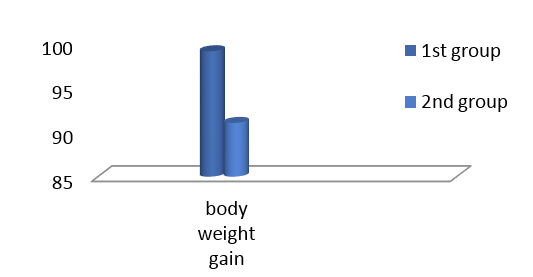Advances in Animal and Veterinary Sciences
Research Article
Effects of Lactobacillus acidophilus on Pituitary-thyroid Axis in Growing Rat
Majida Abdulkhaliq Jaafar Alqayim
Department of Physiology and Pharmacology, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | Several studies have shown that gut microbiota can activate neural pathways and central nervous system signalling systems via gut-brain axis, accordingly the present study was aimed to investigate the interaction of Lactobacillis acidophillus on the activity of pituitary–thyroid axis in the case addressed after weaning of male rats. The study was carried out on growing male rats. At the day of weaning 12 male kids at age of 30 days were divided into two equal groups and treated as following: 1st group considered as control receiving 1ml of water, 2nd – group received 1ml of MRS (5x108 CFU) of L. acidophilus. All the experimental animals were administered orally and daily for the next 32 days. Body weights were recorded at the beginning and end of the experiment. Blood and thyroid gland samples were collected for hormonal assay at the end of the experiment. There was significant differences in body weight gain. Treated group showed less body weight gain than control group. TSH and T3 increased significantly while GH, T4, and TFI were increased slightly in treated group. The analysis of the photomicrograph of thyroid tissue in lactobacillus received group showed increase in the number and size of the thyroid follicles, in addition to a visible hyperchromatic of the follicles epithelia. In conclusion, feeding of Lactobacillus acidophilus to rat kids showed tangible changes in the pituitary- thyroid axis activity suggesting a positive interaction between Lactobacillus acidophilus and host gut-brain axis.
Keywords | Lactobacillus Acidophilus, Thyroid gland, gut-brain axis Thyroid hormones, Growth hormone
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 06, 2015; Revised | March 21, 2015; Accepted | March 22, 2015; Published | April 01, 2015
*Correspondence | Majida Abdulkhaliq Jaafar Alqayim, College of Veterinary Medicine, University of Baghdad, Iraq; Email: [email protected]
Citation | Alqayim MAJ (2015). Effects of Lactobacillus acidophilus on Pituitary-thyroid axis in growing rat. Adv. Anim. Vet. Sci. 3(5): 269-275.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.5.269.275
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Alqayim. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Thyroid gland is the biggest gland in the neck participate in growth and body’s metabolism. The main function of thyroid gland is to synthesis and release the thyroid hormones triiodothyronine (T3) and its prohormone,thyroxine (T4), which is the major form of thyroid hormones in the blood (Irizarry, 2014). The thyroid hormones exert many physiological functions act on most of the body’s cells, increase basal metabolic rate, effect on protein, fat, and carbohydrates metabolism (Gelfand et al., 1987). During fetal life thyroid hormones are synthesized and released from the fetus under pituitary control through thyroid-stimulating hormone (TSH). TSH secretion from fetal pituitary is evident by 12 weeks of gestation, and fetal production of thyroxine (T4) reaches a clinically significant level at 18-20 weeks.
The periventicular neurons in the hypothalamus (PVN) synthesize and release thyrotropin-releasing hormone that stimulate the release of thyroid-stimulating hormone from the pituitary, and TSH stimulate the synthesis and release of T4 from thyroid, this what called the hypothalamus-pituitary- thyroid (HPT) axis (Puymirat, 1992). Regulation of HPT mediated by a negative feedback effects through thyroid hormone receptors in the pituitary and hypothalamus (Scanlon, 1992; Morley, 1981). During the maternal life the thyroid gland activity, like another tissues influenced with maternal environment, but in postnatal life in spite to the master pacemaker in the brain, it influence by local independent circadian oscillators, which is mostly affected by postnatal development (Yamazaki et al., 2009). Although the molecular mechanism of the circadian regulation of the hypothalamus-pituitary-thyroid axis (HPT) not yet analyzed, but it well - known that this HPT axis rhythms, are tightly regulated by the circadian system (Jacques, 2014).
