Advances in Animal and Veterinary Sciences
Review Article
Contagious Pustular Dermatitis (Orf Disease) – Epidemiology, Diagnosis, Control and Public Health Concerns
Rajesh Kumar1*, Raj Narayan Trivedi1, Prakash Bhatt2, Suhaib Ul Haq Khan1, Sandip Kumar Khurana3, Ruchi Tiwari4, Kumaragurubaran Karthik5, Yashpal Singh Malik6, Kuldeep Dhama7, Rajesh Chandra8
1Department of Veterinary Microbiology; 2Veterinary Clinics, College of Veterinary and Animal Sciences, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar, Uttarakhand - 263 145, India; 3Avian Disease Section, National Research Centre on Equines, Hisar, 125 001, Haryana, India; 4Department of Veterinary Microbiology and Immunology, College of Veterinary Sciences and Animal Husbandry, Uttar Pradesh, Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evum Go-Anusandhan Sansthan (DUVASU), Mathura (Uttar Pradesh) – 281001, India; 5Division of Bacteriology and Mycology; 6Division of Biological Standardization; 7Division of Pathology, Indian Veterinary Research Institute (IVRI), Izatnagar (U. P.)- 243 122, India, 8Department of Veterinary Microbiology, College of Veterinary Science and Animal Husbandry, Aizawal, Mizoram, India.
Abstract | Contagious pustular dermatitis (CPD), also known as Orf or contagious ecthyma is an important viral disease of sheep and goats. It is mainly seen as a benign disease but malignant form has also been reported from few parts of the world. The rates of morbidity and mortality are higher, particularly in lambs and kids experiencing the disease for the first time. The causative agent of disease is Orf virus, type species of the genus Parapoxvirus belonging to family Poxviridae. The virus produces localized persistent proliferative skin lesions and affected hosts are infected repeatedly owing to its host immune evasive strategy. These viruses have been found uniformly labile to chloroform but resistant to ether and also exhibit antigenic variations among them. Clinically, the disease is enzootic and occurs in three forms viz. labial, genital / mammary and generalized forms. The incubation period in natural cases is 2-3 weeks. The disease outbreaks mostly occur between autumn and spring but its severity is more in autumn and winter than spring. The virus grows well in cell cultures of caprine, ovine and bovine origin. Confounding symptoms impose laboratory affirmation by serological assays or molecular techniques. The disease can be diagnosed by electron microscopy, serological tests and PCR/ quantitative real-time PCR. Virus specific antibody response to structural proteins of capripox and orf viruses in western-blot analysis readily differentiates these two infections. The antibody response to the 32 kDa and 26 kDa proteins of capripox viruses provides a firm basis for differentiation. Although, the role of humoral immunity is well established but probably cell mediated immunity plays a major role in recovery from natural infections. A number of inactivated and live or live modified vaccines have been tried with variable success. The duration of immunity after vaccination is controversial; outbreaks have occurred in vaccinated animals. The disease is also of public health significance as it causes infection in human beings.
Keywords | Contagious pustular dermatitis, Orf, Contagious ecthyma, Sheep, Goat, Zoonosis
Editor | Muhammad Zubair Shabbir (DVM, M. Phil, Ph D), Quality Operations Laboratory, University of Veterinary and Animal Sciences, Lahore, Pakistan.
Received | August 04, 2015; Revised | September 05, 2015; Accepted | September 06, 2015; Published | November 05, 2015
*Correspondence | Rajesh Kumar, College of Veterinary and Animal Sciences, Govind Ballabh Pant University of Agriculture and Technology, Pantnagar, Udham Singh Nagar, Uttarakhand, India; Email: [email protected]
Citation | Kumar R, Trivedi RN, Bhatt P, Khan SH, Khurana SK, Tiwari R, Karthik K, Malik YS, Dhama K, Chandra R (2015). Contagious pustular dermatitis (orf disease) – epidemiology, diagnosis, control and public health concerns. Adv. Anim. Vet. Sci. 3(12): 649-676.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.12.649.676
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Kumar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Sheep are considered as the moving banks of shepherds which contribute in terms of their meat, wool and hide. Similarly goats are important contributor for the low income persons hence regarded as poor man’s cow but variety of infectious diseases endangers the optimum productivity and led to economic losses (Nadeem et al., 2010; Koufakis et al., 2014). Contagious pustular dermatitis (CPD) is a viral disease primarily of sheep and goats caused by Orf virus belonging to family Poxviridae. As per World Organization for Animal Health, Orf is a notifiable disease and also a zoonotic disease transmitted from animals to humans (Nadeem et al., 2010). It has been known as contagious dermatitis or Orf as early as 1890 by Wallay. The disease affects several other animal species also. The synonyms of disease used in various outbreaks included contagious pustular stomatitis, contagious ecthyma, ecthyma contagiosum, infectious pustular dermatitis, infectious labial dermatitis, sore mouth, scabby mouth and orf (de Wet and Murie, 2011). The disease is prevalent in all the continents with several enzootic areas all over the world. The infection in human beings is still known as ‘human orf’ (Kuhl et al., 2003; Georgiades et al., 2005; Uzel et al., 2005; Pal et al., 2013). Morbidity and mortality is variable. Although, the disease has been considered as a benign disease but it has taken a serious turn in last decade and in certain countries, a severe malignant form of disease has been reported (Elzein and Hausawi et al., 1997; Haig and Mercer, 1998; Ishii et al., 2010; Nandi et al., 2011; Housawi FMT, Abu-Elzein, 1992; McInnes, 2014). At present some vaccines can control the disease, however presence of virus in the environment increases the risk to other animal species. The role of cell mediated immunity in elimination of virus from infected animals is also not fully understood.
Etiology
The CPD is caused by Orf virus which is type species of the genus Parapoxvirus of subfamily Chordopoxvirinae of family Poxviridae. Other members of the genus are Bovine papular stomatitis virus, Parapoxvirus of red deer in New Zealand and Pseudocowpox virus (Nandi et al., 2011; ICTV, 2014). Orf is a dsDNA virus with 138kb long genetic material consisting of approximately 64% G+C content (Li et al., 2012). Presently 4 strains of orf have been sequenced completely namely OVIA82 and OV-SA00 in America, D1701 in Germany and NZ2 in New Zealand (Delhon et al., 2004; Mercer et al., 2006; McGuire et al., 2012). On the basis of antigenic relationship of CPD with vaccinia ectromelia viruses and similarity in size, shape and pathogenicity, CPD virus was included in the mammalian pox group (Webster, 1958; Li et al., 2012). However, sheep orf viruses differed from bovine papular stomatitis and pseudocowpox in the type of cytopathic effects (CPE) produced and indicated that bovine strains formed one closely related group and the sheep strains another (Nagington, 1958). The human and ovine strains of CPD virus are immunologically and culturally similar. They are ether resistant and chloroform sensitive (Liess, 1962; Davies et al., 1975). However, they are distinguishable from bovine papular stomatitis and pseudocowpox on the basis of type of CPE produced as shown by plaque formation and the appearance of stained cells (Nagington, 1968).
Morphology and Morphogenesis
The elementary bodies of CPD virus are short rods with rounded ends and dense sub polar regions (Abdussalam and Cosslett, 1957). The virus is ellipsoidal (Ishii et al., 1953) or elongated and ovoid in shape with diagonally woven bands (Hessami et al., 1979; Hawkins et al., 1990; Mercer et al., 2006; Zhao et al., 2010). The virus measured between 260 and 100 nm when filtered through collodian membranes of known pore diameter (Blanc and Martin, 1949; Frano and Vrtiak, 1957). However, electron microscopy of negatively stained purified orf virus preparations and infected tongue scrapings suggested 220-250 x 120-140 nm (Hessami et al., 1979) and 260 x 160 nm (Hawkins et al., 1991) size. Both apparently complete form of virus with a nucleoid and surrounding envelop and various immature forms of virus particles in different developmental stages have been seen in the cytoplasm of cells (Nagington and Horne, 1962; Kim et al., 1977) in the form of compact microviroplasts as well as fragmented form, often located near to nuclear membrane containing osmophilic fibrils. The viral envelop were seen as arcs around the viroplast and showed a radially arranged toothed appearance like a cog wheel (Saginova and Soslyakov, 1982; Counago et al., 2015).
The characteristic tubular thread structure of wall of the particle in the form of left hand spiral coil of single thread (Nagington, 1964; Mercer et al., 2006; Zhao et al., 2010) with crisscross pattern in negative staining were demonstrated by shadow casting as false and induced by top and bottom images (Buttner et al., 1964: Delhon et al., 2004) the surrounding membrane were thicker, the double element of the peripheral protein layer and inner body with triplet of tube like strands (Schulze and Schmidt, 1964). Electron microscopy of purified CPD virus revealed typical surface structure characterized by an outer layer over a layer of spiral like filament or tubules (Schulze and Schmidt, 1964; Rosenbusch and Reed, 1983; Tan et al., 2009) whereas, innermost designated coat was closely opposite to the tubular surface (Rosenbusch and Reed, 1983). The simplest model of envelop comprises an internal envelop subunit protein associated with the polar ends of an organized layer of phosphoglyceride molecules whose nonpolar parts are in turn associated with a layer of triglyceride molecules and residual space bounded by adjacent subunits and by the external envelop is occupied by cholesterol (Mithiner, 1969). The internal component demonstrated within the particle contains DNA which is 2.85x10-16g/particle equivalent to a mole (Delhon et al., 2004). Wt. of 171x106 / particle (Nagington et al., 1964). However, Robinson et al. (1982) reported the molecular weight of DNA as 88.8x106 daltons. The dry weight of orf virus is 3.69x1015g and nitrogen content is 14.7% of dry weight (Nagington et al., 1964).
Physico-Chemical Properties
The orf virus from different geographical areas have similar physico-chemical properties and were resistant to alternate freezing and thawing up to 4 cycles, U.V. irradiation (Sawhney, 1972), heat stable (Sawhney, 1972), filterable, resistant to pH-3 and behaved similar to pox viruses (Precausta and Stellmann, 1974). The virus in dried scabs was viable from 8 months to more than a year at room temperature (Manley, 1934; Newsom and Cross, 1934), but crusts exposed to external conditions lost their infectivity rapidly (Manley, 1934: Aptow, 2010) during summer. However, during winter infectivity persisted for at least 6 months (Boughton and Hard, 1935). At room temperature, maximum survival of virus in dried state is 15 years (Huang and Li, 1986). The exposure to heat at 1000C for one minute inactivated the virus but it survived 560C for one hour (Saddour, 1989).
Infectivity of CPD was also preserved in the pH range of 4.2-10.9 (Schmidt, 1967a; Mckeever and Reed, 1986). It passed through collodian membranes APD 700 micron (Hart et al., 1949) and loss in potency/infectivity (Manley, 1934; Newsom and Cross, 1934) but completely retained by Mandler Normal candles (Newsom and Cross, 1934).
