Advances in Animal and Veterinary Sciences
Review Article
Biological Effects and Modes of Action of Carvacrol in Animal and Poultry Production and Health - A Review
Mahmoud Alagawany1*, Mohamed Ezzat Abd El-Hack1, Mayada Ragab Farag2, Ruchi Tiwari3, Kuldeep Dhama4
1Poultry Department, Faculty of Agriculture; 2Forensic Medicine and Toxicology Department, Veterinary Medicine Faculty, Zagazig University, Zagazig 44111, Egypt; 3Department of Veterinary Microbiology, College of Veterinary Sciences, Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa Vigyan Vishwa Vidyalaya Evam Go-Anusandhan Sansthan (DUVASU), Mathura (Uttar Pradesh) – 281001, India; 4Division of Pathology, Indian Veterinary Research Institute, Izatnagar, Bareilly, 243122,Uttar Pradesh, India.
Abstract | The carvacrol compound is an antioxidant nutrient, used to enhance growth and productive performance via modification and activation of gastrointestinal tract structure and function and to inhibit/prevent cancer initiations. Numerous studies performed on animal diets supplemented with phytogenic supplements/feed additives containing natural antioxidants such as carvacrol demonstrated its capability to improve performance indices, feed utilization, immune functions and health of livestock as well as reducing the risks of different animal diseases like cancer and other diseases. Such properties could be due to its ability as antimicrobial, antioxidant, antifungal, immunomodulatory, anticancer and anti-inflammatory agents by preventing free radicals and hazardous compounds from interacting with cellular DNA and its ability to change the gut microflora, improving digestion coefficient and absorption of nutrient compounds. The present review illustrates the chemical and physical proprieties, modes of action, metabolism and excretion, biological properties, natural sources and beneficial aspects of carvacrol in animal and poultry production and health.
Keywords | Carvacrol, Nutrition, Antioxidant, Antimicrobial, Anticancer, Immunity, Health, Production performance, Animal, Poultry, Human
Editor | Ruchi Tiwari, College of Veterinary Sciences, Department of Veterinary Microbiology and Immunology Uttar Pradesh Pandit Deen Dayal Upadhayay Pashu Chikitsa, Vigyan Vishvidhyalaya Evum Go-Anusandhan Sansthan (DUVASU), Mathura (U.P.) – 281001, India.
Special Issue| 2 (2015) “Reviews on Trends and Advances in Safeguarding Terrestrial /Aquatic Animal Health and Production”
Received | February 17, 2015; Revised | March 18, 2015; Accepted | March 19, 2015; Published | March 23, 2015
*Correspondence | Mahmoud Alagawany, Zagazig University, Zagazig, Egypt; Email: [email protected]
Citation | Alagawany M, El-Hack MEA, Farag MR, Tiwari R, Dhama K (2015). Biological effects and modes of action of carvacrol in animal and poultry production and health - a review. Adv. Anim. Vet. Sci. 3(2s): 73-84.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.2s.73.84
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Alagawany et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Carvacrol is a monoterpenoid phenol predominantly found in oregano (Origanum vulgare), thyme (Thymus vulgaris), pepperwort (Lepidium flavum) and wild bergamot, also produced naturally by isolation of essential oil from some plants (Kintzios, 2002; Sokmen et al., 2004; Barnes et al., 2007; Tang et al., 2011; Jamali et al., 2012; Kim et al., 2013). The carvacrol amount in marjoram and hop marjoram (Dittany of Crete) are 50 and 70%, respectively. While, the oil extracted from thyme plant contains carvacrol percentage between 5 -75 (León-Rodríguez, 2008). Several in vitro and in vivo studies described different bioactivity of carvacrol nutrient, including antibacterial, antioxidant, antiseptic, antispasmodic, growth promoter, antifungal, antiviral, anti-inflammatory, expectorant, antitussive, immunomodulatory and chemopreventive as well as modifier of rumen microbial fermentation and reduction of methane emission (Luna et al., 2010; Soltanab et al., 2011; Hashemipour et al., 2013; Bravo et al., 2014).
