer in temperature 37°C and 40°C during the 60 minutes. And there was a significant differences between phage titer in temperature 37oC and 40oC during the 60 min in comparison with phage titer in temperature 50oC and 60oC.
Discussion
Isolation and Identification
The importance of Staphylococcus aureus as a successful pathogen resides in its wide genetic diversity and host range and the different pathologies associated with infection. S. aureus is associated with hospital-acquired and community acquired infections and with human carriers (Miller and Diep, 2008) as well as livestock associated infections (Graveland et al., 2011), for which meticillin-resistant S. aureus (MRSA) and meticillin-sensitive S. aureus (MSSA) isolates are important pathogens (Hata et al., 2010).
In the present study, the identification of S. aureus isolates associated with bone infection in hospitals was investigated. Primary isolation and presumptive identification of the pathogen is crucial for microbiological diagnosis and epidemiological surveillance. In order to isolate and identify S. aureus a number of authors have used a variety of selective and/or differential culture media. Among the most common media used are salt–mannitol agar (SMA) and Staphylococcus-110 agar (S110), which are selective and differential media that take advantage of the tolerance of S. aureus to 7.5% (w/v) NaCl as well as its metabolic activities, such as mannitol fermentation in SMA or gelatinase activity in S110 media.
Sheep’s blood agar (SBA) is a rich medium in which the β haemolytic activity of S. aureus isolates was clearly differentiated. In this current biochemical tests such as catalase, coagulase and oxidase tests used for identification of S. aureus.
As the use of differential and/or selective culture media for the isolation and primary identification of S. aureus isolates is not enough to ensure the identity of the isolates, a secondary test is needed to precisely identify the isolates as S. aureus. Determinations of coagulase and β-haemolysin activities are easy and affordable confirmatory techniques to identify S. aureus. When one or both of these confirmatory tests were used in combination with each selective medium, they improved the ability to correctly identify S. aureus
For SBA, only the combination with coagulase test was presented because the criterion of β-haemolysis has been already considered in the positive colony morphology description, although β -haemolysis was confirmed again after primary isolation. All isolates were β-haemolytic. The use of coagulase at 24 h as a secondary test of identification improved sensitivity and specificity in combination with the respective culture medium. However, SMA and coagulase showed higher values of sensitivity compared with the other culture media. Using β-haemolysis as secondary test of identification also increased sensitivity, specificity when combined with the respective culture medium. Our results is confirmed with the work of (Bautista-Trujillo et al., 2013).
MSA is a selective as well as a differential media mannitol is the differential part, it indicates organisms that ferment mannitol. If mannitol fermentation is occurring, lactic acid will be produced, and the pH will drop causing the MSA medium to turn from red to yellow (with phenol red as pH indicator). The salt (7.5%) portion is selective for members of the genus Staphylococcus (Benson, 2002); since they can tolerate high saline levels. Coagulase-positive staphylococci (COPS) will produce luxuriant growth of yellow colonies and may have a yellow halo around the colony. Coagulase-negative staphylococci (CONS) will produce small colourless to pink colonies with no colour change to the medium (Charles and Margi, 2002; Jakee et al., 2008).
Results of antimicrobial susceptibility test of S. aureus isolates revealed that all isolate were resistance to penicillin 100%, this may explain why S. aureus is totally resistant to pencillin G which agreed with Al-Jundiy (2005), Zeidan (2005) and Al-Geobory (2011). The percentage of penicillin G resistant in these studies was 100%, respectively. The present study results were compatible with results of Aghazadeh et al. (2009); likewise, this results were compatible with those obtained by Brady et al. (2007) as they observed that all isolates were resistant to penicillin and other β-lactam antibiotics.
It was found that 92.85 % were resistant to cefoxitin and also 95.23 % of S. aureus were resistant to methicillin. This result is close to work of Kalmus et al. (2011) and Deresse et al. (2012).
Infections due to MRSA are an increasing problem worldwide inside and outside of hospitals, it is clinically and epidemiologically important for laboratories to be able to differentiate MRSA from MSSA. Not only for choosing appropriate antibiotic therapy for the individual patient, but also for control of MRSA transmission (Boyce et al., 2005). Several groups of investigators have reported that the results of cefoxitin disk diffusion (DD) tests correlate better with the presence of mecA than do the results of disk diffusion tests using oxacillin (Swenson et al., 2005). Using cefoxitin and CLSI criteria for disc diffusion, the sensitivity and specificity for recognizing methicillin resistance were both 100 % (Fernande et al., 2005).
Cefoxitin and moxalactam have been reported as surrogate markers for the detection of methicillin resistance (Felten et al., 2002; Skov et al., 2003). The NCCLS has recently reported cefoxitin zone diameter interpretive criteria for the prediction of mecA-mediated resistance (NCCLS, 2000).