The CPD viruses isolated from disease outbreaks in different geographical areas were uniformly labile to chloroform (Sawhney, 1972; Saddour, 1989) but resistant to ether (Trueblood, 1961; Trueblood and Cho, 1963; Sawhney and Toschkov, 1972; Saddour, 1984) and saponin (Sawhney and Toschkov, 1972). The scabs from infected animals became nonvirulent in 2 hours by carbolic acid (1:100), formalin (1:2000) and mercuric chloride (1:20000) but not by potassium permangnate ((1:20000) as observed by Manley (1934). The virus was less penetrable by proteolytic enzymes and stains than vaccinia and related viruses (Abdussalam and Cosslett, 1957) but trypsin treatment slightly increased the virus titer presumably by releasing intracellular virus (Sawhney and Toschkov, 1972). The virus was rapidly destroyed by 50% glycerine (Manley, 1934) but 10% glycerol prolonged the survival time at 4˚C and DSG-72 preserved total viability during lyophilization (Saddour, 1989).
Antigenic Properties
Various strains of CPD virus were antigenically identical in gel diffusion test (Sabban et al., 1961; Preriches, 1980; Dashprakash et al., 2015) with at least 4-5 specific precipitation lines against homologous antiserum (Schmidt, 1961; Rao et al., 1984) but reciprocal cross immunity tests between isolates exhibited some minor antigens in some isolates other that a common major antigen (Horgan and Haseeb, 1947). The purified CPD virus after disintegration with Trion x-100 and -2-mercaptoethanol together, revealed at least 28 polypeptide bands in SDS-PAGE with molecular weight of 4.5, 40.7, 38 and 33.1 kDa (Zuo et al., 1988). The virions treated with NP 40/2ME revealed some 13 polypeptides in soluble fraction and a polypeptide of mole. wt. 38.5 KD believed to be the basic subunit of virion surface tubule (Mckeever et al., 1987). However, after disruption in SDS/2ME, the SDS/ PAGE of purified 5 independently isolated New Zealand orf virus strains exhibited up to 35 polypeptides and had little difference in their polypeptide profiles (Robinson, 1987). The structural analysis of polypeptides from 11 isolates of CPD virus by SDS/PAGE also indicated similar profile except in the molecular weight in the region of 37-40 kDa which appeared to be components of the surface tubules (Buddle et al., 1984). Expression of the virally encoded vascular endothelial growth factor VEGF-E gene and its variants has been documented as cause of proliferative and highly vascularized severe persistent lesions of the orf due to their ability of stimulating endothelial cell proliferation and promoting vascular permeability and significantly contributes to the orf viral pathology. Literature showed interferon stimulated gene expression is inhibited by Orf virus and it modulates the JAK/STAT signaling pathway (Scagliarini et al., 2006; Wise et al., 2007; Harvey et al., 2015).
The PAGE carried out on 15 samples of CPD virus (6 from sheep, 9 from goat) and 2 samples one each of bovine papular stomatitis and pseudocowpox confirmed the differences in composition with major variations between 20 and 44 KD (Gonzalez et al., 1991a). Restriction enzyme analysis with Kpn 1 and hybridization and electroblots confirmed the heterogeneity among different strains of CPD virus due to deletion of DNA segments and changes in nucleotide sequences (Rafi and Burger, 1985; Zamri-Saad et al., 1994). The immunodiffusion and counter immunoelectrophoresis (CIE) of 32 CPD viruses isolates also revealed considerable variation among CPD viruses from different geographical regions which had more influence than host species on its antigenic similarity. Different types of parapox viruses had antigenic similarities suggesting parapox virus a single virus adapted to various ruminants (Gonzalex et al., 1991b). Peralta et al. (2015) described the sequencing and analysis of five molecular markers of orf virus: a partial B2L gene (ORF011), VIR (ORF020), an envelope mature protein (ORF109), vIL10 (ORF127), and GIF (ORF117) from two outbreaks in Argentina. Tseng et al. (2015) showed that the shorter isoform (sh20) of OV20.0 protein of orf virus (ORFV) arises due to use of a downstream initiation codon and is amino-terminally truncated. This form also differs in expression kinetics and cellular localization from full-length OV20.0. Similar to the full-length isoform, sh20 is able to bind dsRNA and PKR, inactivate PKR, and thus act as an antagonist of the interferon response in vitro. In vivo, however, wild-type OV20.0 could not be replaced with sh20 alone without a loss of virulence showing that each has a role. The phylogenetic analyses of orf viruses from Korean black goats most closely related to an isolate (ORF/09/Korea) from dairy goats in Korea, indicating that orf viruses were probably introduced from dairy goats into the Korean black goats (Oem et al., 2013). Lately, Martins et al. (2014) demonstrated that Brazilian orf viruses isolates display differential virulence in lambs which might be associated with genetic changes in putative virulence genes. Subsequently, Yang et al. (2014) provided molecular evidence about the genetic variability of the major antigenic and virulence genes in the virus isolates from epidemic in Xinjiang (China). Zhang et al. (2014) found three genotypes by molecular epidemiology analysis of orf virus from goats and sheep in China during 2009-2011, amino acid substitutions were dispersed among B2 polypeptides from wild and attenuated orf virus strains. The isolation and identification of orf virus from an outbreak in goats in the Rajasthan State of India (Maan et al., 2014). The virus was isolated in Vero cell cultures identified using GIF/IL-2 and B2L gene specific primers in PCR and sequencing. The virus showed high nucleotide similarity with Chinese, far eastern, Brazilian and Indian isolates. Duan et al. (2015) observed the typical structure of the orf virus in cell culture inoculated with GDQY orf virus. Full-length of four genes was amplified and sequenced. Phylogenetic analysis indicated GDQY homologous to FJ-DS and CQ/WZ on ORFV011 nucleotides. ORFV059 was more variable than ORFV011. Comparison of the NA1/11 genome with the sequences of other strains of orf virus revealed highly variable regions indicative of genetic diversity (Li et al., 2015).
Serological Relationship
The isolates of CPD virus from some regions and host species are immunologically identical by cross immunity and reciprocal cross immunity experiments (Horgan and Haseeb, 1947a; Mundu and Mohan, 1961; Precausta and Stellmann, 1973). However, isolates from different geographical regions did not belong to the same antigenic subgroup (Sawhney, 1966) but some of them were similar (Precausta and Stellmann, 1973; Precausta and Stellmann, 1974). The neutralization index of various isolates were different (Precausta and Stellmann, 1973; Buddle et al., 1964) and since, cross reaction among isolates were unilateral, the grouping of CPD virus isolates by this test was impossible (Buddle et al., 1948a).
Although, the goats recovered from goatpox were susceptible to the virus of CPD (Hanley, 1934), it provided some immunity against CPD (Mundu and Mohan, 1961) and perceptible cross reaction in cross complement fixation between CPD and sheep and goatpox viruses and vice-versa (Sharma, 1966; Sharma and Dhanda, 1971). In cross neutralization test, goatpox hyper immune serum neutralized CPD viruses but not the vice-versa revealing one way serological relationship between goat and sheep pox and CPD viruses (Sharma and Bhatia, 1959; Dubey and Sawhney, 1979; Rao and Malik, 1979; Rao and Malik, 1984). On cross agar gel diffusion and immunoelectrophoresis 6 common factors were observed between goatpox and CPD viruses (Dubey and Sawhney, 1979) However, in vitro serum neutralization and cross immunity studies indicated that CPD and goatpox virus from western USA were antigenically dissimilar and could not confer immunity against other (Renshaw and Dodd, 1978).
The virus of milker’s nodule, bovine papular stomatitis, udder pox and CPD of sheep formed a morphologically and serologically uniform group (Liebermann, 1967a). Direct or indirect FAT with 20 strains of these viruses and vaccinia and bovine papular stomatitis revealed that all the viruses were closely related except lumpy skin disease virus (Liebermann, 1967b). Orf and milker’s nodule viruses were indistinguishable by agar gel diffusion (AGPT) and both were closely related to bovine papular stomatitis virus (Freriches, 1980). Indirect FAT and immunoblotting showed significant antigenic overlapping between them as monoclonal antibody against any virus specified cell surface proteins recognized a 40-43 KD protein in orf virus infected cells and also a 45-48 KD protein in cells infected with MNV and BPS virus (Lard et al., 1991; Wang et al., 2015).
The complement fixation, AGPT and virus neutralization tests demonstrated that CPD virus also/ shares antigens with vaccinia and ectromelia viruses (Webster, 1958). It was reported that CPD virus shared a common group specific antigen with vaccinia (Schmidt, 1967c; Mercer et al., 1994) but lacked cross immunity (Robinson, 1988; Mercer et al., 1994). It is also antigenically related to ulcerative dermatosis virus (Trueblood, 1966) and virus of Auzdyk (Roslyakov, 1972) and sheep and goatpox (Rao and Malik, 1984).
Cultivation of Orf Virus
Chicken Embryos
Various attempts to cultivate the virus in developing chicken embryos have failed (Hart et al., 1949; Chodkowski and Zebrowski, 1952; Ishii et al., 1953; Greig, 1956; Gunenkov and Syurin, 1966; Sharma and Dhanda, 1972) or only mild lesions were produced on CAM up to 4th generation (Abdussalam, 1957). However, east German and Bulgarian (B2) strains of CPD virus were successfully propagated on CAM of developing chicken embryos (Sawhney, 1966; Said et al., 2013). The lesions were characterized by haemorrhagic circular lines, oedema and diffuse foci on successive passages until 4th inoculation egg batch (Mazur and Machado, 1989). The susceptibility of chicken embryos to CPD virus is strain dependent, as out of nine strains isolated in Azerbaijan, two could not be adapted to chick embryos (Shakhbazov, 1975).
Table 1: Propagation of CPD virus in different cell cultures
|
Cell culture |
References |
|
Embryonic lamb kidney |
Powright et al. (1959), MacDonald and Bell (1961), Rao and Malik (1982), Burgu and Toxker (1987) |
|
Embryonic lamb testis |
Housawi et al. (1991) |
|
Embryonic lamb lung |
Pospischil and Bachman (1980), Adjid (1992) |
|
Fetal lamb muscle |
Populain et al. (1972) |
|
Fetal lamb skin |
Vdovina et al. (1975) |
|
Lamb kidney |
Sawhney (1966), Ramyar (1973), Precausta and Stellmann (1974), Saddour (1981), Said et al. (2013) |
|
Lamb testis |
Plowright et al. (1959), Sawhney (1966), Traikova (1982), Mazur and Machado (1990) |
|
Lemb epidermis |
Saginova and Roslyakov (1982) |
|
Lamb thyroid |
Burgu and Toker (1987) |
|
Embryonic kid kidney |
Plowright et al. (1959) |
|
Kid kidney |
Housawi et al. (1991) |
|
Kid testis |
Rao and Malik (1982) |
|
Embryonic calf kidney |
Plowright et al. (1959), Trueblood and Cho (1963), Schulze and Schmidt (1967, Ergin and Koklu (1977, Munz (1969) |
|
Embryonic calf lung |
Pospischil and Bachmann (1980) |
|
Embryonic calf muscle |
Munz (1969) |
|
Fetal calf spleen |
Hessami et al. (1979) |
|
Calf kidney |
Sawhney (1966), Saddour (1989) |
|
Calf testis |
Plowright et al. (1959), Schimmelpfenning and Liess (1962), Sawhney (1966), Balassu and Robinson (1987) |
|
Pig testis |
Gerstl (1964) |
|
Pup kidney |
Willayat (1981) |
|
Human amnion and embryonic liver |
MacDonald and Bell (1961) |
|
Chicken embryo fibroblast |
Rossi (1973), Rossi and Casalena (1973), willayat and Garg (1994) |
|
Duck embryo fibroblast |
Rossi (1973), Rossi et al. (1976) |
|
MS cells |
Ramyar (1963) |
|
Vero cells |
Kiva et al. (1977), Hussain and Burger (1979) Housawi et al. (1991), Mann et al. (2014) |
Cell Culture
CPD virus produces similar cytopathic effects in various cell cultures (Table 1). The isolates from sheep and chamois and ovine and human strains of orf virus had no difference in CPE in pig testicular (Gerstl, 1964), calf testicular (Schimmelpfenning and Liess, 1962) and bovine fetal spleen cells (Hessami et al., 1979) respectively. The English and Bulgarian B2 strains multiplied better in lamb and calf testicular and kidney cells (Plowright et al., 1959). The CPD virus isolated from sheep and goats produced CPE within 48-72 hour in primary testis cell monolayers (Mazur and Machado, 1990) which reduced to 24-48 hour after 21 consecutive passages.