Gastrointestinal tract is regulated by two neural circuits and their interaction. The autonomic sympathetic and para sympathetic system on the one hand and enteric nervous system (ENS) on the other hand regulates most aspects of GI physiology, which constitutes a relay station in the bi-directional neuro-endocrine pathways that connect the digestive system and the brain (gut-brain axis) (Collins et al., 2012). Enteric microbiota are essential for the developing ENS during the postnatal life in different regions of the intestine (Collins et al., 2014). Strong new evidences suggest the interference of gut microbiota with gastrointestinal microenvironment regulation and host behavior and brain chemistry. Accordingly it has been found that commensal and probiotics in the gastrointestinal tract can activate neural pathways and central nervous system signaling (Foster and Mcvey, 2013; Bercik and Collins, 2014). Lyer et al. (2004) stated that gut micro biota can produce neurochemicals are exactly the same found in the host tissues and with same pathways. Based on previous reports (Lyte, 2011), Lactobacillus have revealed high concentrations of intracellular neurochemicals such as GABA. These observations arise the possibility of the microbiota- gut-brain inter action is the mechanism by which the microbiota may influence host behavior (Lyte, 2014). Gut micro biota might regulate brain-gut axis through many postulated mechanisms one of the most accepted that through regulation of, serotonin synthesis as serotonin the key neurotransmitter in the both terminal of brain- gut axis (Mahony et al., 2015), furthermore, pyrroloquinoline quinone producing probiotics in rat intestine modulate gut-brain axis by restored neurotransmitter levels (Pandey et al., 2015). At the level of interference with thyroid gland activity specifically, mixed treatment of 2 strains of Lactobacillus with phytosterol-containing treatments induced the increased activity of thyroid glands, as evident by elevated levels of serum total thyroxine, total triiodothyronine, and free triiodothyronine (Awaisheh et al., 2012). Lactobacillus acidophilus, is a lactic acid producing bacteria it is gram positive, non-spore forming cocci, cocco- bacilli or rods in most cases they are anaerobic, they need fermentable carbohydrates for growth and for the production of lactic acid (Cotter and Rose, 2005). Some strains of L. acidophilus considered to have probiotic characteristics that has the potential to protect the oxidative damage mediated by the reactive oxygen species and can act as antioxidative probiotic (Deeplina and Arun, 2015) and promote health benefits (Usman, 2014; Alqayim and Abass, 2014). Thyroid gland function influence health care for example hypothyroidism may also lead to congestive heart failure due to the increased systemic vascular resistance and decreased cardiac contractility. For the purpose of shedding light on side of the interaction of gut microbiota with gut-brain axis, the present study was aimed to investigate the interaction of L. acidophillus on the activity of pituitary –thyroid axis in the case addressed after weaning of male rats.
Material and Methods
Experimental Animal and Diet
Ten healthy Albino Wister pregnant female rats, were kept under suitable condition of (21-25°C) in an air condition room, and were fed freely with standard pellet diet (Table 1). At the day of parturition each dam isolated in a single cage. After parturition these dams with their kids were kept under suitable condition and well fed until day of weaning. At weaning time, 6 male kids from each group were chosen, isolated and placed in a another cages to treated as follow: 1st control group kept on distil water 2nd group began to received (5 × 108 CFU) of Lactobacillus acidophilus. This treatment continued for another 32 days. Body weight (gm) were recorded at the time of weaning and after 32 days, at the end of experiment for calculation of body weight gain. At the end of the experiment all the experimental animals were anesthetized by intraperitoneal injection with 35 mg/kg ketamine hydrochloride. After opening the chest cavity, blood was collected by acupuncture through the left ventricle for hormonal assay. Thyroid gland were isolated and dried with filter paper and weighted, then preserved in formalin solution for histological examination.