Isolation and Purification of S. aureus Bacteriophage
Phage Isolation and Morphology: The common gold standard method for enumerating and differentiating phages is the double agar overlay assay. Therefore this current study are in agreement with (Mansura et al., 2015) in possibility of isolation of phage from sewage by using double agar overlay assay. Oot et al. (2007) emphasized the ubiquitous nature of phage populations, although bacteriophage counts can only rise after an increase in host bacteria population.
In studies involving dairy livestock, milk and udders were primary sources for samples from which phages were isolated (Brüssow and Desiere 2001; Garcia et al., 2007, 2009; Shi et al., 2010; Kwiatek et al., 2012). Alternate sources from which phages could be isolated include sewage or wastewater (Yoon et al., 2010), and feces (Oot et al., 2007; Niu et al., 2009; Santos et al., 2011).
Plaques were formed as clear circular with a size ranged between 2-3 mm in diameter when incubated at 37 Co for 24 hrs. This result agreed with (Aidan et al., 2009; Longping and Zhiying, 2014) due to lyses of the bacterial host (MRSA). Sewage water was observed to be the best environmental source to get lytic phages with aggressive infective qualities; this might be attributed to the fact that phages from sewage tolerate hard conditions in the sewage; thus, these phages show high degree of lysis with high tolerance to harsh physical environment. Therefore, lytic phages from sewage showed larger plaques, higher titers, and clearer plaques than others.
This is in agreement with Marwa et al. (2014), they report that the different sources of phages’ isolation and the finding that each phage showed unique profile of size, shape, clarity, and margin cut of plaques provided preliminary evidence that isolated phages are unique and no phages are identical to each other.
Preparation of Phage Stock and Titration: Agar overlay method was used for preparation of S. aureus phage stocks and obtain a titer between 101 to 109 PFU/ml. this result was in agreement with (Iona and Mark, 2014). Which is achieved a titer in excess of 109 PFU/ml.
From our results, the dilution factor gave the best countable number of plaques was (105), this dilution factor was then used for all other experiments. In addition the optical density of all dilutions were recorded at the same time. The presence of these zones of clearing indicated that is, amplifying bacteriophage from a raw sewage sample and inoculating the phage into S. aureus host was an effective way of isolating and visualizing phage. Amplify (increase the numbers) of phages in the sewage sample by allowing them to infect and reproduce within fresh S. aureus, The infection then “spread” as the viruses reproduced and cells lyses, eventually forming a visible plaque the titer of a phage suspension, therefore, is determined by counting the number of plaques that form from a given volume of suspension. Phage titer is expressed as plaque forming units (PFU) per milliliter (ml), it was also determined at this stage which dilution factor gave the best countable number of plaques (Yoon et al., 2001).
Determination of Host Range (Phage Typing)
Spot test was used to determine the type of bacteriophage, MRSA isolates were successfully lysed by phage, forming a clear lyses zone and inhibition bacterial growth at the site of phage dropped. Approving these S. aureus isolates have specific receptor on its cell wall enabling phage to be attach, this is an agreement with Dias et al. (2013) and Longping and Zhiying (2014), which showed relatively wide spectrum host prophylaxis or treatment of S. aureus associated with osteomyelitis.
The specificity of phages for bacterial cells enables them to be used for the typing of bacterial strains and the detection of pathogenic bacteria. Phage typing is also known as the use of sensitivity patterns to specific phages for precisely identifying the microbial strains. For the detection of unknown bacterial strain its lawn is provided with different phages, and if the plaque (clear zones) appears then it means that the phage has grown and lysed the bacterial cell, making it easy to identify the specific bacterial strain (Clark and March, 2006).
Phage can be isolated from a wide variety of source such as sea water, sewage water/sludge etc. These phage feed mainly on locally available organisms and cell. They have an added advantage in that they are host specific and evolve a long with the host. The development of clear zones of lysis against S. aureus using specific phage lysate indicated that the phage we obtained in this experiment were lytic phage. Therefore this current study focused on the isolation of bacteriophage effective against MRSA, the main causative agent of osteomyelitis. Our results were in agreement with Mahadevan et al. (2009), who determined the host rage of the bacteriophage by (spot test) and they found that each of the tree virulent phages was able to independently lyse the original host bacterium.
Minimum Inhibitory Concentration of Bacteriophage against MRSA
Tube dilution tetrazolium assays (TTAs) have been used to probe the relationships between the cell survival, growth and differentiation that rely on the cellular reduction of tetrazolium salts to their intensely coloured formazans in mammalian cells (Scudiero et al., 1988; Marshall et al., 1995). This reagent has also been used effectively to differentiate between live and dead bacteria because only live bacteria convert the dye into an insoluble purple formazan measured at 560 nm (Stevens and Olsen, 1993). We adopted this method of quantifying dead bacteria by TT assay to estimate the MIC of phages. This adapted procedure is done for the first time in Iraq.