Isolation and Growth Behavior of Orf Virus
The virus from human lesions has been successfully isolated in tissue culture (Nagington and Whittle, 1961), particularly in human amnion cells in medium containing 10% calf serum (MacDonald and Bell, 1961). However, the virus has also been isolated from biopsy samples of human lesions on calf testicle cell culture (Liess, 1962) and bovine fetal spleen cells (Hessami et al., 1979). CRV-102 isolated from a human case of orf produced higher infectivity titer in Vero cells in comparison to ovine orf (CEV-29A and 378) but exhibited similar growth parameters (Hussain and Burger, 1989). The isolates from a common source of infection induced similar CPE in bovine fetal spleen cells (Hessami et al., 1979) but sheep orf virus was initially able to grow in sheep cells whereas sheep virus recovered from human lesions lost this specificity (Nagington, 1968).
The infection is disseminated rapidly and completely but the difference in peak titers in different cell cultures and of appearance of CPE depended on the level of adsorption (Plowright et al., 1959; Schmidt, 1967b) and adaptation of virus in particular cell culture system (Schmidt, 1967b; Saddour, 1989). By indirect FAT, the viral antigen first appeared in the cytoplasm of infected CEF cells at 30 hour PI and reached its greatest diffusion during following 24 hour (Rossi and Casalena, 1973). The virus persisted up to 96 hour in sheep fetal skin culture (Vdovina, 1975).
The CPE produced by CPD virus in bovine fetal spleen cells were characterized by plaque like changes consisting of large round cells with eosinophilic intracytoplasmic inclusions of varying size at 8-18 hour post infection (PI) followed by basophilic inclusions attached to pyknotic nuclei. The cytoplasmic inclusions separated the plasma membrane from nucleus visual complex by 24 hour PI, when the cells detached (Hessami et al., 1979). Almost similar changes were recorded in calf testis monolayer (Schimmelpfenning and Liess, 1962). In calf kidney monolayer, Welcome Institute strain (Wl) exhibited complete adsorption within 16 hour PI with first evidence of virus multiplication at 24 hour and maximum titer at 72 hour which reduced to 24 hour after 47 passages (Schmidt, 1967b). Similar results were also obtained with duck embryo fibroblast (DEF) cell culture infected with three strains of CPD virus (Rossi, 1973). The CPD virus in BFS cells exhibited increased virus infectivity at 8 hour PI with maximum titer at 36 hour PI (Hessami et al., 1979). The cell free and cell associated titers of CPD virus in kid testis cell culture was maximum at 96 hour PI (Rao and Malik, 1982) with higher titers of virus in cell associated fraction than respective cell free fractions (Plowright et al., 1959; Hessami et al., 1979; Rao and Malik, 1982).
One step growth experiment of CPD virus in bovine testis cells revealed presence of newly replicated viral DNA at 4-6 hour PI, accumulated rapidly between 8-16 hour PI and reached plateau between 25-30 hour PI. Most viral induced polypeptides were first detected during 2 hour beginning at 10 hour and reached peak rate of synthesis between 14-16 hours PI and continued at that rate for at least 10 hour. The host polypeptide synthesis declined to very low levels by 20 hour PI. The transition between early and late events occurs between 8-10 hours PI. The infective virus was first detected between16-18 hour and produced at steady state till 40 hour PI (Balassu and Robinson, 1987).
The ovine (29 A and 378) and a human orf (CEV-102) exhibited similar growth pattern in Vero cells and sheep but differed in plaque morphology and ability to induce vesicle formation in sheep. In-vitro latent period and highest infectivity titers of CEV-102, 29 A and 378 were 96, 48 and 120 hour and 1.4x109 , 6.8x107 PFU/gm respectively, and viruses were no longer detectable at the site of inoculation by 288 hour PI. Hence, plaque morphology, ability to induce vesicle formation and growth curve in skin can be considered as important criteria to differentiate CPD virus isolates (Hussain and Burger, 1989).
TRANSMISSION
Natural transmission of disease occurs through direct or indirect contact with infected animals (Kummeneje and Krogsrud, 1979) particularly with dried crusts that falls on the pastures during grazing (Harriss, 1948). The disease was also transmitted from wild to domestic goats when infected herds had prolonged contact with areas where salt blocks were provided artificially on highways and camp grounds. Fewer infected sheep were observed annually when salt blocks were removed from Jasper National park (Samuel et al., 1975). However, rapidity of spread and extent of lesions of CE were ascribed to penetration by sharp stems of the edible shrub (Temoletonia retusa) (Gardiner et al., 1967) that prepares the path for infection (Rubino and Espantoso, 1937). In outbreaks of CE, it was suggested that with the presence of spiky weeds in the pastures, the ecthyma virus was being inoculated into the mouth (Hawkins et al., 1991). The pest of small ruminant’s virus in West Africa causing erosions of lips and mouth also provided an entry for orf virus (Obi and Gibbs, 1978). Aerosol transmission of virus has also been reported (Bowden et al., 2008). The disease can also be transmitted by use of contaminated gavage feeding tube (Moore et al., 1983) or by ear tagging (Housawi and Elzein, 1992). The disease from infected sheep and goats has been transmitted to other species of animals by feeding them raw sheep carcasses (Wilkinson et al., 1970). Transmission cycle of orf virus is depicted in Figure 1.
DISTRIBUTION
Contagious pustular dermatitis of sheep and goats was reported initially by Zellor in 1920 from South West Africa. Since then, it has been reported from almost all parts of world involving not only sheep and goats but cattle, dogs, camel and both free living wild and captive animals also. According to Animal Health Year Book (1992) published by FAO/OIE/WHO in 1992, the disease exists in countries listed in Table 2. In addition, the disease outbreaks have also been reported from Norway (Vikoren et al., 2008), China (Wu and Sun, 1992), Indonesia (Adjid, 1992), Iraq (Hussain et al., 1992), Brazil (Nobrega et al., 2008) and Spain (Mariscal-Estrada et al., 1992). Cases are reported from Pakistan (believed to be endemic) but prevalence reports are very few (Abbas and Mughal, 2014). Orf is endemic in China though vaccination has been practiced. Between the period of 1980- 1990 almost 8 provinces (Gansu, Heilongjiang, Hebei, Jiangxi, Liaoning, Tibet, Qinghai and Xinjiang) were affected with orf (Zhang et al., 2010).
HOST RANGE
Contagious pustular dermatitis has been noticed primarily in ovine and caprine species (Animal Health Year book, 1992;
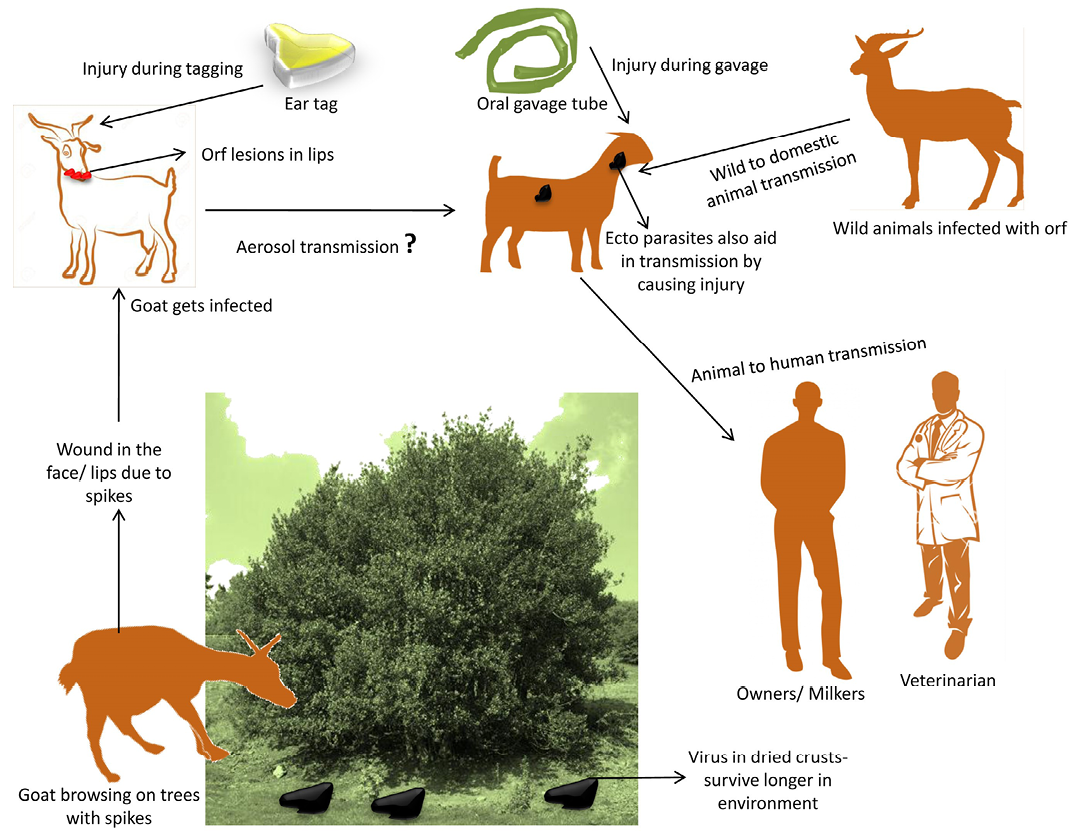
Figure 1: Transmission cycle of orf virus
Table 2: Occurrence of CPD in sheep and goats in different countries
|
Continents |
Species |
Occurrence of disease |
||
|
Low sporadic |
Enzootic |
High |
||
|
1. Africa |
Sheep |
Algeria, Burundi, Congo, Ethiopia, Mauritania, Namibia |
Guinea Bissau |
- |
|
Goat |
Ghana, Kenya |
Burundi, Namibia |
- |
|
|
Sheep & goat |
Benin,* Botswana, Comoros,* Cote d’ Ivoire, Djibouti, Lesatho, Liberia, Morocco, Mozambique, Nigeria,* Senegal, Tanzania,* Tunisia, Zimbabwe |
Africa |
- |
|
|
2. America |
Sheep |
Argentina, Belize, Guatemala,* Peru, Nicaragua, Venezuela*** |
Cuba, Uruguay |
- |
|
Goat |
Cuba |
Antigua and Barbuda |
- |
|
|
Sheep & goat |
Bahamas, Bolivia, Brazil, Canada,*** Dominican republic, * Mexico, USA |
Costa Rica, Guyana |
- |
|
|
3. Asia |
Sheep |
Bahrain, Syria, Yemen, UAE,*** |
- |
- |
|
Goat |
Sri Lanka, Bangladesh |
- |
- |
|
|
Sheep & goat |
Cyprus, Israel, Jordan, Lebanon, Turkey, Malaysia |
India, Oman, Mongolia |
- |
|
|
4. Europe |
Sheep |
Bulgaria, France, Germany,*** Iceland, Ireland |
- |
- |
|
Sheep & goat |
Albania, Hungary, Netherland, Switzerland, UK |
Norway |
Greece |
|
|
5. Oceania |
Sheep |
Fiji, New Caledonia |
Australia |
- |
|
Sheep & goat |
- |
New Zealand |
- |
|
* Disease exists but occurrence unknown; ** Disease in imported animals only; *** Exceptional occurrence of disease
Mondol et al., 2006; Isshi et al., 2010; Zhang et al., 2010; Kinley et al., 2013; Joseph et al., 2015) but occasionally it occurs in chamois (Gerstl, 1964; Veternik and Zednik, 2013), reindeer (Kummenje and Krogsrud, 1979; Klein and Tryland, 2005), serow of bovidae family (Okada et al., 1984; Guo et al., 2004), muskox (Zarhkl et al., 1983; Mathiesen et al., 1985; Vikoren et al., 2008), camel (Dashtseren et al., 1984; Munz et al., 1986; Azwai and Carter, 1995), cattle, cats (Kertzdorn, 1985; Guo et al., 2004) and dogs (Wilkinson et al., 1970; Paiba et al., 1999) also. Experimental infection of CPD has been established in sheep and goats (Selbie, 1944; Ishii et al., 1953; Vrtiak and Vrzgula, 1955; Greig, 1956; Sharma and Bhatia, 1959; Makeever et al., 1987; Savory et al., 2000; Housawi et al., 2010), buffaloes (Huang and Li, 1986), dogs, cat and man (Borcilla and Isopescu, 1937) and rabbits (Blaizot, 1937; Borcilla and Isopescu, 1937; Selbie, 1944; Marrone, 1948; Abdussalam, 1953; Ishii et al., 1953; Abdussalam ,1957; Mundu and Mohan, 1961; Sharma and Dhanda, 1972; Huang and Li, 1986; Willayat and Garg, 1992).
