Advances in Animal and Veterinary Sciences
Short Communication
Progesterone Profile of Goats Subjected to Oestrus Synchronization and its Relevance in Predicting Pregnancy Outcome
Sneha S Panicker1, Raji Kanakkaparambil2*, Promod Kanjirakuzhiyil3, Ramachandran Koodathil3
1Department of Animal Reproduction, Gynaecology & Obstetrics, College of Veterinary & Animal Sciences, Pookode, Wayanad, Kerala, India; 2Department of Veterinary Physiology, College of Veterinary & Animal Sciences, Mannuthy, Kerala-680651, India, 3Department of Animal Reproduction, Gynaecology & Obstetrics, College of Veterinary & Animal Sciences, Pookode, Wayanad, Kerala-673576, India.
Abstract | Successful oestrous synchronization protocol must support a reasonable level of pregnancy in the synchronized cycle. Premature luteal regression and a drop in progesterone, the pregnancy hormone and concentration is a common phenomenon in goats following oestrus synchronization. Therefore, this study was undertaken to evaluate the comparative efficacy of two oestrus synchronization protocols on the progesterone profile of the Malabari cross bred goats which would be of practical significance for estrous synchronization of goats. It was observed in this study that the progesterone profile in goats did not vary with the treatment regimens to which they were subjected. Curiously, we observed that the progesterone concentrations on the day of insemination showed significant difference (P<0.05) between the goats those conceived and those failed to conceive. We could make the earliest detection of pregnancy using progesterone estimation by ELISA by day 17th post insemination, while it could be confirmed only on day 20th by ultra-sonography.
Keywords | Goats, Oestrus synchronization, Progesterone, ELISA, Conception
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | March 03, 2015; Revised | March 07, 2015; Accepted | March 08, 2015; Published | March 18, 2015
This article is a Part of MVSc thesis of Dr. Sneha S Panicker
*Correspondence | Raji Kanakkaparambil, College of Veterinary & Animal Sciences, Mannuthy, Kerala, India; Email: [email protected]
Citation | Panicker SS, Kanakkaparambil R, Kanjirakuzhiyil P, Koodathil R (2015). Progesterone profile of goats subjected to oestrus synchronization and its relevance in predicting pregnancy outcome. Adv. Anim. Vet. Sci. 3(4): 207-210.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.4.207.210
ISSN (Online) | 2307-8316; >ISSN (Print) | 2309-3331
Copyright © 2015 Panicker et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
India stands second in the world concerning with goat population and constitutes an important role in rural livestock as it provides employment on a large scale. It also provides livelihoods of low and medium input farmers and hence, it is essential to improve the reproductive efficiency using different scientific techniques in these small ruminants.
Estrus synchronization plays a major role in fixed time breeding and it is essential when oestrous detection is not very efficient. The value of estrus synchronization is vital in goats as the duration of both estrous cycle and estrus is variable (Rahman et al., 2008) and as they exhibit silent oestrous (Greyling and van der Nest, 2000). Oestrus synchronization can be carried out by the conventional methods like alteration in the light exposure period, buck exposure and use of hormonal treatments. Synthetic Gonadotropin Releasing Hormone (GnRH) preparations, equine chorionic gonadotropin (eCG), human chorionic gonadotropin (hCG), progestagens administered by different routes (oral, injections, vaginal pessaries) and prostaglandin (PGF2α) therapies are all used for oestrus synchronization in ruminants. A drop in progesterone concentration shortly after insemination in Ovsynch and Progestagen protocol has been reported by some which indicates premature corpus luteum regression (Holtz et al., 2008).
Reproductive efficiency depends on follicle development, achievement of oocyte competence for fertilization as well as the capacity to carry pregnancy to term. Successful techniques must not only help to achieve synchrony, but also should support a reasonable level of pregnancy in the synchronized cycle. However, none of the estrus synchronization protocols in use to date assures all expectations concerning predictability and reliability of the method for successful pregnancy outcome. Progesterone, the pregnancy hormone, is essential for maintaining pregnancy. The approaches to minimize the occurrence of premature corpus luteum regression and low progesterone levels at the time of prostaglandin treatment was found to maximize fertility of does synchronized by Ovsynch protocol (Holtz et al., 2008). Therefore, it is essential to monitor the progesterone profile after oestrous synchronization and insemination. Hence, this study is undertaken to evaluate the efficacy of oestrous synchronization protocols on progesterone profile to appreciate whether these protocols have any influence on luteal regression.
MATERIALS AND METHODS
A total of 24 number of healthy Malabari crossbred goats aged between 3-4 years were selected randomly for the study. The animals were exposed to natural lighting conditions at latitudes between 11°47’ N & 15°58’ N where maximum temperature and relative humidity is ranging between 27-29oC and 78-88 mm/Hg respectively during the breeding season. They were fed with a daily ration of 200 g concentrate per animal (Table 1). In addition, free access to green grass and water was made available.
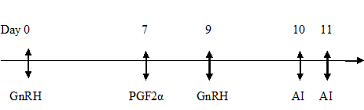
The goats were divided into two groups of eight animals each. Goats belonging to Group I were subjected to oestrus synchronization by using Ovsynch Protocol with first dose of GnRH analogue (0.004mg) Buserelin injection (1ml Receptal®, Intervet, Holland) on day 0 intramuscularly followed by an intramuscular injection of prostaglandin (12.5 mg) Dinoprost (2.5 ml Lutalyse®, Pfizer, Belgium) on 7th day. Forty eight hours after prostaglandin treatment (day 9th) a second dose of Buserelin (0.004mg) was again administered intramuscularly.