|
Percentage |
Nutrient Composition |
|
88% |
Dry matter |
|
14-16% |
Crude protein |
|
2.5-3.0% |
Crude fat |
|
2.5-3.0% |
Crude fiber |
|
10% |
Crude Ash |
|
10% |
Moisture |
|
1-3% |
Calcium |
|
1% |
Phosphorus |
|
1% |
NaCl |
|
5000 IU/KG |
Vitamin A |
|
700IU/KG |
Vitamin D3 |
|
30 mg/KG |
Vitamin E |
|
2400 Kcal/KG |
Metabolizable Energy |
Serum Hormonal Assay
Serum T3, T4, TSH, and GH were measured by radioimmunoenzymetric assay using commercial kits supplied from Accu-bind Monobind –USA.
Thyroid Function Index (TFI)
The TFI is an index for the clinical evaluation of thyroid function described by (Mamtani et al., 2014). Depending on the calculated value of TFI in control group, we can distinguish whether there is a hyperthyroidism or hypothyroidism TFI =
= TSH/ (FT3 × FT4).
TSH/ (FT3 × FT4).
Histological Analysis
Sections of the thyroid gland were obtained from the experimental groups and were fixed with 10% for histopathological evaluation. Serial sections were prepared as described in (Luna, 1968).
Statistical Analysis
The Statistical Analysis System- SAS (2012) was used to analyze effect of different factors in study parameters. Least significant difference –LSD test was used to significant compare between means in this study.
Results
Thyroid Weight and Body Weight Gain
Results shown in Figure 1 revealed that there were significant differences in body weight gain between groups. The greatest body weight gain recorded in the present study was in control group, in addition feeding L. acidophilus to growing rats did not increase body weight in the present study.
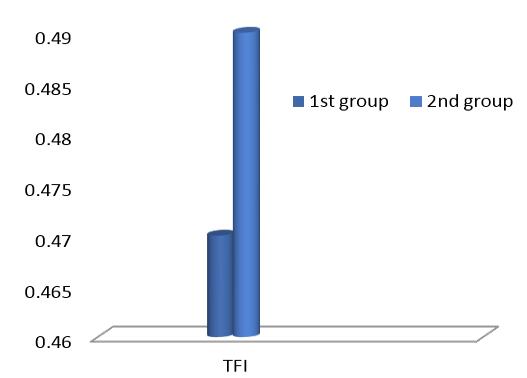
Figure 2: Effects of feeding L. aciaophilus on Thyroid Function Index (TFI) during the 32 day spot weaning
Pituitary-thyroid Axis
Results of the present study shown in Table 2 represent the differences in GH, TSH, T4, and T3 in the serum of the different experimental rats. Results reveled a significant (P<0.05) elevation in TSH and T3 and non-significant in GH and T4 in rats kids fed Lactobacillus acidophilus for 32 days post weaning when compare with control. When the TFI account showing the existences of differences in rats had fed L. acidophilus in comparison with control group as shown in Figure 2, the non-significant TFI increase in 2nd group suggesting a functional hyperthyroidism.
Table 2: Effects of feeding L. acidophilus on GH, TSH, T4, and T3 for 32 days post weaning in male rats
|
Animal groups |
GH |
TSH |
T4 |
T3 |
|
1stcontrol |
0.923 ± 0.05 |
1.48 ± 0.04b |
2.46 ± 0.23 |
1.26 ± 0.03c |
|
2nd group lactobacillus (5 × 108 CFU) |
1.08 ± 0.08 |
2.27 ± 0.28a |
2.88 ± 0.23 |
1.65 ± 0.07a |
|
LSD value |
0.194 NS |
0.514 * |
0.744 NS |
0.142 * |
* (P≤0.05); (mean ± SE); n=6
The relationship between serum TSH and thyroid weight in rats fed L. acidophilus for 32 days shown in Figure 3. The R2 value (0.503) show significant regression of variable of y on variation of x values. The histological examination of thyroid gland sections from control and L. acidophilus administered groups were presented in Figure 3.