Experimental infection could also be established in moose calf (Alces aloes), caribon (Bangifer tarandus) by Zarhkl et al. (1983) and mule deer dawns (Odocoileus hemionus), white tailed deer dawns (Odocoileus verginionus), proughorn daens (Antilocapra americana) and wapiti calves (Cervus elaphuselsoni) by Lance et al. (1983).
Pigs, horses (Slagsvild, 1938; Ishii et al., 1953), poultry (Slagsvild, 1938), guinea pigs (Slagsvold, 1938; Harries, 1948; Ishii et al., 1953; Greig, 1956; Abdussalam, 1957; Mundu and Mohan, 1961; Trueblood and Cho, 1963; Sharma and Dhanda, 1972), rats (Harries, 1948), mice (Greig, 1956; Abdussalam, 1957; Mundu and Mohan, 1961; Trueblood, 1961) and hamsters (Trueblood and Co, 1963; Sharma and Dhanda, 1972) were found refractory to CPD virus infection.
EPIDEMIOLOGY
Contagious pustular dermatitis was recognized as a benign disease (Thorp, 1942; Fontanellli and Caparrini, 1955; Buchner et al., 1963) as infected animals, particularly adults recovered spontaneously (Fontanelli and Caparrini, 1955; Tontis et al., 1981) within 3-5 weeks without treatment (Coates and Hoff, 1990; Giangaspero et al., 2013). In the last decade, the classical picture has changed in GPR and Australia where the disease exhibited malignant form with high mortality in lambs experiencing the disease for the first time and persisted over 9 months. The losses were very high due to reduced weight gain and mortality even in adult sheep (Valder et al., 1979). Sometimes, the disease may occur in acute form (Chadkowski and Zebrowski, 1952; Volkova et al., 1967; Munz, 1969) or changes into a chronic form (Nolikuwera et al., 1992) when complicated by secondary invaders.
All the age groups are equally affected (Harriss, 1948; Kozlowski and Dziekonski, 1956; Sharma and Bhatia, 1959; Obi and Gibs, 1978; Kummeneje and Krogsrud, 1979; Tontis et al., 1981; Munz et al., 1986; Mazur and Machado, 1989; Housawi et al.,1991; Yeruham et al., 1991; Billinis et al., 2012; Bouznach et al., 2013) irrespective of breed and sex of the animals (Flament and Martin, 1938; Sharma and Bhatia, 1959; Kater and Hansen, 1962; Gerstl, 1964; Samuel et al., 1975; Kumar et al., 1974; Debouck, 1979; El-Dahaby et al., 1980; Pekelder et al., 1980; Ilic et al., 1986; Zanin et al., 1987; Coates and Hoff, 1990; Nooruddin and Barik, 1989; Nolikuwera, 1992; Yeruham et al., 1991; Wu and Sun, 1992). United Kingdom has documented 2.167 million orf affected sheep, leading to £10 million loss, includes both treatment and production losses as per the report of Bennett and Ijpelaar (2005). Onyango et al. (2014) reported prevalence of orf in UK to be 1.88% and 19.53% in ewes and lambs respectively. The morbidity and mortality is variable depending on the age group and prevalence of disease in the area (Onyango et al., 2014). In an outbreak of CPD in Hungary, the morbidity and mortality averaged 11 and 6.5% but 74% of the affected sheep were under 4 months of age and 73% of these were under 4 weeks of age (Olah and Elek, 1953a). The morbidity in lambs of less than 1 month old was recorded to be 77.6% in Spain (Mariscal-Estrada et al., 1992). The mortality of up to 78% was observed in a severe form of CPD in lambs in U.K. (Darbyshire, 1961) whereas in Guatemala, it reached upto 90% in a flock of sheep (Rosales and Loarca, 1971). In one year old sheep in Germany (GFR), 70% out of total 300 in a farm suffered from CPD in a severe outbreak (Theil and Rudolph, 1981) but 100% morbidity and 93% mortality were recorded by Mazur and Machado (1989) in a herd of 117 goats including 38 unweaned kids. In Israil also, 80% mortality in yaez was noticed due to CPD by Yeruham et al. (1991).
The morbidity and mortality rates were 10 and 3% in Switzerland (Tontis et al., 1981), 2 and 1.3% in India and Bangladesh (Mundu and Mohan, 1961; Nooruddin and Barik, 1989), 70-80 and 5-15% in Saudi Arabia (Housawi et al., 1991) and 60.66 and 8.95% in Iraq (Hussain et al. 1992). A mortality of 24.7% (162 out of 655) was documented in free ranging goats in China even after administration of antibiotics to prevent secondary bacterial infections (Zhang et al., 2010).
In spite of the widespread nature (Thorp, 1942; Mikami et al., 1978), the economic importance of CPD was thought to be limited (Mikami et al., 1978). However, loss of mammary gland in lactating ewes (Rosales and Loarca, 1971), under development (Tontis et al., 1981) and lean and stunted growth of survivors (El-Dahaby et al., 1978) caused great economic losses (Valder et al., 1979). If lambs with CPD are rejected for slaughter, 1.25 million lambs would have lost in New Zealand alone (Robinson, 1983). The economic cost of an outbreak in Spain was estimated to be ptas 87000 due to losses in production and cost of labour and drugs (Mariscal-Estrada et al., 1992).
Incorporation of fresh lot of animals with history of disease at a farm (Mazur and Machado, 1989) or reincorporation of animals taken away from the farm for some time acts as source of disease (Coates and Hoff, 1990). Scagliarini et al. (2012) carried out a seven month study in South Africa, from the period of September 2009 to March 2010 and recorded 54 outbreaks of orf during that period, showing the impact of this disease. Boer goats were reported to be commonly affected by orf. This study also reported that plants of the species Acacia was linked to the outbreaks of orf. These plants have woody sharp thorns which may damage the mucosa in lips and also can cause damage to the facial muscles leading to the viral entry and infection (Scagliarini et al., 2012). The outbreaks are also recorded from the survival of virus without a new source being introduced (Slagsvold, 1938) in Morocco, Sabban et al. (1961) in Egypt, Rodrignez et al. (1983) in Mexico, Kumar et al. (1974) in India and Debouck (1979) in Belgium. The disease once imported into a farm, may become endemic there (Nfi, 1992) and serves as source of infection to other indigenous animals. In Norway, the disease among captive vuskox is causing heavy losses and before 1982, it was the main cause of death in a research herd formed from animals imported from Greenland (Mathiesen et al., 1985). The infection in sheep and goats puts other species at risk as dogs acquired disease trough feeding on raw sheep carcass (Wilkinson et al., 1970), in camel, it was transmitted from sheep (Munz et al., 1986) and in reindeers also, it came most likely from sheep and goats as the disease is very common in that area. It was concluded that sheep plays an important role in maintaining the virus in the environment (Housawi et al., 1993; Bhanuprakash et al., 2006). Genomic studies based on DNA amplification, sequencing and phenotypic correlation suggested that genetic variation and movement of the circulating orf virus strains occur in between different geographical areas (Kottaridi et al., 2006a; Billinis et al., 2012; Zhang et al., 2015).
The outbreaks of CPD mostly occur between autumn and spring (Slagsvold, 1938; Gerstl, 1964; Pekelder et al., 1980; Tontis et al., 1981; Larson and Zahoory, 1983; Sinha et al., 1986; Zanin et al., 1987) but its severity was more in autumn (Tontis et al., 1981; Zanin et al., 1987) and winter (Gerstl, 1964; Zanin et al., 1987; Sarr et al., 1988) than in spring (Tontis et al., 1981). However, some outbreaks do occur in summer (Pekelder et al., 1980; Tontis et.al., 1981; Hussain et al., 1992) also. Serological evidence also revealed high neutralizing antibody titres frm December to March and low from May to July (Saar et al., 1988). In an extensive study conducted over 6.3 million lambs, Robinson (1983) reported highest incidence of CPD (2.2%) in December and January.
