Advances in Animal and Veterinary Sciences
Research Article
Investigation of Sex Reversal in Layer Chickens in Bangladesh
Amir Hossan Shaikat1*, Md. Ahasanul Hoque2, SKM Azizul Islam1, Mohammad Mahmudul Hassan1, Shahneaz Ali Khan1, AKM Saifuddin1, Mohammad Yousuf1, Md. Al Mamun1, Mohammad Belayet Hossain1
1Department of Physiology, Biochemistry and Pharmacology (DPBP); 2Department of Medicine and Surgery, Chittagong Veterinary and Animal Sciences University (CVASU), Chittagong, Bangladesh.
Abstract | Sex reversal is evolutionary a rear event in animal and human. To understand the magnitude of occurrence, a total of 28 sex reversed layer chickens were identified in two poultry farms during 2011 over a total population of 3000 layer chickens in Ramu, Cox’s Bazar. The chickens were randomly divided into two equal groups. Case chickens (Group I) were separated from normal chickens for 15 days and were monitored for phenotypic characteristics such as body weight, comb length, comb width, and wattle length. These parameters were measured and compared with the corresponding characteristics of normal chickens. After monitoring period, blood samples were taken from studied chickens, plasma was collected to quantify the sex hormones. Moreover, studied chickens were sacrificed for gross and histopathological examination. Results indicate that case chickens had atrophied ovaries containing seminiferous tubules and Leydig cells. These results were linked with higher plasma testosterone (8.1 ng/ml) and lower estrogen (5 pg/ml) and progesterone concentration (318.1 pg/ml). Case chickens also have spurs, higher body weight, comb length and width, and wattle length. It is likely that farm chickens might have been exposed to nitrobenzene and dichlorodiphenyltrichloroethane, which could lead the formation of Leydig cells in the ovary. The Leydig cell secretes testosterone and subsequently they become high in plasma, causing appearance of secondary sexual characteristics of male in female chickens. The study highlights the important of environmental chemicals on the physic-chemical characteristics in chicken and therefore extreme caution should be practiced to avoid any contact or exposure to animals.
Keywords | Sex reversal, Layer chicken, Arrhenoblastoma, Hormone, Bangladesh
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 03, 2015; Revised | March 14, 2015; Accepted | March 16, 2015; Published | March 24, 2015
*Correspondence | Amir Hossan Shaikat, Chittagong Veterinary and Animal Sciences University, Chittagong, Bangladesh; Email: [email protected]
Citation | Shaikat AH, Hoque MA, Islam SKMA, Hassan MM, Khan SA, Saifuddin AKM, Yousuf M, Al Mamun M, Hossain MB (2015). Investigation of sex reversal in layer chickens in Bangladesh. Adv. Anim. Vet. Sci. 3(4): 245-252.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.4.245.252
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Shaikat et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sex reversal is an important as well as an interesting event in biological fields. Sex reversal has been occurred earlier in humans and animals through surgical, pharmacological and psychiatric procedures (Hodgkin, 1990). Sex reversal has also been reported in Tilapia fish by providing feed containing androgen methyl testosterone (Ronald, 2001) and in Gonostoma gracile (Sassa et al., 2004). Genetic factors mostly determine the sex of humans and animals. However, water pH, salinity, social interaction and importantly temperature may have contributory effects on sex reversal in animals (Baroiller and D’Cotta, 2001).
Sex reversal has been encountered in a variety of birds and mammals (Manolakou et al., 2006). In birds sex several occurs due to the result of the unique laterality or asymmetry of gonad that occurs during embryogenesis of male and female genital systems, where greater numbers of primordial germ cells migrate to left gonad than to the right (Smith and Sinclair, 2004). Sex reversal in domestic chickens (Gallus domesticus) is well known and has occurred naturally and experimentally (Thomas and Marion, 2003). Sexual changes occur most commonly in female birds that subsequently develop masculine characteristics following regression of a functional ovary.