The analysis of the thyroid light microscopic photomicrograph obtained from control group (Figure 4) reveled a normal size and number of follicles lined by squamous to cuboidal shape follicular cells and filled with light to moderate colloid material. Thyroid sections from 2nd group that fed with L. acidophilus (Figure 5) showed that there were two types of follicles some of them are widely distended and filled with well stained colloidal material lined by hyper plastic squamous epithelia, also there were the finger like projection indicating a the hyperplasia of flat epithelial cells. In addition there were another type of small follicles filled with tense colloid material and lined by clear cuboidal epithelial cells characterized by a visible basophilic hyperchromatic, other section showed a vacuolar changes in the peripheral regions of the follicles epithelial.
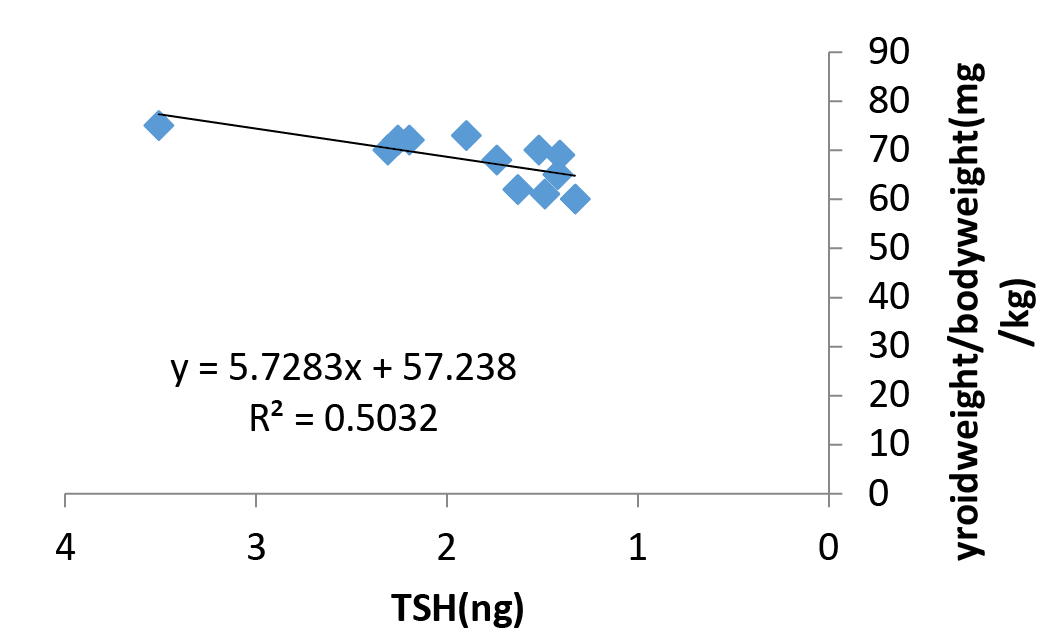
Figure 3: Relationship between serum TSH and thyroid weight in rats fed L. acidophilus for 32 days. The R2 value show significant regression of variable of y on variation of x values
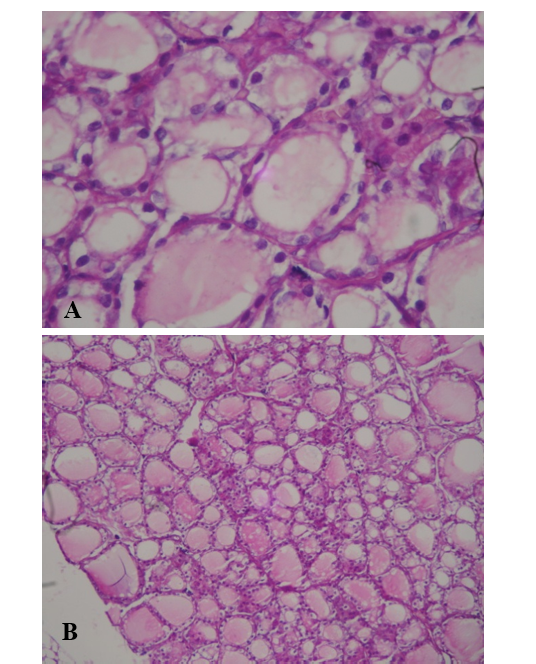
Figure 4: Light microscopic photograph of thyroid gland in control group (A-10x, B-40x). It shows a normal size and number of follicles lined by squamous to cuboidal shape follicular cells and filled with light to moderate colloid material (H&E)
Discussion
Body Weight Gain
Gut micro biota feeding play a significant role in regulation of energy balance of the host, one of the postulated mechanism for this regulation defined by Turnbaugh et al. (2006), suggesting a metabolic pathway in food energy extraction. On the other hand dietary inclusion with different strains of Lactobacillus was potential and safer anti- obesity natural bioactive material (Torres et al., 2015). Based on the above, the results of weight gain in the present study suggest an active role for L. acidophilus as anti-obesity bioactive material. In spite of the reduced body weight gain in the L. acidophilus fed rat there was increase in thyroid weight /body weight, which indicate increase of thyroid growth.