THE DISEASE
Clinical Signs and Gross Lesions
The lesions of CPD are primarily perilabial (Slagsvold, 1938; Roncero et al., 1987; Zanin et al., 1987; Housawi et al., 1991) involving oral commissures (Kummeneje and Krogsrud, 1979; Nooruddin and Barik, 1989; Hussain et al., 1992), lips (Ishii et al., 1953; Darbishire, 1961; Kater and Hansen, 1962; Gardiner et al., 1967; Verdes et al., 1970; Obi and Gibbs, 1978; Valder et al., 1979; Rodrignez et al., 1985; Munz et al., 1986; Nooruddin and Barik, 1989; Hussain et al., 1992), muzzle (Obi and Gibbs, 1978) and mouth (Darbyshire, 1961; Obi and Gibbs, 1978; Thel and Rudolph, 1981; Hawkins et al., 1991) including oral mucosa (Marsh and tunnicliff, 1937; Darbyshire, 1961; Morales and Van Kruiningen, 1971; Valder et al. 1979; Tontis et al., 1981; Munz et al., 1986; Ilic et al., 1987; Hawkins et al., 1991), tongue (Thorp, 1943; Chodkowski and Zebrowski, 1952; Darbyshire, 1961; Gardiner et al., 1967; Morales and Van Kruiningen, 1971; Valder et.al., 1979; Theil and Rudolph, 1981; Zanin et al., 1987; Hawkins et al., 1991), gums and dental pad (Gardiner et al., 1991; Hussain et al., 1992) and palate (Thorp, 1942; Morales and Van Kruiningen, 1971; Rodrignez et al., 1985; Zanin et al. 1987; Hussain et al., 1992). Small erythematous to larger coalescing ulcerated papules were observed on the gingiva, tongue and over dental pad and hard palate of young lamb (McElroy and Bassett, 2007). Sometimes, the disease may be seen in part of gastro-intestinal tract (GIT) (Darbishire, 1961) particularly in pharynx and larynx (Thorp, 1943; Valder et al., 1979), oesophagus (Valder et al., 1979; Tontis et al., 1981) and rumen (Morales and Van Kruiningen, 1971; Tontis et al., 1981; Theil and Rudolph, 1981) or throughout alimentary tract (Thorp, 1943). The lesions on the skin of head (Tontis et al., 1981; Greig et al., 1984; Ilic et al., 1987; Coates and Hoff, 1990), face (Greig et al., 1984), ears (Freig et al., 1984; Nooruddin and Barik, 1989), neck, chest and flank (Coates and Hoff, 1990), legs (Greig et al., 1994) and nose (Ilic et al., 1953; Nooruddin and Barik, 1989) and mucosa of nostrils (Kater and Hansen, 1962; Munz et al., 1986; Alian et al., 2015) are also not uncommon. Erythema multiforme (EM) is a hypersensitivity reaction occurs as complication of orf infection in humans. It develops approximately after 1-2 week post-infection and characterized as small red ring-like blisters. Sometimes less defined toxic erythemas may also appear (Joseph et al., 2015).
In some outbreaks, the lesions were localized to mammary gland (Slagsvold, 1938; Fontanelli and Caparrini, 1955; Verdes et al., 1970; Hessami et al., 1979; Larsson and Zahoory, 1983; Rodrignez et al., 1985) particularly teats (Peres, 1932; Barrat, 1934), inguinal region and inner surface of thigh (Fontanelli and Caparrini, 1955; Larsson and Zahoory, 1983), vulva (Hessami et al., 1979; Valder et al., 1979; Roncero et al., 1989) and scrotum (Ohman, 1941). On udder, these may be seen alongwith perilabial lesions (Carre, 1931; Aldasy and Suveges, 1964). Involvement of foot (Kater and Hansen, 1962; Valder et al., 1979), interdigital space and coronet (Zanin et al., 1987; Hawkins et al., 1991) leading to loss of hooves (Valder et al., 1979) had been reported in some unusual outbreaks. Occasionally, the disease has been observed in generalized form (Slagsvold, 1938; Munz et al., 1986; Nooruddin and Barik, 1991; Wu and Sun, 1992) particularly in young animals (Munz et al., 1986). An ocular form of disease with lesions confined to eyes was reported by Gillain (1936).
The lesions of CPD commonly appears as cauliflower like papillomatous growth or proliferative type (Selbie, 1944; Kummeneje and Krogsrud, 1979; Valder et al., 1979; Tontis et al., 1982; Greig et al., 1984; Munz et al., 1986; Zanin et al., 1987; Coates and Hoff, 1990; Zamri-Saad et al., 1992), scabby and granulomatous (Hussain et al., 1992) or dry and crust thick scabs (Verdes et al. 1970; Obi and Gibbs, 1978; Noruddin and Barik, 1989; Hawkins et al., 1991) on the mucocutaneous junction and perilabial areas. However, blisters or pearl like lesions, pustule and crusts on the teats and around the mammary gland (Peres, 1932; Slagsvold, 1938; Fontanelli and Caparrini, 1955; Verdes et al., 1970; Larsson and Zahoory, 1983) and papular and pustular lesions around the mouth (Roncero et al., 1989; Housawi et al., 1991; Hussain et al., 1992) and multiple erosive and pustular erruptions in the mouth, cheeks, tongue, lips and part of GIT (Darbyshire, 1961) have also been reported. In some cases, scrotum appeared enlarged and contained watery clear fluid (Ohman, 1941). Sometimes, superficial and deep abscesses of udder may also be seen (Fontanelli and Caparrini, 1955). If udder was involved along with perilabial lesions, it was of lesser degree and did not cause any ill effect or loss in milk yield (Aldasy and Suveges, 1964). In malignant form of disease, cauliflower like growth on oral mucosa and necrotic or deep ulcerative lesions in the buccal cavity, pharynx, larynx, oesophagus or throughout the alimentary tract were noticed by Thorp (1943). However, foot and genital lesions in association with oral lesions enabled the differentiation from FMD (Valder et al., 1979). In the labial form of disease in addition to diptheroid necrotic lesions of mouth and tongue, severe ulcerations in oral cavity, oesophagus (Tontis et al., 1981) and rumen (Theil and Rudolph, 1981; Tontis et al., 1981) were observed.
Many a times, the disease was complicated by Fusiformis necrophorus (Marsh and Tunnicliff, 1937; Thorp, 1942), streptothricosis (Munz, 1969) and Dermatopilus congolensis (Abu-Samra and Walton, 1981; Cuuz et al., 1990; Yeruham et al., 1991). Resulting into severe generalized lesions or prolonged outbreaks of CPD (Abu-Samra and Walton, 1981; Cruz et al., 1990) with exudative dermatitis (Yeruham et al., 1991).
The incubation period in natural cases of CPD is 2-3 weeks (Coates and Hoff, 1990; Zamri-Saad et al., 1992). Experimentally, the skin lesions in sheep appeared in 4-5 days (Grishaev and Shchepetova, 1970; Willayat and Garg, 1992) and in cats in 8-11 days as a papule followed by degenerative changes leading to pustule formation without undergoing stage of vesiculation (Grishaev and Shchepetova, 1970) and produces erythema multiforme. Initially fever (Ilic et al., 1987; Housawi et al., 1991), restlessness (Ilic et al., 1987), anorexia (Housawi et al., 1991), hypersalivation, swelling and reddening of gums and tongue (Zamri-Saad et al., 1992) followed by papular/pustular, nodular or cauliflower like lesions (Zamri-Saad et al., 1992) around the mouth (Housawi et al., 1991) and other parts of body (Ilic et.al., 1987) were observed. In severe outbreaks, predominant signs were pneumonia with mucopurulent nasal discharge followed by erosive stomatitis and gastroenteritis of varying severity. In addition, acute myocarditis, hemorrhagic pneumonia and liver degeneration or focal necrosis were also present (Darbyshire, 1991). In ocular form, the affected animals showed purulent conjunctivitis often succeeded by keratitis and ulceration (Gillain, 1936). In cases of involvement of mammary gland, the lambs starved as ewes refused milk followed by retention of milk (Carre, 1931) and mastitis (Barrat, 1939). The clinical signs in captive wild animals were similar to domestic sheep and goats which had poor general body condition and difficulty in feeding (Samuel et.al. 1975) and loss of body weight (Zamri- Saad et al., 1992; Ozturk et al., 2012).
Course of Infection
Natural cases of human infection occurred within 3-6 days post exposure and ran a course of 3-4 weeks (Carne et al., 1946) but lesions may also appear by day 12 (Dyar, 1951) with a course of 5-8 weeks (Dyar, 1951; Hodgson-Jones, 1951) irrespective of the treatment given (Hodgson-Jones, 1951). Experimentally, the lesions were seen after an incubation period of 4-6 days, reaching maximum on days 17-24 and ran a course of 22-28 days. The highest titer of virus in human lesions ranged from 10-4 to 10-6 on days 12-14 PI (Park et al., 1951). Recovery from infection was soon under local treatment (Oppermann and Stumpke, 1937) and there was spontaneous regression of lesions (Carne et al., 1946) which cleared up rapidly (Fontanelli and Caparrini, 1955) with no scars left (Kozlowski and Dziekonski, 1956).
Histopathology and Pathogenesis
The histopathological changes in CPD resemble those of variola (Salyi, 1939) and classical pox diseases (Abdussalam, 1957b). Hyperkeratosis (Morales and Van Kruiningen, 1970; Coates and Hoff, 1990), acanthosis of epidermal cells (Abdussalam, 1957b; Morales and Van Kruiningen, 1971) and degenerative changes in the epidermis (Abdussalam, 1957b; Roncero et al., 1989) particularly in the stratum spinosum (Coates and Hoff, 1990), were seen due to virus multiplication in the prickle cells (Okada et al., 1984) leading to necrosis (Abdussalam, 1957b). Lesions of epidermal hyperplasia, hyperkeratosis, intra-epithelial pustules and ulcers were evident upon post-mortem in semi-domesticated reindeer (Trylanda et al., 2013). The ballooning degeneration forming vesicles or pustules (Wheeler and Cawley, 1956; Morales and Van Kruiningen, 1971; Kluge et al., 1972) acanthosis with pseudoepitheliomatous hyperplasia and superficial multiloculated vesicles and pustules were found at an early stage followed by granulomatous and papillomatous character (Wheeler and Cawley, 1956; Nobrega et al., 2008). During degeneration, globular structure containing chromatin material was seen in cytoplasm which disintegrated as the cell approached keratinization. The nucleolus is enlarged and frequently surrounded by halo or clear space (Abdussalam, 1957b). The characteristic eosinophilic intracytoplasmic inclusions (Roncero et al., 1989) were present in early stage (4-6th day) of exanthema (Sodyi, 1939) or in hyperplastic epithelium at the edge of lesions but were rarely seen in granulomatous lesion or late stages of infection (Kluge et al., 1972). The same was confirmed in experimentally infected cats (Grishaev and Shcheptova, 1970). However, Ishii et al. (1953) failed to observe inclusion bodies. The corium exhibited oedema and hyperemia of superficial dermis (Coates and Hoff, 1990) and polymorphonuclear and mononuclear inflammatory cells (Abdussalam, 1957b).