Carvacrol is molecule that has crucial bioactivities on poultry and animal physiology and metabolism (Reiner et al., 2009), this compound could have antioxidant action on poultry meat when added in the diet. Carvacrol plays a critical role as natural antioxidant in the reduction of lipid peroxidation which leading to oxidative destruction of cellular membranes (Rhee et al., 1996; Yanishlieva et al., 1999). Moreover, the deleterious effect of these compounds may lead to increase in the production of toxic metabolites (free radicals) and also to apoptosis. On the other hand, Bavadekar (2012) reported that carvacrol promotes cell death in prostate cancer cells.
Several studies have been reported the addition of some phytogenic additives or their products such as cold pressed oil, essential oil or extracts to animal and poultry diets that improved live body weight, body weight gain, feed conversion ratio, immune response, antioxidant status, carcass traits and quality, and lowered morbidity and mortality rates (Ashour et al., 2014; Farag et al., 2014; Alagawany et al., 2015a, 2015b; Dhama et al., 2015). The current review covers many important aspects including the mechanisms of action, metabolism and excretion, biological activities and beneficial applications of carvacrol in animal and poultry production and health.
CARVACROL SOURCES
Carvacrol is a component of some medicinal plants, such as black cumin (Nigella sativa), oregano (Origanum compactum), Monarda didyma, Origanum dictamnus, Origanum microphyllum, Origanum onites, Origanum scabrum, Origanum vulgare, thyme (Thymus glandulosus), savory (Satureja hortensis) (Aligiannis et al., 2001; De Vincenzi et al., 2004; Coskun et al., 2008; Liolios et al., 2009; Figiel et al., 2010). Also, carvacrol has been produced by chemical and biotechnological synthesis via metabolic engineered microorganisms (More et al., 2007).
CHEMICAL AND PHYSICAL CHARACTERISTICS
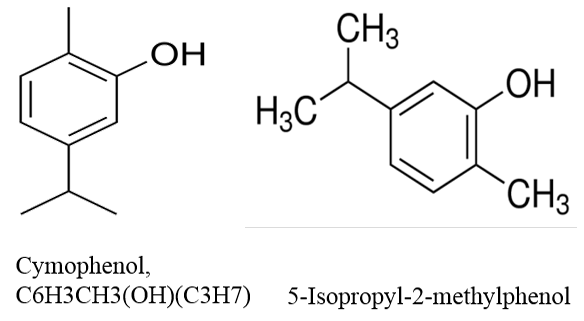
Carvacrol is an isomer and derivative of phenol, the chemical formula of carvacrol (cymophenol) is C6H3CH3 (OH) (C3H7), a monoterpenoid phenol (Bouchra et al., 2003; De Vincenzi et al., 2004). Carvacrol is also named 5-isopropyl-2-methylphenol or 2-Methyl-5-(1-methylethyl)-phenol according to International Union of Pure and Applied Chemistry (IUPAC). The structural formula of carvacrol is shown in Figure 1.
Carvacrol is a liquid and has the same taste of thymol. The density of carvacrol ranges from 0.976g/cm3 at 20°C to 0.975g/cm3 at 25°C. Carvacrol boils at 237~238°C, while its melting point is 1. It can be volatile with steam. Carvacrol is highly lipophilic; the solubility of carvacrol is very high in carbon tetrachloride, ethanol, diethyl ether, acetone; but insoluble in water (Ultee et al., 2000). Yadav and Kamble (2009) reported that formation of carvacrol could be resulted from alkylation of o-cresol with propylene or isopropyl alcohol (IPA) over solid acid catalysts.