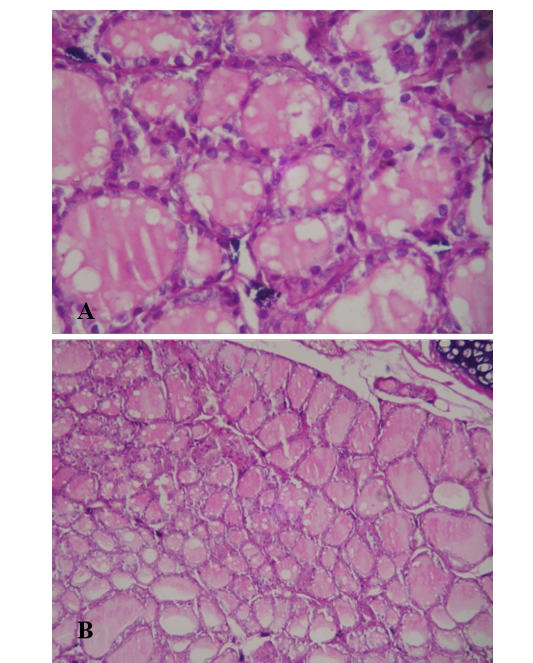
Figure 5: Light microscopic photograph of thyroid gland in L. acidophilus group (A-10x, B-40x). Show widely distended and filled with well stained colloidal material lined by hyper plastic, basophilic hyper chromatic epithelia, (H&E)
Pituitary –Thyroid Axis
In the present study the significant increase in serum TSH indicate a coordinator role for L. acidophilus on the brain –pituitary –thyroid axis. In spite of regulation of gastrointestinal physiology, the enteric nervous system or what called the little brain, also modulate communication between the gut and central nervous system (Sharma et al., 2009). The coordination between the brain, the intesti nal tract and endocrine systems is well described (Mayer, 2011). The enterochromaffin cells in the lamina propria work as a bidirectional transducer between nervous system and gut lumen (Rhee et al., 2009). Body of recent evidences discuss the role of probiotics in the organization of this brain-gut axis and modulating of endocrine system (Cryan and Dinan, 2015; Hemarajata and Versalovic, 2013; Pandey et al., 2015). The high level of TSH and GH denoted in the present study encourage the suggestion that the L. acidophilus used in this trial instrumental in organization the pituitary gland. A close relationship between thyrotropin-releasing hormone (THR), thyroid stimulating hormone (TSH), and growth hormone (GH) had been found and this axis plays an important role in growth (Coghurn et al., 1995). Normally the activity of hypophysiotropic TRH neurons is regulated by the negative feedback effects of thyroid hormone to ensure stable, circulating, thyroid hormone concentrations, but in the current experiment, observed persistence of high TSH despite rising T3in the circulationthis may be explained by the occurrence alteration in the set point for negative feedback regulation by thyroid hormones.