The damage to the skin is essential for virus infection and development of typical lesions. The virus replicates in the cells of an underlying replacement epidermal layer (Mckeever et al., 1988; Jenkinson et al., 1990b) derived from the walls of the wool follicle (Mckeever et al., 1988). Histologically, cutaneous responses were identified as (1) repair of the injury and (2) reaction to viral replication occurring within the newly repaired epidermis (Jenkinson et al., 1990b). The virus initially infects the cells of stratum basale at margins of wound and is transferred to daughter cells which traverse across the exposed dermis to form basis of new epidermis. The replication begins during differentiation of new epidermis and infection spreads laterally and uniformly to outer stratum spinosum and subsequently to entire depth of epidermis except stratum basale. A similar pattern of spread occurs in hair follicles also but above the level of sebaceous gland duct (Jenkinson et al., 1990c).
The skin reaction consists of a cellular response with necrosis and sloughing of the affected epidermis and underlying stratum papillare of dermis (Mckeever et al., 1988). The healing starts by epidermal proliferation (Jenkinson et al., 1990b) and is completed by the formation of third epidermis derived from deeper portions of wool follicles (Mckeever et al., 1988). The inflammatory cells included neutrophils, basophils and possibly mast cells but not eosinophils (Jenkinson et al., 1990a and b). In early viral replication neutrophils are accumulated in response to the initial trauma but cutaneous mast cells are not significantly changed (Jenkinson et al., 1990a). As a result leucocyte count increased from 7000 to 14000 on day 6 PI and returned to normal by 20th day (Dzhanibekov, 1969).
In mixed infections of CPD and D. congolensis, skin revealed congestion and oedema of epidermis and infiltration of neutrophils resulting in purulent dermatitis (Yeruham et al., 1991). The chronic CPD associated with Actinomyces pyogenes and Corynebacterium pseudotuberculosis also revealed prominent area of hyperkeratosis (Nolikuwera, 1972).
DIAGNOSIS
The diagnosis of CPD is mainly based on benign nature of the disease and characteristic skin lesions (Coates and Hoff, 1990), clinical signs and histopathology of skin lesions, transmission experiments and demonstration of a pox virus by electron microscopy (Samuel et al., 1975; Rodrignez et al., 1983) of skin biopsies of CPD infected skin (Coates and Hoff, 1990). The transmission of disease into susceptible sheep (Marsh and Tunnicliff, 1937; Aldasy and Suveges, 1964; Sinha et al., 1986) and goats (Sinha et al., 1986; Nolikuwera et al., 1992) or lambs and kids (Thilakrajan et al., 1975; Mazur and Machado, 1989 and 1990) leading to exanthema (Marsh and Tunnicliff, 1937; Sinha et al., 1986; Nolikuwera et al., 1992) followed by development of antibodies to CPD virus (Mazur and Machado, 1989) but not in animals which has recovered from buccal form of disease (Gillain, 1930), have been widely used as a diagnostic tool. Biological tests in rabbits (Abdussalam, 1957; Sinha et al., 1986; Cargnelutti et al., 2011), cats (Grishaev and Shchepetova, 1970) and kittens (Thilakrajan, 1975; Sinha et al., 1986) were done but kittens were most suitable. The rabbits were less susceptible than sheep for in vivo virus neutralization tests (Abdussalam, 1957). The cats developed skin lesions in 8-11 days PI (Grishaev and Shchepetova, 1970).
Agar gel precipitation (Abdussalam, 1953; Romero-Mercado et.al., 1992), agglutination (Abdussalam, 1953), haemagglutination (Chodkowski and Zebrowski, 1952; Abdussalam, 1953) and indirect haemagglutination (Maeda and Scott, 1985), complement fixation (Rottgaroldt et al., 1949; Abdussalam, 1953; Schmidt, 1967d; Romero-Mercado et al., 1973a and b; Koptoulos et al., 1982; Maeda and Scott, 1985; Mazur and Machado, 1990) and immunofluorescence tests (Kluge et al., 1972; Rossi and Casalena, 1973; Koptopoulos et al., 1982; Chubb and Couch, 1985; Mazur and Machado, 1990) and ELISA (Koptopoulos et al., 1982; OIE, 2010) have been used for detecting orf virus antigen and antibodies. Diagnosis between capripox virus (CPV) and CPD virus infections have been made possible by western-blot analysis as CPV hyperimmune sera did not react with any CPDV protein which corresponds to 26Kd protein of CPV and significantly CPDV hyperimmune serum did not recognize the 32Kd protein of CPV. Similar patterns were also observed with western-blot cross analysis (Chand et al., 1994).
Multiplex PCR and PCR targeting B2L or virus interferon resistance gene (VIR) gene has been employed to diagnose the parapoxvirus infections (Inoshima et al., 2000; Torfason and Gunadottir, 2002; Kottaridi et al., 2006b; Abraho et al., 2012). A PCR assay developed for rapid diagnosis of contagious ecthyma virus specific primers targeting a highly conserved GIF/IL-2 and A32L gene was found sensitive to detect minimum DNA concentration of 5 ng (Ramesh et al., 2008; Chan et al., 2009). A semi-nested PCR based on the major enveloped protein (B2L) gene has been reported to detect low copy number of virus particles from clinical samples. The efficacy of PCR was comparable (85–87%) to the cell culture/neutralization methods. A duplex PCR assay using A29 gene (413bp) and H3L gene (708bp) has been reported to have potential as a diagnostic tool for detection and differentiation of pseudocowpox and orf viruses (Zheng et al., 2007). Recently, PCR targeting B2L gene has been developed for confirmatory diagnosis in sheep (Ferede, et al., 2014). A real-time PCR has also been developed based on B2Lgene to detect and differentiate from pseudocowpox virus, bovine popular stomatitis virus and seal parapox virus in clinical samples (Gallina et al., 2006; Nitsche et al., 2006). Bora et al. (2012) developed a quantitative PCR assay to evaluate viable virus content in live attenuated orf vaccine. Studies demonstrated that TaqMan probe based duplex real-time PCR (drt-PCR) has been optimized with two different sets of primers and probes targeting the highly conserved DNA pol gene of capripox, sheeppox and orf virus genome. In comparison to real-time PCR for detection of orf virus, drt-PCR assay has proved to be more specific (100% diagnostic specificity) and highly sensitive (diagnostic sensitivity of 98.7%) (Balinsky et al., 2008; Balamurugan et al., 2009; Bora et al., 2011; Venkatesana et al., 2014a, b). Schmidt et al. (2013) performed partial nucleotide sequencing and phylogenetic analysis of B2L gene of seventeen Brazilian orf viruses and concluded that discrete nucleotide changes in B2L gene may help in grouping orf viruses according to host species. Li et al. (2013a) did phylogenetic analysis of two Chinese orf virus isolates based on B2L and VIR gene sequences. Li et al. (2013b) developed a method for rapid detection of orf virus by LAMP assay based on DNA polymerase gene. LAMP assay is an easy, quick, precise and sensitive technique for detection of orf virus infection without any cross reactivity with other viruses (Tsai et al., 2009; Wang et al., 2013; Venkatesan et al., 2015).
Restriction fragment length polymorphism (RFLP) has been used for molecular characterization of different strains of para poxviruses. However, it is less useful in species differentiation but molecular hybridization using terminal region specific primers is extensively useful. Phylogenetic analysis of parapoxvirus virus isolates has been proved useful to deduce the genetic relationship between isolates (Hosamani et al., 2009; Chu et al., 2011; Nandi et al., 2011).
IMMUNITY
The resistance of animals plays an important role in the disease causation and virus becomes virulent especially after lambing (Carre, 1931), surgical operations, blood protozoan infections and unusual change in weather (Munz, 1969). Due to lowered resistance, infection becomes chronic or persistent (Nolikuwera, 1992) and other concurrent infections like caseous lymphadenitis and streptothricosis (Munz, 1969; Nolikuwera et al., 1992) may also be observed.
The animals recovered after artificial or natural infection of CPD became immune for reinfection (Borcila and Isopescu, 1937; Slagsvold, 1938; Chodkowski and Zebrowski, 1952; Yu et al., 2012) for atleast 3 months (Ishii et al., 1953) or 9-12 months in certain cases (Borcila and Isopescu, 1937; Richter and Jansen, 1968) with absolute immunity for 8 months after recovery (Mundu and Mohan, 1961), Abdussalam (1957) observed only partial resistance to reinfection for 50 days. The immunity varied considerably according to the site of experimental reinfection independent of site of original infection (Schmidt, 1962 and 1967d; Haig, 2006; Yu et al., 2012). The sheep completely resistant to experimental reinfection of mouth by even a heterologous strain of orf virus for one year PI, were partly susceptible to infection of the thigh within one month of recovery and were fully susceptible within a year (Freriches, 1980) suggesting the role of local immunity in orf virus infection (Schmidt, 1962).
No evidence of transfer of maternal immunity from vaccinated ewes to their offspring was obtained under laboratory conditions (Glover, 1936) and immunity of lambs born to immune sheep was practically nil (Richter and Jansen, 1968). However, ewes vaccinated against CPD at the end of pregnancy or during lactation, transferred antibodies against CPD through colostrum and milk (Jansen et al., 1968) and when colostral antibodies disappear, the disease occurs in lambs (Poulain et al., 1972). It was also observed that lambs getting colostrum had higher levels of passive antibody than lambs vaccinated at 1-4 days of age, only the vaccinated lambs could be protected against challenge with CPD virus at one month of age. Presence of maternal antibodies prevented active antibody response but not the delayed type hypersensitivity (DTH) response to vaccination and/or challenge with CPD virus (Buddle and Pulford, 1984).
The naturally recovered or vaccinated (With attenuated strain) animals were protected against reinfection or repeated challenge irrespective of the inhibition of growth of virus by immune serum (Manley, 1934; Khanduev et al., 1970). Detection of specific antibody to orf virus by ELISA (Mckeever et al., 1987; Yirrel et al., 1989), western blotting (Mckeever et al., 1987) and peripheral blood lymphocyte (PBL) proliferative response (Yirrel et al., 1989) revealed seroconversion of infected animals. The proliferative response of PBLs was observed between day 7 and 14 PI and always preceded specific antibody detection. Following secondary infection, all animals showed 4 fold rise in specific antibody titre and greater PBL proliferative response (Yirrel et al., 1989). The nodal response is also directed mainly towards induction of specific antibody (Mckeever and Reed, 1987) which in the presence of complement, lysed CPD virus infected cells in vitro as detected by 51cr release assay (Demartini et al., 1978; Pearson et al., 1979). However, circular antibody against CPD virus were detected late and the level was also variable (Robinson and Balassu, 1981; Koptopoulos et al., 1982). Hence, no definite conclusion can be drawn about the mechanism which decreased infectivity titres in skin lesions or disappearance of CPD virus from skin by 12th day PI (Hussain and Burger, 1989). Also, there are controversial reports regarding relationship between the titres of circulating antibodies and protection against virus (Poulain et al., 1972; Freriches, 1980), the later appears to be of local nature (Khanduev et al., 1970) because CPD evokes tissue immunity only (Schmidt, 1967c).