MODES OF ACTION AND BIOLOGICAL ACTIVITIES
Several modes of action could be obtained by using phytogenic additives, such as affecting feed consumption, enhancing digestive enzymes secretion and increasing the motility of the digestive tract; antimicrobial activity, antiviral activity, antioxidative activity, endocrine and immune stimulation; anthelminthic, coccidiostat and anti-inflammatory activity (Akyurek and Yel, 2011). Basmacioğlu et al. (2010) affirmed that antioxidative and antimicrobial efficacy of the active component of plant extracts or essential oils had been used in a lot of in vitro or in vivo experiments, but some questions still remain unanswered concerning the mode of action, optimal dosage, and pathway of metabolism of these additives in poultry.
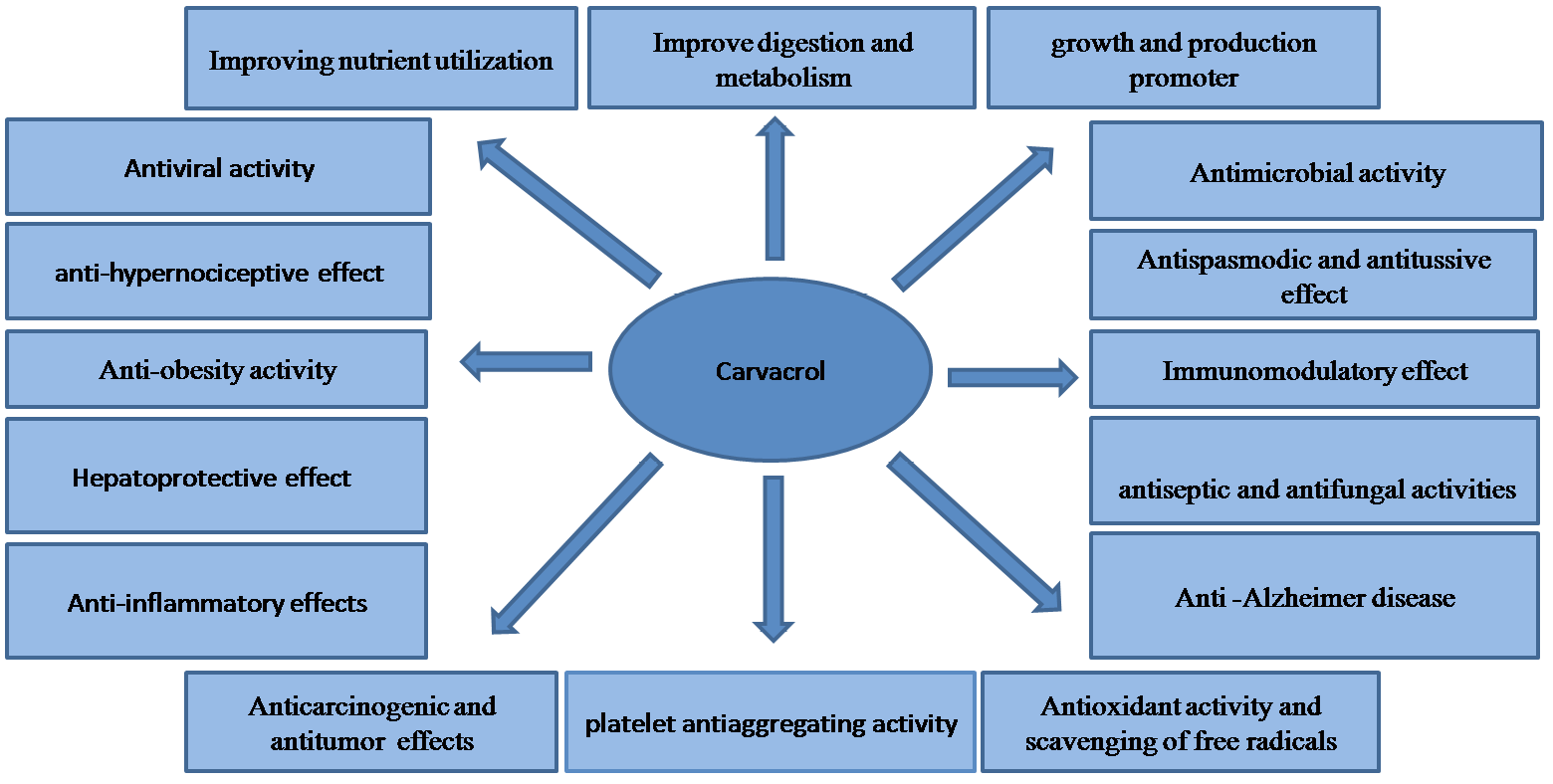
In fact, carvacrol component is added to various ingredients, such as nonalcoholic beverages (28.54 mg/kg), baked goods (15.75 mg/kg), chewing gum (8.42 mg/kg), etc. (Fenaroli, 1995). However, the mode of action of this compound was unknown by many researchers. A good knowledge of carvacrol mode of action is very required regarding application in nutritional systems. Formerly, Ultee et al. (1998) reported the antimicrobial activity of carvacrol on pathogen Bacillus cereus. Carvacrol is a hydrophobic compound and has an effective impact on biological membranes. The modes of action and beneficial aspects of carvacrol are shown in Figure 2.
METABOLISM AND EXCRETION
The metabolism and excretion of carvacrol in the body is very quick. The major metabolic pathway of carvacrol is related to the esterification of phenolic group with glucuronic acid (C6H10O7) and sulfuric acid (H2SO4). But, a minor route of carvacrol metabolism is transformation of the end methyl groups to primary alcohols. In an experiment on male albino rats, Austgulen et al. (1987) found that rats excreted a major percent of administered carvacrol (1 mmol/kg) in urine, as its sulphate and glucuronide conjugates, with extensive oxidation of the methyl groups mainly and this gave derivatives of benzyl alcohol and 2- phenylpropanol in addition to their corresponding carboxylic acids. A minor metabolite resulting from ring hydroxylation has also been detected. Moreover, the residual of carvacrol or its derivatives in urine was very small after one day only; this phenomenon is a strong indicator of the high speed of the carvacrol metabolism and excretion during the first day.