Group I (Ovsynch protocol)
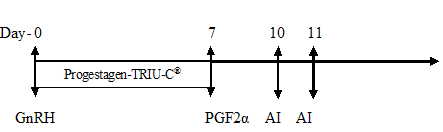
Animals in Group II were subjected to the intra-vaginal placement of progesterone device (TRIU-C®, Virbac, India) containing 160 mg of natural progesterone along with intramuscular injection of GnRH (Buserelin,0.004 mg) on the first day of the experiment (day 0). The intravaginal device was retained for seven days and at the time of withdrawal (day 7th) an intramuscular injection of 12.5 mg of Dinoprost, an analogue of PGF2α (2.5 ml Lutalyse®, Pfizer, Belgium) was administered.
Group II (Progestagen-TRIU-C® protocol)
The animals were inseminated using fresh semen 72 hours after the administration of prostaglandin in group I and II respectively followed by a re-insemination after 24 hours. The blood was collected and serum was separated for the progesterone assay on days 0, 5, 7, 17 and 22 post insemination. The progesterone levels were analysed using ELISA kit (Pathozyme®) and the data were compiled and subjected to statistical analysis using Least squares analysis of variance as described by Harvey (1987). Analysis was done to study the effect of synchronization protocols on the progesterone levels on days 0, 5, 7, 17, 22 post insemination and compare the level of progesterone between pregnant and non-pregnant animals on the above mentioned days. Duncan’s Multiple range test as modified by Kramer (1957) was used to test the differences among least squares means.
Table 1: Nutritive value of concentrate feed in percent
|
Chemical composition |
Crude protein |
Crude fibre |
Fat |
Calcium |
Acid insoluble ash |
Total ash |
Moisture content |
|
Nutritive value |
17.57 |
36.64 |
2.12 |
1.82 |
3.4 |
11.9 |
6.2 |
Table 2: Mean (± S.E.) Progesterone concentrations (ng/ml) at different intervals
|
Groups |
Day S (Pre-treatment) |
Day 0 |
Day 5 |
Day 7 |
Day 17 |
Day 22 |
|
Group I (Ovsynh) |
6.35a ± 1.30 |
3.62a ± 0.86 |
8.05a ± 1.20 |
10.32a ± 1.14 |
10.11a ± 1.40 |
11.32a ± 1.25 |
|
Group II (Progest gen-TRIU-C®) |
8.67a ± 1.30 |
4.30a ± 0.86 |
7.50a ± 1.20 |
12.32 a ± 1.14 |
13.87a ± 1.40 |
10.54a ± 1.17 |
|
Non pregnant |
8.77a ±1.30 |
6.14a ± 0.86 |
8.25a ± 1.20 |
9.73a ± 1.14 |
9.77a ± 1.40 |
7.17a ±1.17 |
|
Pregnant |
6.25a ±1.30 |
1.78b ± 0.861 |
7.30a ± 1.20 |
12.91a ±1.14 |
14.21b ±1.40 |
14.70b ± 1.25 |
a, b across columns - Means with different superscript vary significantly (P< 0.05)
RESULTS AND DISCUSSION
There was no significant difference in the mean (± S.E.) serum progesterone profiles of animals in group I (Ovsynch) and group II (Progestagen-TRIU-C®) (Table 2). This is in agreement with the results obtained by Titi et al. (2008) who have reported that the progesterone profiles remain similar in goats synchronized with different protocols.
The progesterone levels of goats that had conceived (1.78 ± 0.86 ng/ml) was much lower than those that failed to conceive (6.14 ± 0.86 ng/ml) on day 0 of insemination (Table 2). From the present study, it could be suggested that the animals with lower level of progesterone on the day of insemination (day 0) may have greater probability for conception. There have been no reports regarding the co-relation between the progesterone levels on the day of insemination with the conception rate following synchronization protocols in goats. It needs further studies to confirm whether the level of progesterone on the day of insemination can be a crucial factor deciding successful outcome.
In the present study, there was no significant difference in the serum progesterone level between pregnant and non-pregnant animals on days 5 and 7 post insemination (Table 2). This is in agreement with reports from Thorburn and Schneider (1972) and Pennington et al. (1982). However, there was significant difference between the progesterone profile of pregnant and non-pregnant goats (P<0.05) on days 17 and 22. The pregnant animals exhibited higher progesterone levels by day 17. Thus the earliest diagnosis of pregnancy in Malabari goats in this study with the serum progesterone estimation was done on day 17th post insemination. The progesterone level was low in non-pregnant goats from day 16 to 21 as reported by Zarkawi and Soukouti (2001), Medan et al. (2004) and Titi et al. (2008). Padilla- Rivas et al. (2005) reported the detection of amniotic vesicle by day 19.5 ± 0.3 post insemination in Boer goats as the first indication of pregnancy. In the present experiment, the earliest possible detection of embryonic vesicle was made on day 18th in few animals, but only by day 20th it could be detected tans-rectally in all the pregnant animals. However, significant increase in the progesterone level was evident from day 17 post insemination in pregnant goats. Therefore, serum progesterone profile may be taken as a method to detect pregnancy as early as 17th day post insemination in goats.
CONCLUSION
The study suggested that there was no significant difference in mean serum progesterone profiles of Malabari crossbred goats synchronized with two different protocols. However, this study suggests that the level of progesterone on the day of insemination may be a crucial factor deciding successful outcome and this warranties further study. Serum progesterone estimation on the day 17th post-insemination can be a method to detect pregnancy in goats.
REFERENCES