The percentage of efferent lymphocytes expressing MHC class II antigens and surface immunoglobulins increased with no change in T cell subset in the efferent lymph or lymph node. The short term nature of local T cell response explains the incompleteness of immunity to orf virus in sheep (Yirrel et al., 1991). However, lymphocytes collected from sheep on day 12 PI, responded specifically to stimulation from CPD virus antigen about the time when infectious virus disappeared from the site (Hussain and Burger, 1988). It was also observed that immunization of lambs with polypeptide bands of 4.5, 40.7, 38.0 and 33.1 KD obtained in SDS/PAGE stimulated positive intradermal DTH reaction indicating their immunogenicity for lambs (Zuo et al., 1988). Following dermal infection of previously sensitized sheep with orf virus, there was early memory T cell response in the dermis and afferent lymph followed by recruitment of antigen specific lymphocytes from the blood and activation within the lymph node (Haig et al., 1992). The infection of orf virus by local scarification also induced an influx into the dermis of significantly greater numbers of T cells (T4 and T8 subsets) underneath the early lesions and later within epidermal pustules. Although, dermal B cells also increased in number but fewer cells were present within pustules (Jenkinson et al., 1992; Yu et al., 2013).
Therefore, it appears that CMI plays a larger role in recovery from infection or virus clearance from site of skin (Mckeever et al., 1987; Hussain and Burger, 1989). Further, class 11 dendritic cells develop after orf virus infection in the exposed necrotizing dermis adjacent to infected hair follicles and under infected degenerating epidermis. These cells interact to form a barrier to invasion and provide the basis of highly integrated local dermal defence system (Jenkinson et al., 1991).
Rohde et al. (2012) showed that ORFV down-regulates MHC I surface expression in infected cells by targeting the late vesicular export machinery and the structure and function of the Golgi apparatus, which possibly helps to escape cellular immune recognition. Plasmacytoid dendritic cells are involved in the immunological protective response against Orf infection (Saadeh et al., 2014).
PREVENTION AND CONTROL
The prophylactic vaccination controls CPD (Barrat, 1939; Dayus, 1939; Hart et al., 1948; Hardy, 1964). Though, certain commercial virus vaccines failed to protect sheep against infection sometimes (Gardiner et al., 1967) yet it appears that vaccination is of some value in large flocks (Mortelmans and Vercruysse, 1953).
Initially, the vaccines were mainly prepared from dried or desiccated scabs collected from infected animals (Boughton and Hardy, 1935; Flament and Martin, 1938; Thorp, 1942; Rottgardt and Pirazzi, 1951; Richter and Jansen, 1968; El-Dahaby et al., 1980) which retained their infective power for more than 14 years but the material most suitable as vaccine also included extensive oedema in addition to normal lesions (Rottgadt and Pirazzi, 1951). Such vaccines provided protection to reinfection/challenge (Flament and Martin, 1938) on inoculation by scarification (Pekelder et al., 1980) in brisket area (Flament and Martin, 1938), skin of the inner surface of thigh (Manley, 1938; Thorp, 1942) and tail (Huang and Li, 1986). The vaccine was safe (Thorp, 1942) in all age groups including unweaned animals (El-Dahaby et al., 1980). In previously infected animals, the vaccine shortened course of infection and reduced its severity (Boughton and Hardy, 1935) and induced a good immune response (Richter and Jansen, 1968; El-Dahaby et al., 1980; Huang and Li, 1986). The vaccination of pregnant ewes 6 weeks to 2 months before lambing reduced the incidence of disease (Meynink et al., 1987) but outbreaks still occur particularly in young lambs which are not normally vaccinated until six weeks of age. Lambs removed from their dams at birth or 24-48hour after birth and housed separately, were protected (Carre, 1931) by scarification of axila of foreleg without any apparent ill effects and with no evidence of spread to their dams. The suggestion of this practice into field vaccination scheme (Kerry and Powell, 1971) when used in real practice, controlled CPD in all pregnant ewes, yearlings and lambs (3-4 weeks or earlier) but nonreacters (about 1% lambs) needed revaccination (Kuhne and Meixner, 1984) after 2-5 months (Pekelder et al., 1980). The immunity lasted for one (El-Dahaby et al., 1980) to two years (Boughton, 1935).
In controlled vaccination experiments with scab material in lambs, incidence of disease was very less with very mild lesions in comparison to controls. A vaccine trial on 1,500,000 lambs yielded extremely satisfactory results (Boughton and Hardy, 1935). The strict sanitary measures along with vaccination reduced the disease to nil by 1969 in Egypt (El-Dahaby et al., 1969). However, local reaction following vaccination may be severe (Richter and Jansen, 1968) particularly in lambs where it can cause generalized infection also (Kerry and Powell, 1971).
The vaccines prepared from powder scab preserved in glycerol (Peres, 1932), chloroform treated dried scabs suspended in pure glycerol (1:100) (Canchemez, 1933) or crusts ground in glycerinated serum were also effective (Rapuntean et al., 1975) when inoculated in the inguinal region of sheep (Peres, 1932) by scarification (Vrtiak and Vrzgula, 1955) and conferred immunity to natural/challenge/experimental infection (Rapuntean et al., 1975). However, powder scab mixed with immune serum and treated with chloroform and suspended in 50% glycerol did not yield satisfactory results (Tatarevic and Petrovic, 1977).
The lyophilized live vaccine using CPD virus adapted to lamb kidney (Ramyar, 1973) and DEF cells (Rossi et al., 1976) provided protection of sheep against challenge (Ramyar, 1973) and exposed animals by single dose of 1 ml. The lambs born during subsequent two weeks were also protected when vaccinated 15 days later (Rossi et al., 1976). The LK adapted CPD virus vaccine produced transient nodules at the site of injection and provided protection for 1 year (Ramyar, 1973). In affected animals, the disease regressed more quickly after vaccination and the vaccine was harmless for newborn lambs (Rossi et al., 1976). A recent field isolate adapted to cell culture produced large lesions, detectable antibody response and protection against challenge virus similar to that of traditional vaccine. This isolate was distinct on the basis of restriction enzyme analysis but provided cross protection in sheep (Pye, 1990). In malignant form of disease, tissue culture derived vaccine with an interferon inducer gave promising results and was superior to traditional vaccine (Valder et al., 1979). Immunogenicity and protective efficacy of three locally developed live orf virus vaccines with field strain of orf virus was assessed in Saudi Arabia over Noemi sheep for direct comparison of vaccine efficacy (Housawi et al., 2012).
To induce immunity without any ill effects, inactivated vaccines using various physical and chemical agents were tried (Glover, 1936; Olah and Elek, 1953; Richter and Jansen, 1968; Sawhney et al., 1972) but the results were not promising. However, a formalized field virus (Mundu and Mohan, 1961) or heat inactivated (At 600C for 45 min.) virus vaccine with no live virus in it evoked a reasonable immunity (Richter, 1969). Such vaccine has been recommended for flocks not yet infected and very young lambs (Richter and Jansen, 1968). A vaccine consisting of homogenized glutaraldehyde inactivated musk oxen papilloma tissue in Freund’s complete adjuvant injected two weeks after birth, protected musk ox calves from CPD (Mathiesen et al., 1985). Comparative evaluation of a live tissue culture vaccine prepared from B2 strain in calf kidney cells and the same virus inactivated by UV irradiation for 20 min and 30 min or with 1% formaldehyde, revealed protection of lambs against challenge 6-8 weeks post vaccination except formaldehyde vaccine (Sawhney et al., 1972).
A live modified freeze dried vaccine prepared by propagating orf virus in primary LK cell culture protected 90% and 84-88% lambs by single dose under mucosa of lip or two doses given 7-11 days apart by scarification respectively with a total dose of 2-3x105 TCID50 (Kovalev et al., 1971). The attenuated strains of CPD virus particular I4 strain by 4 serial passages in chick embryos (Shakhbazoy, 1975), Pendik strain by 28 passages in calf kidney cells (Ergin and Koklu, 1977) and D 17 d strain by 135 passages in sheep embryo kidney cell culture (Zach, 1979) reported that modified vaccine (D 17 d strain was highly effective in sheep and milk fed and new born lambs when injected @ 1ml/animal subcutaneously with a total dose of log 106.5-7.5/ The vaccine was safe in pregnant ewes and new born lambs even in 5 fold dose. It also reduced the morbidity and mortality in already infected flocks. The attenuated vaccine protected sheep, pregnant ewes and lambs (Mayr et al., 1981; Verdes et al., 1989). Ergin and Koklu (1977) observed that lambs inoculated with modified Pendik strain were immune for six months but resistance depended on the route of inoculation. The lambs injected intradermally on thigh and abdomen were resistant for 3 and 5 months respectively while lambs inoculated beneath the mucosa were resistant for 6 months (Kovalev et al., 1971). The freeze dried virus could be stored for several months at -20˚C (Ergin and Koklu, 1977).
An attenuated orf vaccine gave a strong cellular and weak humoral immune response but provided protection even with heterologous virus infection like bovine papular stomatitis and orf in calves, cows and steers with no virus excretion after subcutaneous application (Buttner, 1981). The comparative evaluation of live modified and a standard orf vaccine prepared from mild virus propagated on the skin of sheep, revealed no difference in immunity (Kovalev et al., 1971). During serial passage in cell culture, orf virus was contaminated with endogenous viruses and chlamydia of sheep and they remain present in the vaccine (Mayr et al., 1981). An outbreak of border disease in goats was reported by pestivirus contaminated orf vaccine (Loken et al., 1991). Orf virus has been used for developing a recombinant bivalent vaccine to provide dual protection to the lambs against orf and E. granulosus, parasitic cause of cystic hydatid disease in sheep and humans also (Tan et al., 2012).
Field trials of vaccines revealed neutralizing antibodies in the serum of most of the vaccinated sheep (Mayr et al., 1981) and also in colostrum and milk from ewes. However, passive immunity had a local effect and protected the lambs for 2-3 months against natural infection (Verdes et al., 1989). The vaccine was effective particularly as an emergency measure in already affected flocks and in lambs reared artificially in lamb bars. In the latter, combination of vaccine and multipotent parammunity inducer was effective (Mayr, 1981). Disease occurrence in lambs from vaccinated ewes maintained in an infected environment showed that reimmunization was necessary at about the age of 5 months (Verdes et al., 1989). Vaccine containing caprine strains of contagious ecthyma virus provide better protection for goats from contagious ecthyma than currently available vaccines suggested for sheep (Musser et al., 2008).
In spite of control of disease by vaccination, there are reports of vaccination failure which may be attributed to faulty vaccination procedure or wrong assessment of degree of protective immunity. As this virus was suggested to multiply in macrophages, the current procedures of inducing a localized skin reaction may not be effective in some cases (Buddle, 1981). Complete protection against even homologous strain was not achieved. Although, antigenic differences between vaccine and field strains appeared an unlikely cause of failure (Buddle et al., 1984) but this possibility cannot be completely ruled out particularly when vaccine strain belonged to a different geographical region. Studies revealed that immunization with the use of tissue culture-based ORFV vaccines is promising in terms of protection indices (Candice et al., 2012). The epidemic of CPD has also been controlled by culling and controlling the population after an annual census in Italy (Zanin et al., 1987). A DNA vaccine pcDNA3.1-ORFV011/ORFV059 expressing ORFV011 and ORFV059 chimeric-proteins has been developed, which can significantly improve the potency of DNA vaccination and could be served as more effective and safe approach for new vaccines against ORFV (Zhao et al., 2011).