Michiels et al. (2008) carried out some experiment with gastric fermentation simulation of pig. Results reported 29% degradation of carvacrol in cecum, while jejunum was not affected. When piglets received oral feeding of 13.0, 13.2, 12.5 and 12.7 mg carvacrol /kg body weight, they showed half-life between 1.84 and 2.05 hours in the whole digestive tract. Carvacrol was almost absorbed completely in the proximal small intestine and the stomach. The concentrations of plasma (total of free and conjugated compound) maximized at 1.39 hours and followed by high concentrations in the urine.
BENEFICIAL ASPECTS OF CARVACROL
Improving Nutrients Bioavailability and Growth/Productive Performance
A number of animal and poultry trials have been conducted previously to establish the dependency and functionality of carvacrol and supplementation of it in animal and poultry diets. Hashemipour et al. (2013) reported that feed supplementation with thymol + carvacrol mixture by 60, 100, and 200 mg/kg of diet improved growth performance, digestive enzyme activities, and antioxidant enzyme activities besides inhibiting lipid peroxidation in broiler chicks. The concentration of IgG in serum was enhanced in the diet supplemented with different plant extracts such as thyme and oregano compared to the control diet in mice and pigs (Namkung et al., 2004).
In 17 days old poults which ate a diet with supplementation of a plant extract containing carvacrol at 300 mg/kg diet, Jamroz and Kamel (2002) observed daily body weight gain and feed conversion ratio to be improved by 8.1 and 7.7 %, respectively. Lee et al. (2003a) reported that increased efficiency of feed utilization could be the explanation of thymol and carvacrol effects on performance. Hernandez et al. (2004) stated that the improvement of broiler performance fed diets supplemented with several additives such as thymol and carvacrol, pepper essential oils (200 mg/kg), and rosemary extracts (5,000 mg/kg) could be attributed to its beneficial effects on digestibility.