Antibiotic resistance amongst bacterial pathogens is a cause of widespread concern. Despite continuous and vigorous efforts, the discovery of new antibacterial has not kept pace with the requirements of the medical profession. Bacteriophage therapy, all but forgotten in the wake of the antibiotic era, has been attracting the attention of many research groups (Sulakvelidze et al., 2001) and efforts are being made to develop this naturally occurring agent into a preventive or therapeutic product. Virulent phages appear to be somewhat similar to antibiotics because they display remarkable antibacterial activity. Recent reports on the effects that such agents and their derivatives (Fischetti, 2010; Appaiah et al., 2012; Vipra et al., 2012) have on antibiotic resistant strains suggest a significant clinical advantage. Because a phage is a complex molecule and does not diffuse like an antibiotic, it is not possible to use a test similar to the disc diffusion test for this particular purpose.
In this study, we examined whether a test similar to the determination of minimum inhibitory concentration can be applied to phage preparations so that clinical laboratories might test the sensitivity of clinical isolates to identify the causative organism and future courses of action and treatment. Therefore, it is important to determine their concentrations quickly and reliably, and the proposed MIC method appears to be both reliable, and reproducible.
This method is laborious and time consuming, and the proposed MIC method appears to be an interesting alternative. In addition, one of the main factors affecting plaque formation is the density of the indicator cells on the plates, because a very dense confluent lawn would affect infection by phage, resulting in very small plaques (Rabbani et al., 2004). None of these factors affect the MIC method of detecting phage adsorption, which is novel, time-saving, easy and could even be applied to mammalian viruses for titer estimation (Goldberg et al., 1994). The TTA colorimetric bactericidal assays have been shown to produce comparable results with S. aureus, Salmonella, E. coli and Klebsiella (Vipra et al., 2013), supporting our observations regarding the applicability of this dye in measuring viability in MRSA. The MIC test can be conducted manually or it can be completely automated, depending on particular lab situations to handle several samples simultaneously. The MIC method could prove very useful in the diagnosis of bacterial strains through phage typing, and the results of this study determine the presence of prophages in any bacterial host, which is both novel and interesting.
Effect of Temperature and pH on the Infectivity of Phage
pH values of 2, 7.4 and 8 were used to determine whether the bacteriophage are affected by exposure to different pH values. The values were chosen in accordance with the range of pH (2-8) obtained in Rabbit GIT. Plaque assays were performed on every sample to register the change in abundance of infectious phages over time.
The impact of bacteriophage treatment of bacterial pathogens in infected rabbits depends on the ability of the phages to reach the target organs at sufficient density and lytic potential to control the pathogen. Numerous factors affect the survival and lytic properties of the phages administered to the rabbits, e.g., delivery mechanism, multiplicity of infection, animal’s immune response, physical and chemical environment, and development of phage-resistant bacteria. However, little is known about the fate of bacteriophages after addition to infected rabbits.
The aim of this experiment was to examine the regulation of bacteriophage survival/decay in controlled laboratory experiments at various environmental pH that influenced the decay rates of phage and the infectivity of the bacteriophage.
Our results in agreement with Jonczy et al. (2011), which they approved that some phage can be stored for a long period in neutral pH (6-8). This experiments testing the pH tolerance of the bacteriophages confirmed that the pH was very important for the infectivity of the phages.
The effect of temperature on the infectivity of phage was also evaluated. The temperature at which a phage loses viability is called thermal inactivation temperature. This loss of viability has been shown to result from disruption of head proteins and the subsequent release of DNA, and the alteration of phage binding receptors (adsorption sites) (Slopek et al., 1987). These results were in agreement to Al-khafaji (2012).
Conclusion
S. aureus was the most frequent isolated bacteria from bone infection in all age patients. Sewage water was observed to be the best environmental source to get lytic phages with aggressive infective qualities and the isolated phages show high degree of lysis with high tolerance to harsh physical environment such as pH and temperatures in vitro. The Tube dilution tetrazolium assays (TTAs) have been used first time in country to estimate the MIC of bacteriophage and it appears to be both reliable, and reproducible. This method is laborious and time consuming, and the proposed MIC method appears to be an interesting alternative.
Acknowledgement
Authors acknowledges to the Department of Physiology and Pharmacology and Surgery, College of Veterinary Medicine, Baghdad University, Baghdad, Iraq.
Conflict of Interests
There exists no conflict of interest.
Authors’ Contribution
All the authors have contributed equally in terms of giving their technical knowledge to frame the article.
References