TREATMENT
The lip lesions of CPD infected animals were treated with 10% Potassium permanganate solution (Van De Kerk, 1954) and antibiotics like penicillin in cases complicated with stomatitis or enteritis (Mortelmans and Vervruysse, 1953). Antibiotics and topical application of antiseptics were found useful by Bragazai (1959). The affected animals were successfully treated with parenteral administration of antibiotics (Tontis et al., 1981) like chloramphenicol (Lansade, 1959) in association with its topical application as ointment (Lansade, 1959; Larsson and Zahoory, 1983) or sodium permanganate and salicylic acid ointment (Tontis et al., 1981). Two to three local applications of chloramphenicol gave good results in the treatment of CPD in sheep and lambs. The IM or IV treatment was suitable for cases with complications (Lansade, 1959). However, orf infection treated with parenteral streptomycin sulphate, procaine penicillin and topical oxytetracycline hydrochloride and gentian violet and later with topical use of salicylic acid, had no beneficial effect (Greig et.al., 1984). Topical preparations found effective in order were lotagen, methylene blue, a racemic chloramphenicol ointment, glycerine iodate and Terramycin ointment. Lotagen on buccal mucosa rapidly blanched affected tissue which subsequently fell off leaving nearly normal epithelium, but it was less effective though still best for peribuccal areas of skin (Rapuntean et al., 1975). Stibophen (antimosan) given to 86 affected sheep @ 3 ml to lambs and 5 ml to ewes reduced severity and produced a striking improvement in 8-10 days (Zettl, 1966). Recently, when severely affected bottle fed lambs failed to respond to local treatment with oxytetracycline and gential violet, they were treated surgically following anaesthesia by slow I.V. injection of a solution containing 9 mg alphaxalone and 3 mg alphadolove/ml at a dose of 6 mg total steroid/kg. After debridement, the resultant bleeding was controlled by diathermy and the submucosa was treated twice by liquid nitrogen spray cryotherapy. This treatment required 5 min for each lamb followed by rapid recovery without any evidence of recurrence (Meynink et al., 1987 and 1990). Several farmers in South Africa use orally and topically the extracts of Cassia italica root which has proven antipyretic, analgaesic, antimicrobial, anti-inflammatory, purgative and antitumor properties but its antiviral properties are not well elucidated, needing further studies (Scagliarini et al., 2012).
PUBLIC HEALTH CONCERNS
Human Orf
Ovine and caprine CPD, a disease of worldwide occurrence is transmissible to man also and is commonly known as human orf or sore mouth. The disease occurs in man handling sheep and goats (Newsom and Cross, 1934; Carne et al., 1946; Nagington and Whittle, 1961; Fleming et al., 2015). However, professionals are at higher risk as several cases were reported in shepherds (Oppermann and Stumpke, 1937; Kozlowski and Dziekonski, 1956), shearers (Australia, 1946), meat porters and butchers (Hodgson-Jones, 1951), farmers (Muir, 1951; Royer et al., 1970), stock auctioneer (Purdy, 1955) and employees of slaughter house handling skin and wool (Robinson and Peterson, 1983). The researchers (Moore et al., 1983), working with goats (Ilic et al., 1986), public who visits zoo, students having contact with sheep and cattle (Hessami et al., 1979), laboratory workers who have prolonged exposure with infected sheep (Fastier, 1957, workers handling experimental animals (Mundu and Mohan, 1961), veterinarians and veterinary attendants (Liess, 1962) were also found to contract the infection by handling the orf virus infected material or animals (Bodilsen and Leth, 2013). Reports does not exist regarding the occurrence of orf due to consumption of unpasteurized milk but Food and Agricultural Organization recommends discarding of the milk from affected animals (FAO, 2011). Several countries report incidences of orf viral infections in human, among which UK reports on an average 3 cases per year from 2004- 2014 (Onyango et al., 2014).
Transmission
The human orf infections generally occur naturally (Lloyed et al., 1951; Verdes et al., 1970) by direct contact of animals (Peterkin, 1937; Schoch, 1939; Nomeland, 1940) through cuts or minor injuries on the finger and skin of hands (Carneetal, 1946; Dyar, 1951; Royer et al., 1970) or from the mother who milked the sheep to her child (Fontanelli and Caparrini, 1955) through scratching and during Islamic religious worship practice of Eid ul-Adha from infected sheep to in contact humans (Verdes et al., 1970; Nougairede et al., 2013; Shahmoradi et al., 2014). Though there is no demarcation between the sexes regarding orf infection in human, male are at higher risk compared to female as male comes with direct contact with the infected animals in slaughter houses and other places (Nadeem et al., 2010; Bayındır et al., 2011).
Lesions and Symptoms
Incubation period of orf in humans can vary from days to a week (Uluğ et al., 2013). Orf in humans usually occurs as a locally, similar to benign neoplasm but on rare occasions systemic signs like mild fever and lymphadenitis can occur (Gurel et al., 2002). Some authors report the cutaneous form in five different phases: phase 1 consist of formation of small papule, phase 2 consist of larger lesions with red centered, white margins and lens shaped nodules, phase 3 consist of exudation, phase 4 is the regenerative phase leading to formation of black spots and crusts in the area of lesions and finally phase 5 is healing stage (Taghipour et al., 2015). Lesions generally appear on the fingers (Peterkin, 1937; Nagington and whittle, 1961; Ilic et al., 1986) and skin of hands (Oppermann and Stumpke, 1937; Carne et al., 1946; Dyar, 1951; Van De Kerk, 1954; Purdy, 1955; Kozlowski and Dziekonski, 1956; Mundu and Mohan, 1961; Liess 1962; Verdes et al., 1970; Hessami et al., 1979; Ilic et al., 1986; Fleming et al., 2015), mostly in the non dominant hand (Uzel et al., 2005) and occasionally on the chin (Kozlowski and Dziekonski, 1956), face (Peterkin, 1937), neck (Oppermann and Stumpke, 1937; Mundu and Mohan, 1961), wrist (Schoch, 1939) and umbilicus (Kozlowski ans Dziekonski, 1956). Sometimes, extensive eruptions may be seen on almost all parts of body particularly hands (Fleming et al., 2015), forearms, feed and legs with regional glandular swelling and thrombosis of retinal vein leading to permanent blindness of the eye (Royer et al., 1970; Bayindir et al., 2011).
The lesions begin as reddish circular papules increasing gradually in size (Carne et al., 1946) followed by maculo-papular pock like lesions (Hessami et al., 1979), vesicular (Newsom and Cross, 1934; Wheeler and Cawley, 1956; Royer et al., 1970) and vesiculo-pustular eruptions (Royer et al., 1970). The granulomatous nature of lesions (Peterkin, 1937; Dyar, 1951) covered by blister (Dyar, 1951) and scabby lesions (Oppermann and Stumpke, 1937) have also been recorded. Generally, the lesions are painless (Dyar, 1951) with no swelling of regional lymph node and without any evidence of systemic reaction (Kozlowski and Dziekonski, 1956). However, in some cases throbbing pain may be felt with axillary lymphadenitis and a general systemic reaction (Carne et al., 1946). Lesions may resolve naturally within 4-6 weeks from the onset of the symptoms (Ünal et al., 2002; Uzel et al., 2005). Secondary bacterial infections are the common complications noticed in orf infected humans. Other complications like Stevens Johnson syndrome, erysipelas, generalized mucocutaneous eruption, eyelid edema and erythema multiforme are also recorded in immune compromised individuals (Karakaş et al., 2010). Erythema multiforme is a type of delayed type hypersensitivity reaction occurring due to infection or drugs (Joseph et al., 2015).
On incision, the lesions appeared to be granulomatous with some central softening and accumulation of serous fluid in the late stages (Carne et al., 1946). In man, the lesion of orf are characterized by vesiculation which is a result of ballooning degeneration of the cells of prickle layer but granulomatous character occasionally dominates the microscopic appearance (Wheeler and Cawley, 1956).
Diagnosis/Confirmation of Infection
Confirmatory diagnosis can be made by electron microscopic examination of the keratinocytes which may reveal the typical structure of virus in the cytoplasm or isolation of virus on lamb fibroblast (Lo and Mathisen, 1996). The material from lesions of human infection was able to set untypical reaction in susceptible experimental sheep (Newsom and Cross, 1934; Lloyed et al., 1951; Mahmoud et al., 2010), lambs (Oppermann and Stumpke, 1937) and guinea pigs (Kozlowski and Dziekonski, 1956). Likewise, suspension of crusts from udder of goats and from the lesions of the woman and her child produced typical lesions when applied to scarified skin of young goats (Verdes et al., 1970). The failure of development of lesions in immunized lamb confirms the diagnosis (Purdy, 1955). Reciprocal cross immunity tests using human and ovine orf virus isolates also indicated that it was due to ovine virus (Carne et al., 1946; Liess, 1962). The neutralizing antibodies in the patient and convalescent sheep (Nagington and whittle, 1961) and allergic test on the farmer (Patient) 10 months later proved it to be CPD virus (Royer et al., 1970; Bayindir et al., 2011).
Immunity and Control
The recovered human volunteers (11/12 subjects) exhibited solid immunity on challenge but 12th had mild lesions (Primary) and developed severe infection following her second challenge inoculation (Parke et al., 1951). The human infection was very common in shearers in Australia, therefore, to avoid interference with shearing of affected sheep, extensive vaccination has been carried out (Australia, 1946).
CONCLUSION
Contagious pustular dermatitis (CPD) also known as contagious ecthyma or orf or ecthyma contagiosum, is an exanthematic debilitating disease of sheep and goats with zoonotic potential. It is caused by a parapox virus and responsible for producing ulcerative stomatitis in susceptible host. At present, the CPD has the status of disease of worldwide occurrence and is also changing into malignant form. Orf infection and its allied impediments can arise in rural as well as urban areas due to less awareness, casual negligence and through religious or cultural practices involving animal handling and slaughter. Orf has gained higher importance throughout the world in the recent years due to its zoonotic prospective, affecting mainly immune compromised patients. Persons involved in small ruminant rearing occupation are at risk. Though the disease is self limiting, secondary bacterial infections lead to complications that may lead to mortality. Determination of pathophysiology and relative contributory risk factors may help in keeping away the disease. Differential diagnosis, prompt treatment and isolation of affected animals can prevent spread of virus to other animals in the herd. Although, current vaccines can control the disease but continuous presence of virus in the environment is increasing the risk of disease to other species of animals. The role of cell mediated immunity in elimination of virus from infected animals is also not conclusively established. Therefore, attempts should be made to overcome these problems on the basis of recent developments in molecular biology and immunology particularly the recombinant vaccine.
Conflict of interest
There is no conflict of interest.
Authors’ Contribution
All authors contributed equally.
REFERENCES
http://dx.doi.org/10.1016/0166-0934(91)90068-B
http://dx.doi.org/10.1016/0378-1135(94)90033-7