Jaafari et al. (2012) fed one day female broilers with cholesterol-free or cholesterol-rich diets supplemented with 200 ppm carvacrol for four weeks of age and observed that feed intake and weight gain were reduced but feed conversion got improved. Moreover, plasma concentration of triglyceride was lowered with no effect on plasma content of cholesterol due to dietary carvacrol addition. When carvacrol was supplemented with corn-soybean meal basal diet supplemented with carboxymethyl cellulose, there were no any significant effects on feed efficiency or plasma content of cholesterol. Lee et al. (2003b) reported a negative effect on broilers body weight when feeding on 200 ppm carvacrol /kg diet with the same dose of cinnamaldehyde. A study of Lee et al. (2004) confirmed the non-significant impact on productive performance traits i.e. live body weight, feed consumption, feed efficiency and ileal content of microbiota by feeding broiler chickens on carvacrol with thymol and organic acids. These previous studies showed an indecisive influence of carvacrol on the efficiency of feed in broiler chickens and additional studies are wanted to get assured results as suggested by Umaya and Manpal (2013). Supplementing the diet of one day old broilers with 5.0 ppm carvacrol for seven days of age, caused an improvement in body weight gain and depressed oocyte shedding, gut lesions in addition to lowering gene expression of proinflammatory cytokine during coccidiosis when challenging birds by Eimeria acervulina (Lillehoj et al., 2011). Moreover, lipid metabolism, estrogen and androgen metabolism in intestinal intraepithelial lymphocytes were regulated due to carvacrol supplementation in broilers. Feeding chickens on diet supplemented with 5.0 ppm carvacrol/ kg altered the expression of 74 genes in intestinal intra epithelial lymphocytes. Treatment with carvacrol also led to an upregualtion of many genes associated with the metabolic and endocrine system such as protease serine 3 (PRSS3) and selenoprotein X, 1 (SEPX1) (Lillehoj et al., 2011). Based on the aforementioned results, several studies should be taken place to help in understanding the carvacrol molecular mechanism in the digestive tract of chicken and simplify the development of novel dietary ways to immunomodulate host response in disease or normal cases.
The consumer attention has been raised within the past decades regarding the quality of meat and its products. The poultry meat content of polyunsaturated fatty acids is high; so it is susceptible to oxidative deterioration, which negatively impacts the meat quality. Broilers fed diet contained 150 ppm of carvacrol through the experimental period (1 day - 42 days of age) caused a reduction in thiobarbituric acid production which is a proof of lipid peroxidation in samples of thigh (stored for 5-10 days). Similar results obtained by Yanishlieva et al. (1999); Annalisa et al. (2009); Mastromatteo et al. (2009); Kim et al. (2010) and Akalin and Incesu (2011) revealed that carvacrol supplementation minimized lipid oxidation and microbial load in chicken patties stored at low temperature (0-3°C), as well as improving shelf life and quality of poultry meat. So, using carvacrol as a natural antioxidant could improve quality of poultry products.
Antiviral Activity
Herbal plants and their derivatives or extracts have been evaluated for their possible antiviral prosperities, including the cold pressed or essential oils of certain commonly used culinary herbs (Jassim and Naji, 2003; Sokovic et al., 2010). Carvacrol plays a key role as antiviral component against human rotavirus (RV). On the same context, Mexican oregano (Lippia graveolens) extract and oil as well as carvacrol component are able to reduce/inhibit the viral diseases in animal and human. Specifically, the antiviral activity of oregano and its phenolic components on acyclovir resistant herpes simplex virus type 1 (ACVRHHV-1) and human respiratory syncytial virus (HRSV) and of carvacrol on RV have been documented (Bernstein, 2009; Pilau et al., 2011).