A rare ovarian tumour of arrhenoblastoma developed in female birds could change the sexual characteristics (phenotypic and sex hormonal) of the birds (Bigland and Graesser, 1955). Arrhenoblastoma causes atrophied ovarian follicles and subsequently the decline of egg production. Sex reversal due to ovarian cyst or tumour, adrenal hypertrophy etc. has also been reported in animals and humans where the left ovary becomes regressed and therefore, residual tissues of right ovary proliferate as ovotestis (Jacob and Mather, 2000).
Pesticides like dichlorodiphenyltrichloroethane (DDT), 1, 1-dichloro-2, 2-bis (4-chlorophenyl) ethylene (DDE), Di-(2-ethylhexyl) phthalate (DEHP), mono-2-ethylhexyl phthalate (MEHP) and nitrobenzene have carcinogenic effect which may facilitate in developing arrhenoblastoma and other tumors in ovary (Xu et al., 2010). As in sex reversal, functional ovary of layer chickens become regressed due to arrhenoblastoma, leading to decrease egg production that may lead to economic loss in layer farming. The aim of the current study was to determine the phenotypic changes, sex hormonal status and histopathological changes in the ovary of sex reversal commercial layer chickens in Bangladesh. The study also aimed to explore possible causal factors associated with sex reversal of layer chicken in Bangladesh.
Material and Methods
Study Area
The present investigation was conducted in chicken layer farms of Ramu belonging to Cox’s Bazar district under the Chittagong division, located at the eastern part of Bangladesh. On February 2011, the owner of Motaher Poultry Farm (MPF) reported that he observed certain unusual sex characteristics in some of his layer chickens (age 32 weeks) in the farm. These were changed-voice, increased comb and wattle length and spur developed in layer chickens. In addition, egg production was declining from 85% to 70% within 15 days of peak laying period. According to farmer’s complains, the authors of the paper in a team visited the affected farm and explored the similar history of unusual sex characteristics developed in some of layer chickens as farmers reported. In order to conduct a prospective study 10 layer farms were randomly selected within 1-2 km of index case farm to further investigate whether the occurrence of sex reversed chickens for 1 year study period. Farmers were initially trained on recognition of sex reversal characteristics on their farms and how to inform if cases of sex reversed chickens were observed. We maintained a constant communication with the farmers and visited at 1-2 months interval. On September, 2011 another Parents Poultry Farm (PPF) under the monitoring area had similar unusual phenotypic sex characteristics identified in some of its birds (age 34 weeks).
Grouping of Birds, Collection of Blood Sample and Hormonal Assay
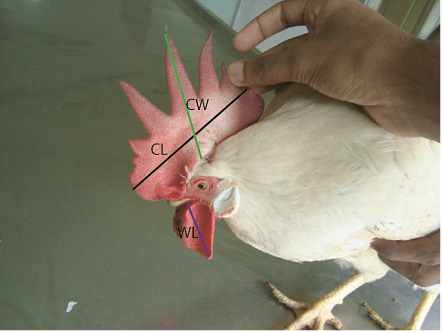
A total of 28 phenotypically modified layer chickens (defined as case birds) (18 from MPF and 10 from PPF) and 30 randomly selected normal layer chickens (defined as control birds) (20 from MPF and 10 from PPF) were included in this investigation. They were intensively monitored for 15 days after the occurrence of sex reversal events. Farmers’ consent was taken for on-farm study. Case and control birds were tagged with unique identification numbers and kept them in separate cages. Comb length and width, wattle length (Figure 1), distance between two pubic bones and ova ry length were measured according to the procedure described by Francesch et al. (2011). Appearance of spur was also inspected regularly. Body weights of all studied chickens were measured by a digital balance.
Measurement of Phenotypic Traits
Blood samples (approx. 2 ml/per chicken) were taken from the studied chickens under intensive monitoring on day 0 only. The samples were then transferred into heparinized tubes (GD005LH, Gongdong, China) after puncture the jugular vein. Samples were put on ice eskie (BX2012, China) and transported to laboratory. Plasma samples were separated by spinning at 3000 rpm for 10 minutes. Samples were then stored in -80ºC until the final analyses were done.
