Advances in Animal and Veterinary Sciences
Research Article
Comparison of Phenotypic Characteristics and Virulence Traits of Klebsiella pneumoniae Obtained from Pneumonic and Healthy Camels (Camelus dromedarius)
Sandeep Kumar Sharma1*, Naresh Kumar Mudgal2, Pradeep Sharma2, Brij Nandan Shrngi3
1Department of Veterinary Microbiology & Biotechnology, Post Graduate Institute of Veterinary Education and Research- Jaipur, Rajasthan University of Veterinary and Animal Sciences, Bikaner, India; 2Department of Animal Husbandry; 3Department of Veterinary Microbiology & Biotechnology, College of Veterinary & Animal Science- Bikaner, Rajasthan University of Veterinary and Animal Sciences, Bikaner, India.
Abstract | The present study was aimed to compare Klebsiella pneumoniae isolates obtained from nasal discharge of pneumonic and healthy camels in context of phenotypic characteristics and virulence traits. Total 65, 16S-23S rDNA internal transcribed spacer (ITS) region based confirmed isolates were included with species-specific amplicon of 130 bp in this investigation. Out of which 47 were from pneumonic and 18 from healthy camels. All K. pneumoniae isolates showed typical inherent phenotypic pattern except six isolates from pneumonic camels were found ability to produce indole. For virulence traits, all isolates were non hemolytic with prominent capsule. All the isolates from pneumonic camels were urease positive and most of (93.6%) isolates positive on first three days while all isolates from healthy camels were urease negative on first three days. None of the isolates from healthy camels showed hypermucoviscosity while 25 (53.19%) isolates from pneumonic camels showed existence of virulent hypermucoviscosity trait. It may indicate differences in virulence properties of studied isolates and further suggest some genotypic studies regarding virulence characterization of K. pneumoniae.
Keywords | Klebsiella pneumoniae, Camels, Phenotypic characteristics and virulence traits
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 22, 2014; Revised | January 10, 2015; Accepted | January 12, 2015; Published | January 21, 2015
*Correspondence | Sandeep Kumar Sharma, Rajasthan University of Veterinary and Animal Sciences, Bikaner, India; Email: [email protected]
Citation | Sharma SK, Mudgal NK, Sharma P, Shrngi BN (2015). Comparison of phenotypic characteristics and virulence traits of Klebsiella pneumoniae obtained from pneumonic and healthy camels (Camelus dromedarius). Adv. Anim. Vet. Sci. 3(2): 116-122.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.2.116.122
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Sharma et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Klebsiella pneumoniae was recognized in 1887, when Trevisan (1987) has described a bacterium from the lungs of a patient who had died of pneumonia. K. pneumoniae is a lactose-fermenting, facultative anaerobic, non-motile with a prominent capsule bacillus in the family Enterobacteriaceae. This opportunistic pathogen found in a variety of environmental sources such as soil, vegetation, water and industrial wastes including mucosal surfaces of mammals with common association of a broad range of infections viz. peritonitis, septicemia, pneumonia, urinary tract infections, and meningitis (Podschun and Ullmann, 1998). It is an important pulmonary pathogen for camels, horses, dogs, foals, sow and calves (Arora and Kalra, 1973; Boguta et al., 2002; Henton et al., 1994; Selbitz et al., 2002). In camels, K. pneumoniae reported from acute destructive bronchopneumonia and community-acquired bacterial pneumonia with increased tendency to develop abscess, cavitation and empyema (Arora and Kalra, 1973; Zubair et al., 2004; Kane et al., 2005; Abubakar et al., 2010).