Oregano essential oils including carvacrol compound have been strongly promoted as natural antiviral factors effective against many viral diseases such as the pandemic H1N1 virus (Vimalanathan and Hudson, 2012). On the contrary, Sokmen et al. (2004) noted that anti-influenza virus activity was not affected by supplementation of oils or extracts derived from oregano. Gilling et al. (2014) reported that carvacrol as a natural food is very effective in inhibiting human norovirus within one hour of exposure by acting directly on the viral capsid.
Antimicrobial Activity
The antimicrobial effects of essential oils have been due to the presence of phenolic compounds, such as carvacrol, thymol, eugenol, curcumin and cinnamaldehyde which are presented in essential oils of oregano, thyme, clove, turmeric and cinnamon, respectively (Tsao and Zhou 2000; Lambert et al. 2001; Veldhuizen et al., 2006; Alagawany et al., 2015b). Sikkema et al. (1995), Adam et al. (1998), Weber and de Bont (1996), Ben-Arfa et al. (2006) and Nostro and Papalia (2012) mentioned that the beneficial/inhibitory effects of phenolic compounds could be attributed to the interactions between the effective compounds and cell membrane of microorganisms and is usually associated with the hydrophobicity of these compounds.
Arsi et al. (2014) showed that campylobacter numbers were reduced with 1% carvacrol supplementation, or a combination of both thymol and carvacrol at 0.5%. Friedman et al. (2002), Sokovic et al. (2002), Nastro et al. (2004), and Baser (2008) found antimicrobial influences of carvacrol against many species of microbes such as Pseudomonas, Aspergillus, Salmonella, Streptococci, Listeria, Bacillus and Fusarium. Burt et al. (2005 and 2007) observed that carvacrol supplement as antimicrobial component has a significant impact on harmful bacteria including Escherichia coli and Salmonella numbers in chickens, this effect may be attributed to inhibit the growth of pathogenic bacteria by carvacrol vapour. Johny et al. (2010) postulated that carvacrol and eugenol decreased (P≤ 0.05) Salmonella Enteritidis and C. jejuni counts in chicken cecal contents to <1.0 log10 cfu/ml at 50 and 75 mM and 20 and 30 mM, respectively.
Antioxidant Activity and Scavenging of Free Radicals
Free radicals or reactive oxygen intermediates are generated by cells during the normal metabolism. When free radicals such as superoxide radical (O2.-), hydrogen peroxide (H2O2) and hydroxyl radical (OH.) are accumulated excessively, this leads to a damage in tissue and privation of many cellular functions. Carvacrol as an antioxidant protects the cells against free radicals. Moreover, antioxidants inhibit prostaglandin synthesis and induct drug-metabolizing enzymes in addition to many biological activities as reported by Azirak and Rencuzogullari (2008).
Some studies assured the efficiency of carvacrol in scavenging free radicals i.e. nitric oxide, superoxide radicals, peroxyl radicals and hydrogen peroxide (Kohen and Nyska, 2002; Aristatile et al., 2010). The existence of hydroxyl group (OH) which linked to aromatic ring is suggested to be the reason for the highly antioxidant activity of carvacrol either in vitro or in vivo as explained by Aeschbach et al. (1994) and Guimaraes et al. (2010). The reaction of carvacrol with a free radical is facilitated due to its weak acid character, so donating hydrogen atoms to an unpaired electron, producing another radical that is stabilized by electron scattering generated at a molecule resonance structure (Aristatile et al., 2010).
Supplemental oregano by 50 to 100 mg/kg to broiler chick diets exerted an antioxidant effect in the broiler tissues (Botsoglou et al., 2002). Ruberto et al. (2000), Alma et al. (2003), and Luna et al. (2010) reported that the diet unsupplemented with carvacrol or thymol has similar effectiveness to inhibit the oxidation of lipids than the synthetic antioxidant supplementation such as butylated hydroxytoluene, ascorbic acid and vitamin E, and could be considered good natural additives to be applied in animal and poultry industry to improve the performance and health. Animals fed diet supplemented with carvacrol had greater concentrations of SOD and GSH-PX and more level of poly unsaturated fatty acids (PUFA) in the brain phospholipids than the unsupplemented control (Youdim and Deans, 2000).