Analytical Procedures with the Blood Plasma to Assess Sex Hormones
The plasma samples stored at -80°C were thawed and homogenized properly. An enzyme linked immune assay (ELISA) protocol previously described by Elder and Lewis (1985) with some modifications by Elder et al. (1987) was used to determine the level of plasma testosterone, 17β estradiol (estrogen) and progesterone. Reagents supplied by ALPCO immunoassays, Germany and EAGLE BIOSCIENCE were used to perform ELISA testing on plasma samples obtained.
Gross and Histopathological Examination
All studied chickens were euthanized by Pentobarbital Na (100mg/bw, I/V) at the end of 15 days of intensive monitoring. The visceral and reproductive organs of euthanized chickens were examined grossly and recorded the lesions. Fat deposition status on abdominal cavity was also assessed. Whole ovary from each euthanized bird was immediately taken in 10% neutral buffered formalin with unique identification number and left for 15 days in order to fix the tissues. The tissue sections were then stained with hematoxilin and eosin according to the procedure explained by Gridley (1960). Stained slides were mounted using Canada balsam and covered with cover slip. Slides were then air-dried before microscopic examination. The microscopic photos of the stained sections were taken using the Cannon G 100 digital camera mounted on Light microscope (Olympus BX51, Japan) in 10x, 40x and 100x objectives to record histological features of ovary and any replacement of ovarian cells by testicular cells. The image bars were set using the stage micrometer and Canvas 9 image editing software.
Statistical Analysis of Data
The data obtained from the studies were entered into the Microsoft Excel 2007 programme (Microsoft Corporation). The data were then sorted and cleaned in the MS-2007 and exported to STATA/IC- 11.0 (STATA Corporation, USA) for statistical analysis. Independent t test was performed for mean values of body weight, comb length, width, various sex steroid hormone levels such as testosterone, estrogen, progesterone etc between case and control groups of layer chickens. Results were expressed as mean, standard error, 95% confidence interval and p value.
Results
Characteristics Features of Sex Reversed Chickens
Changes of phenotypic characters’ of layer chicken have been presented in Figure 2. The range of comb length (cm), comb width (cm) and wattle length (cm) were significantly higher (p< 0.001) in case chickens comparing with control chickens (Table 1). Overall, sex reversed chickens gained significantly (p< 0.001) greater body weight than the chickens belonging to control group (Table 1, Table 2 and Table 3). The sex reversed layer developed distinct spur of variable sizes (Figure 3).
Estimation of Sex Steroid Hormone
Level of sex hormones estimated from studied chickens is presented in Table 1. Farm based sex steroid level is presented in Table 2 and 3. The testosterone level of sex reversed chickens was significantly higher (p< 0.001) than that of control chickens. On the contrary, the level of estrogen and progesterone was significantly lower (p< 0.001) in sex reversed layer chickens than that of control chickens.

Figure 2: Sex reversed female bird

Figure 3: Spur in sex reversed chicken
Table 1: Comparative phenotypic trait values and summary estimates of sex hormones in studied layer chickens
|
Key Variable |
Parameter assessed |
Case (N=28) Mean ( CI) |
Control (N=30) Mean (CI) |
p |
|
Phenotypic traits |
Body weight (gm) |
1503.5 (1460.9-1546.1) |
1340.5 (1311.4-1369.2) |
< 0.001 |
|
Distance between pubic bones (cm) |