Organisms adapted to changing in environmental conditions via different mechanisms, one of these is the thyroid axis mediating suppression of Hypophysiotropic TRH neurons (Fekete and Lechan, 2014). Raising of TSH induce effects on thyroid gland and increase in the synthesis and release of thyroid hormones, T4 and T3. But in the present study the significant increase in T3 at the expense of T4 in 3rd and 2nd groups leads us to the assumption that there were increasing in the conversion of blood circulation T4 to the active T3. T4 converted to T3 within cells by deiodinases (5’-iodinase), in thyroid and liver , and kidney tissues, but the liver is the major tissue for this conversion .Among the factors that inhibits the liver conversion of T4 to T3 is the presence of free radical load (Marit et al., 2000; Vadhanavikit and Ganther, 1993). The thyroid gland is a unique endocrine gland that requires hydrogen peroxide for thyroid hormone synthesis. Oxidative reactions in thyroid gland are predisposing for thyroid hormones synthesis correlated with production of ROS particularly H2O2 under physiological conditions (Kale, 2015). Thyroid gland contain a defined H2O2 generating system, this H2O2 is required as a substrate control the thyroperoxidase, the enzyme catalyzes the Iodide-protein binding during the thyroid hormones synthesis (Corvilain et al., 1991). Thyroid epithelial cells are well adapted to endogenously produced ROS in basal and goitrous conditions (Jacobet al., 2015). Using antioxidants in improving thyroid gland function were studied (Karan et al., 2014). Reducing lipid peroxidation and protecting the liver from oxidative effects of free radicals will be one of the main strategies to promote conversion of T4 to T3 by using herbs (Sage, 2013). Meanwhile L. acidophilus proved its effectiveness as antioxidant (Abu-Elsaad et al., 2015) this has resulted in appositive way to increase the conversion of T4 to T3 in the present experiment. Same results recorded by (Chenhui et al., 2014) who found that feeding Se-probiotics caused increase in T3 and not T4.
The histological examination of the thyroid gland revealed that the probiotic-fed rats in the present study have increase of follicular number and follicular cell hyperplasia (increased cell number) and follicular cell hypertrophy (increased cell size) as well as reduces size and become more regularly- shaped ,dense colloid space. Changes observed in thyroid tissue of rats fed with L. acidophilus that indicate increase in thyroid hyperplasia and activity specifically the finger like projection and the peripheral of the vacuolated follicles which considered a signed of active resorption. By contrast the appearance of proliferative epithelial of small follicles filled with tens colloid product could explain the L.acdiophilus –associated increase in T4 and T3in the present study. These findings are signs of thyroid activity may be resulted from the hypersensitivity of the thyroid to increased TSH denoted in the present study. As the TSH considered the major regulating factor for thyroid growth (Rapoport and Spaulding, 1996), results confirmed this correlation as mention by the R2 values, the same pattern of thyroid response to probiotic was recorded by (Hood et al., 1999), who found that moderate increase in serum TSH resulted in increased number of proliferating thyroid follicles. Furthermore Lactobacillus other species supplementation improved thyroid function (Bernard et al., 2014).
In conclusion, the present finding suggest a significant role of feeding probiotic(Lactobacillus-acidophilus) in regulation of the brain-gut axis resulting in increase of pituitary –thyroid axis activity ,thyroid sensitivity to TSH. It is concluded from the increase in thyroid activity associated with no increase in body weight gain that probiotis feeding in one way or another modifying metabolic rate, to make sure of this we suggest further studies concerned with.
Acknowledgment
The authors would like to express their deep gratitude and appreciations to Dr. Anaam Badder for her help in the analysis and description of the histological part of this research.
References