Immunomodulatory Effect
Improving poultry immunity is one of the main goals to prevent infectious diseases. Immunodeficiency could occurr by several factors including abuse of antibiotics, vaccination failure or immune-suppressive infectious diseases. To improve the bird’s immunity and decrease the susceptibility to infectious diseases, immune stimulators could be used. Acamovic and Brooker (2005) and Silveira et al. (2013) reported that herbs rich in flavonoids such as thyme and carvacrol could improve the immune functions through acting as antioxidants and extending the activity of vitamin C.
Botsoglou et al. (2002) expected to find an improvement in the immune responses of chicks because of the assured antioxidant, antibacterial and antiviral activities of carvacrol which had been reported by many researchers. Lillehoj et al. (2011) pointed out that feeding birds with diets containing plant-derived phytonutrients such as carvacrol, thymol, cinnamaldehyde, capsicum and oleoresin significantly improved the immune response in chickens and lowered poultry infectious diseases. Furthermore, Hashemipour et al. (2013) reported that feeding birds with diets contained carvacrol plus thymol linearly increased (P<0.01) the primary and secondary response against SRBC antigen and IgG.
Anti-carcinogenic and Antiplatelet Effects
Some natural antioxidants such as carvacrol exert anticarcinogenic and platelet antiaggregating impacts (Karkabounas et al., 1997 and 2002; Evangelou et al., 1998; Liasko et al., 1998; Ipeka et al., 2005). Also, Karkabounas et al. (2006) and Michiels et al. (2008) affirmed the effect of carvacrol as anticancer and antiplatelet in vivo and in vitro during hepatocellular carcinoma, pulmonary tumors and chemical induced carcinogenesis. As well, Aydin et al. (2007) and Jayakumar et al. (2012) suggested that carvacrol exhibits antigenotoxic activity at nontoxic concentrations (<0.05 mM) but it needs more investigation.
Arunasree (2010), Yin et al. (2012), and Al-Fatlawi et al. (2014) pointed out genomic DNA fragmentations and caspase-3, -6 or -9 enzymes gene expression were induced by carvacrol supplementation; also carvacrol addition induced apoptosis regulatory genes in human breast cancer cell line (MCF-7) cells and retarded growth. Carvacrol plays an important therapeutic role in treating cancer including cervical cancer cells (Arunasree, 2010; Mehdi et al., 2011).
Hepatoprotective Effect
In a D-galactosamine induced rat model, carvacrol exhibited a hepatoprotective role either in vivo or ex vivo. Aeschbach et al. (1994), Aristatile et al. (2009a) and Guimaraes et al. (2010) revealed that using carvacrol at the level of 80 mg/kg body weight in rat helped restoring the concentrations of lipid peroxidation products, lipids content in kidney, liver and blood plasma to its normal values. In addition, enzymic and non-enzymic antioxidants concentrations induced by D- galactosamine also restored to normal by carvacrol. The aforementioned authors added that the treatment with carvacrol restored and controlled the damage of DNA and the reductions in mitochondrial enzymes which induced by D-galactosamine.
In fact, lake of glucose and oxygen needed for metabolism of the cell could be happened if blood flow to an organ was insufficient or stopped resulting in ischemia. Reperfusion is a term of the restoration of blood flow to the tissue after the elimination of the causative agent for ischemia. As a result to reperfusion, toxic products pass to the circulation system. During liver surgery, renal I/R injury and liver transplantations, hepatic ischemia is a frequent problem. Canbek et al. (2008), Aristatile et al. (2009b) reported that carvacrol protects liver during renal I/R injury and hepatic I/R injury through improving liver antioxidant defence and minimizing the products of lipid peroxidation.