4.6 (4.3-4.8) |
4.8 (4.5-5.1) |
0.22 |
|
|
Comb length (cm) |
11.2 (10.6-11.9) |
5.2 (5.1-5.4) |
< 0.001 |
|
|
Comb width (cm) |
7.5 (7.3-7.8) |
4.9 (4.8-5.0) |
< 0.001 |
|
|
Wattle length (cm) |
4.9 (4.8-5.1) |
3.1 (2.9-3.2) |
< 0.001 |
|
|
Sex hormones |
Testosterone (ng/ml) |
8.1 (6.7-9.5) |
0.9 (0.7-1.2) |
< 0.001 |
|
Estrogen (pg/ml) |
5 (4.5-5.3) |
36.8 (34.9-38.6) |
< 0.001 |
|
|
Progesterone (pg/ml) |
318.1 (302.4-333.7) |
646.9 (621.7-672.2) |
< 0.001 |
Case: Birds showing phenotypic changes of sex reversal, Control: Birds having no changes of sex reversal, CI: 95% Confidence Interval
Table 2: Evaluation of phenotypic traits and estimated sex hormones in studied chickens of MPF, Ramu
|
Key Variable |
Parameter assessed |
Case (N=18) Mean ( CI) |
Control (N=20) Mean (CI) |
p |
|
Phenotypic traits |
Body weight (gm) |
1579.3 (1561.7-1596.8) |
1380.8 (1356.9-1404.6) |
< 0.001 |
|
Distance between pubic bones (cm) |
4.9 (4.8-5.0) |
4.9 (4.6-5.2) |
0.74 |
|
|
Comb length (cm) |
12.2 (11.5-12.8) |
5.3 (5.1-5.5) |
< 0.001 |
|
|
Comb width (cm) |
7.9 (7.7-8.2) |
4.9 (4.8-5.0) |
< 0.001 |
|
|
Wattle length (cm) |
4.9 (4.8-5.1) |
2.9 (2.8-3.0) |
< 0.001 |
|
|
Sex hormones |
Testosterone (ng/ml) |
8.5 (6.4-10.6) |
1.1 (0.7-1.4) |
< 0.001 |
|
Estrogen (pg/ml) |
5.1 (4.9-5.5) |
38.1(35.7-40.5) |
< 0.001 |
|
|
Progesterone (pg/ml) |
310.9 (289.4-332.5) |
651.4 (614.6-688.3) |
< 0.001 |
Case: Birds showing phenotypic changes of sex reversal, Control: Birds having no changes of sex reversal, CI: 95% Confidence Interval
Table 3: Evaluation of phenotypic traits and estimated sex hormones in studied chickens of PPF, Ramu
|
Key Variable |
Parameter assessed |
Case (N=10) Mean (CI) |
Control (N=10) Mean (CI) |
p |
|
Phenotypic traits |
Body weight (gm) |
1367 (1338.7-1395.3) |
1259.5 (1220.9-1298.1) |
< 0.001 |
|
Distance between pubic bones (cm) |
3.9 (3.6-4.1) |
4.3 (3.7-5.0) |
0.111 |
|
|
Comb length (cm) |
9.5 (9.0-9.9) |
5.2 (5.0-5.4) |
< 0.001 |
|
|
Comb width (cm) |
6.9 (6.5-7.2) |
4.9 (4.7-5.0) |
< 0.001 |
|
|
Wattle length (cm) |
4.8 (4.5-5.1) |
3.3 (2.9-3.7) |
< 0.001 |
|
|
Sex hormones |
Testosterone (ng/ml) |
4.7 (4.2-5.23) |
0.7 (0.5-0.9) |
< 0.001 |
|
Estrogen (pg/ml) |
4.7 (4.2-5.3) |
34.1 (31.5-36.3) |
< 0.001 |
|
|
Progesterone (pg/ml) |
330.9 (307.5-354.3) |
637.92 (610.1-665.8) |
< 0.001 |
Case: Birds showing phenotypic changes of sex reversal, Control: Birds having no changes of sex reversal, CI: 95% Confidence Interval
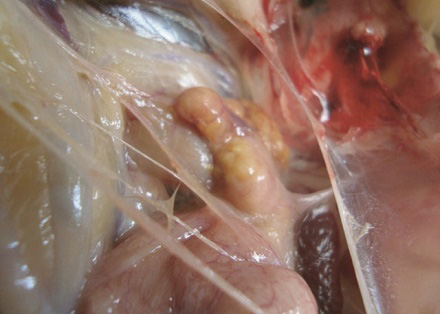
Figure 4: Atrophied ovary in sex reversed bird
Gross and Histological Features
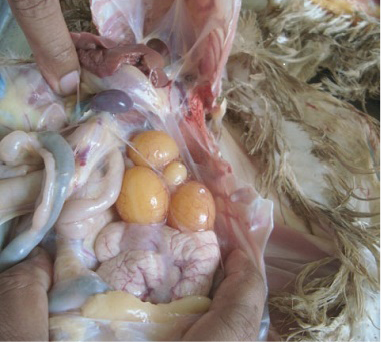
Fat deposition was observed in the abdominal cavity in sex reversed layer chickens. Appearance and structure of proventriculus, liver, kidney and intestine were normal regardless of types of studied chickens. Atrophied ovary with no grossly visible hierarchical follicles (Figure 4) was observed in sex reversed chickens, whereas normal layer chickens had 4-5 hierarchical follicles (Figure 5). A mass of cells surrounded by connective tissue capsule and seminiferous tubules like structures (Figure 6) were observed in ovarian sections of sex reversed layer chickens. Each tubule was lined by one or more layer of germinal epithelium i.e. large cells with pale, eosinophilic cytoplasm and containing a large, often vesicular nucleus. Spermatids and spermatozoa were not evident. Presence of Leydig cells (Figure 7) were also observed in ovarian section of sex reversed chickens. Variable sizes of follicles in a focus were also evident in the ovarian section of sex reversed chickens.