Anti-inflammatory and Anti-hypernociceptive Effects
Hypersensitivity of nociceptive pathways causes inflammatory hyperalgesia or it could be called hypernociception. The immune system cells release the mediators such as interleukins, cytokines or tumor necrosis factor-α during inflammation. The previous action activates the higher order neurons which exist in the transmission of the nociceptive input and also activates the primary nociceptors. Trabace et al. (2011) stated that pain sensitivity increased in laboratory animals as a result to the aforementioned hypersensitivity of nociceptive pathways which contributes to hypernociception. The threshold sensitivity of mice exposed to carrageenan was improved by using carvacrol at the dose of 50 and 100 mg/kg comparing to indomethacin and standard drug. Marchand el al. (2005) reported that the method of carvacrol in inhibiting hypernociception is to inhibit the migration of mononuclear cells and neutrophils concluded in the production of proinflammatory cytokines such as nitric oxide and consequently a decrease in prostaglandins. The morphology of the cells did not affect due to carvacrol treatment excepting a decrease in the TNF-α levels in pleural lavage. Hotta et al. (2010), and Guimaraes et al. (2012) found an anti-hypernociceptive effect of carvacrol by decreasing the levels of enzyme responsible for inducing nitric oxide synthase and in turns the macrophages content of nitric oxide. Marchand et al. (2005), Kaufmann et al. (2011), and Uyanoglu et al. (2011) had contradicting reports regarding including the antioxidant effect of carvacrol in controlling nitric oxide production and lipid peroxidation during hypernociception.
Anti-obesity Effect
Obesity is a medical condition in which excess body fat accumulates to the extent that it may have a negative effect on health, leading to increased health problems. It is like other chronic diseases such as hyperlipidemia, cancers and diabetes. Umaya and Manpal (2013) stated that the main factor in enhancing obesity and attributed to the metabolic diseases in humans and animal models is the consumption of high levels of fat in the diet and they found that carvacrol caused an inhabiting of fat accumulation between cells and adipocyte differentiation in mouse embryo 3T3- L1 cells. Also, results showed that diet high in fat and supplemented with carvacrol decreased total visceral fat, plasma and liver total cholesterol, HDL-cholesterol, triglyceride and free fatty acids of mice. Moreover, carvacrol decreased the expression of adipogenesis related genes- fibroblast growth factor receptor in visceral adipose tissues. Also, carvacrol decreased the expression of receptors which stimulates the intake of fat rich diet such as galanin receptor 1 and 2.
Wieten et al. (2010), and Cho et al. (2012) found that free fatty acid levels and the mRNA and protein levels of toll-like receptors were reduced by carvacrol. Free fatty acids in high levels are reported in obese animals, because of their release either from high fat diet or from adipose tissues. Carvacrol as anti-obese drug needs for more detailed studies to be recommended for this purpose.
Conclusion
This review highlights the beneficial applications of the dietary addition of carvacrol as a natural antioxidant or growth enhancer with useful activities on feed efficiency, nutrient bioavailability, immunity, oxidative status, egg quality parameters and growth/productive performances. Furthermore, useful impacts of lowered serum and meat MDA, lipid peroxidation have been noted in poultry and animal fed rations supplemented with carvacrol, indicating the beneficial effects and important role of carvacrol dietary supplementation which could be due to its pharmacological effects and beneficial health effects, such as antimicrobial, antioxidant, anticancer, antiplatelet, antiviral, anti-inflammatory, antifungal, and growth promoting properties. Exploration of the carvacrol modes of action like nutritional, pharmacological, health benefits and biological properties may play crucial role in its beneficial usages in poultry farm and animal management systems by providing further understanding of the health applications and increasing performance parameters in agriculture species.
ACKNOWLEDGEMENTS
All the authors of the manuscript thank and acknowledge their respective Universities and Institutes.
REFERENCES