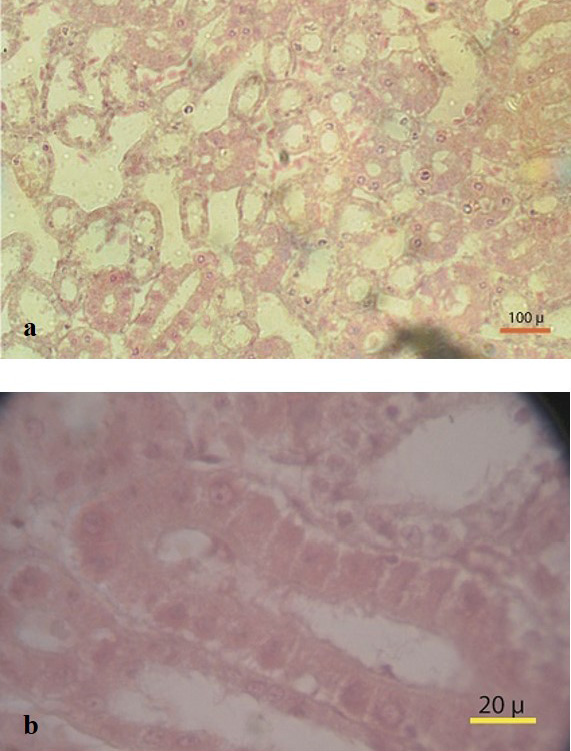
Figure 6: (a) Massive seminiferous tubule like structure in ovary section (10x) (b) Seminiferous tubule like structure with connective tissues (100x)
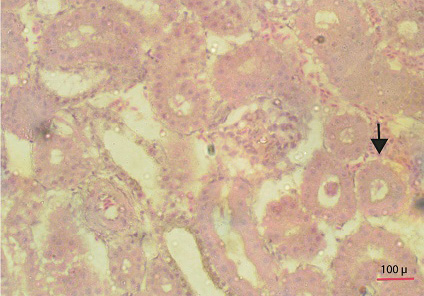
Figure 7: Section showing ovarian follicles and seminiferous tubule like structure with leydig cells (Thick arrow) (40x)
Response of the farmers
Owners of MPF reported that Flora® (ACI Pharmaceuticals Limited, Bangladesh) (Nitrobenzene) was used by a spray gun for early flowering of lemon trees surrounded by his farm and the same gun was then being used for spraying cold water to the chickens in his farm for approximately 3 months without prior washing of gun. Besides that, DDT was used as insecticide in a paddy field surrounding the other studied farm “PPF”. The farm owner responded that water from that field entered into the farm and farmers also speculated DDT mixed water might have contaminated the handmade chicken ration. DDT was also used against different pests in litter used for the farm.
Discussion
To the best of author’s knowledge, identification of sex reversed layer chickens under the present study was the rare event and recognized for the first time in Bangladesh. The present investigation was performed to elucidate the phenotypic changes, hormonal status and histopathological changes in sex reversal commercial layer chickens.
Sex reversal can occur due to hermaphroditism (presence of functional ovarian and testicular tissues) or pseudohermaphroditism (coexistence of both male and female sexual accessory organs) (Drescher, 2007). Sex reversed chickens had atrophied ovary in this study which correspond to the finding of Jacob and Mather (2000) in chickens. The presence of seminiferous tubule like structure accompanied with numerous Leydig cells in ovarian sections of the sex reversed chickens in the present study suggest these birds had arrhenoblastoma (Hughes, 2008) which is supported by an earlier study (Javert and Finn, 1951). Leydig cells secrete a sufficient level of testosterone (Dillard et al., 2008) to suppress the action of female sex hormones. Therefore, phenotypic characteristics of female chickens could be changed and aligned with the characteristics of male chickens (Bigland and Graesser, 1955). Arrhenoblastoma is generally believed to originate from undifferentiated male directed cells in the rete ovary (Novak, 1951). In general the cell mass destined to become sex glands was at first indistinguishable as male or female and eventually cords of cells appear beneath the germinal epithelium. These structures become permanent as seminiferous tubule in male and remain as abortive and atrophied in female (Fred and Wolferman, 1941). Owners of MPF reported the possible exposure of nitrobenzene on chickens approximately for 3 months during cold water spraying. Chickens or animal exposed to nitrobenzene is related to increase testosterone production (Gupta et al., 2010). Studied sex reversed chickens had 4 times higher testosterone concentration than that of normal layer chickens (2.4 ng/ml) which is supported by Schrocksnadel et al. (1971). This hormone can influence or regulate male fitness by developing or expressing various physiological, morphological and behavioral traits (Adkins-Regan, 2005) and changing the secondary male sexual characteristics on female chickens (Heikkila et al., 2005).
In earlier studies there was much evidence that DDT was used as insecticide in a paddy field surrounding the other studied farm “PPF”. The farm owner responded that water from that field entered into the farm and farmers also speculated DDT mixed water might have contaminated the handmade chicken ration. DDT was also used against different pests in litter used for the farm. This chemical agent could reduce progesterone synthesis (Crellin et al., 2001) and increase testosterone production (Wo´jtowicz et al., 2007) in porcine granulosa cells followed by musculinization in human (Younglai et al., 2004). In the present study, plasma progesterone concentration of sex reversed layer chickens were found significantly lower in case chickens than that of normal chickens. This may be due to less number of luteal cells and decreased oviposition and this explanation is coincided with the findings of Silva et al. (2012). It can also decrease aromatase activity which is responsible for conversion of testosterone to estrogen. Therefore, it is likely that DDT can disrupt endocrine homeostasis by altering both synthesis and metabolic breakdown of the ovarian steroids (Craig et al., 2011). DDT and nitrobenzene have effects on undifferentiated male directed cells in the rete ovary. Individual response and exposure of concentration of chemicals may have effects on lower percentage of sex reversed birds over the populations (Crews et al., 2000).
Estrogen has long been considered a factor in the determination of gonadal sex in some non-mammalian species (Akazome and Mori, 1999). Average estrogen concentration (5pg/ml) in case chickens was subnormal and similar to level of estrogen (6-7.3 pg/ml) in adult male chicken (Schrocksnadel et al., 1973). Atrophied ovary observed in sex reversed chickens under the present study might be a cause of having subnormal level of estrogen.
Egg production was declined in both farms in the study which could be due to dysfunctioning of ovary of sex reversed chickens. This possible logic in declining egg production is supported by a number of earlier studies (Rozenboim et al., 2007).
Sertoli-Leydig cell tumour accompanied with sex hormone alteration might have led to development of secondary male sexual characters in female chickens in the present study. This explanation is concurred with the findings of Ketterson et al. (2005). The lower percentage of sex reversal (28 out of 3000) may be due to in MPF, the birds which exposed to the sprayer first regularly got maximum exposure of nitrobenzene and we isolated maximum number of birds from there (11 out of 18 of MPF) and in PPF it may be due to individual variation of physiology of birds.
Acknowledgement
We thankfully acknowledge that Chittagong Veterinary and Animal Sciences University had given financial support and Department of Pathology and Parasitology for technical support in research works. We would like to extend our thanks to owners of the studied farms who had the full cooperation with the study. This manuscript is not submitted in any other journal for publication and all authors significantly contributed during manuscript preparation.
Conflict of interest
Authors has no conflict of interest.
References