Klebsiella pneumoniae strains exhibit many virulence factors such as capsular polysaccharides (CPS), lipopolysaccharide (LPS), iron-scavenging systems (siderophores), adhesins and hypermucoviscosity (Podschun and Ullmann, 1998) along with other virulence factors such as urease production, hemolysins, protein-tyrosine kinase, phosphotyrosine-protein phosphatase and heat-labile & heat-stable endotoxins (Dworkin et al., 2006). The capsule is an important virulence factor to govern pathogenesis, which protects the bacteria from phagocytosis and bactericidal effects of serum factors (Highsmith and Jarvis, 1985, Struve and Krogfelt, 2003). Sometime K. pneumoniae also exhibits large amounts of mucopolysaccharide web of capsular and extracapsular polysaccharides to produce more virulent hypermucoviscosity strain (Wiskur et al., 2008). Klebsiella is a non-hemolytic for human red blood cells but found to be hemolytic for rabbit blood agar and urease production of this organism has significant role in colonization and survivability in various systemic infections (Dworkin et al., 2006; Maroncle et al., 2006).
Camel (Camelus dromedarius) is a uniquely morphological & physiological adapted animal in desert ecosystem with good potential to thrive well on meager resources under extreme climatic conditions. It has important utilities in human society such as drought, farming, milking and many other important farming purposes but sometime certain sudden environmental variations make this animal susceptible to various infections (Kebede and Gelaye, 2010). In India, Klebsiella pneumoniae has been also reported from cases of “khurak” (local name of pneumonia) in camels. “Khurak” is an infectious respiratory disease characterized by alveolar serofibrinous exudation, capillary hyperaemia and microbial bronchopneumonia with respiratory distress, pyrexia, prolonged cough and septicemia in camels. It is a highly morbid, causing considerable loss of production and deaths especially in adult camels (Arora and Kalra, 1973; Sharma, 2012). K. pneumoniae found as commensal in healthy individual as well as pathogenic agent in diseased animals. So it is required to detect variations among Klebsiella pneumoniae strains obtained from healthy and diseased individuals. Thus this study has designed to compare Klebsiella pneumoniae isolates obtained from pneumonic and healthy camels on the basis of primary and secondary biochemical characters and virulence properties.
Materials and Methods
Sample Collection and Identification
A total of 163 severe nasal discharge samples from pneumonic (96) and healthy (67) camels were collected. The pneumonic and healthy camels were determined on the basis of clinical symptoms viz. the camel with severe nasal discharge, respiratory distress, depression and pyrexia up to 41°C temperature in early stages. After a week or so, the temperature tends to drop down to normal, but cough persists for a long time was considered as pneumonic camel. While the camels, which were not show any clinical and medication history since last six month considered as healthy. Samples of pneumonic camels has been collected from clinical complex of college of veterinary and animal science, Bikaner (Rajasthan) and samples of healthy camel has been from surrounding villages of Bikaner area. All samples were collected without any discrimination of age, sex and breed. The samples were collected directly from nasal cavity aseptically with the help of sterile cotton swabs. And proceeded on selective medium Simmon’s citrate agar with 1% inositol (SCAI) (Van Kregten et al., 1984) and single yellow dome shaped colonies were further checked for genotypic confirmation with 16S-23S rDNA internal transcribed spacer (ITS) region based specific primers (Liu et al., 2008). For genotypic confirmation genomic DNA was used as template and isolation was carried out by method of Chen and Kuo (1993). All amplified PCR products were analyzed by 8% polyacrylamide gel electrophoresis (PAGE).
Phenotypic Characterization and Detection of Virulence Traits
Genotypically confirmed 65 isolates were included, out of which 47 from pneumonic and 18 isolates were from healthy camels. All were subjected to various primary and secondary biochemical reactions such as catalase, oxidase and motility then checked for lactose fermenter mucoid colonies on MacConkey agar (MCA) and nonmetallic dark colored mucoid growth on EMB agar further secondary biochemical characters such as IMViC pattern, growth on TSI, gelatin liquefaction, aesculin hydrolysis, nitrate reduction, malonate utilization, lysine decarboxylation and ONPG (o-Nitrophenyl-β-d Galactopyranoside) tests were conducted (Quinn et al., 1994 and Garcia and Isenberg, 2007). For phenotypic detection of virulence traits, Maneval’s capsular staining method was used for capsular detection, haemolysin production was observed on sheep blood agar, urease production was tested by change in color of media from yellow to red observed for seven days in urease broth as described in literature and virulent hypermucoviscosity trait checked by string test (Wiskur et al., 2008).
Results
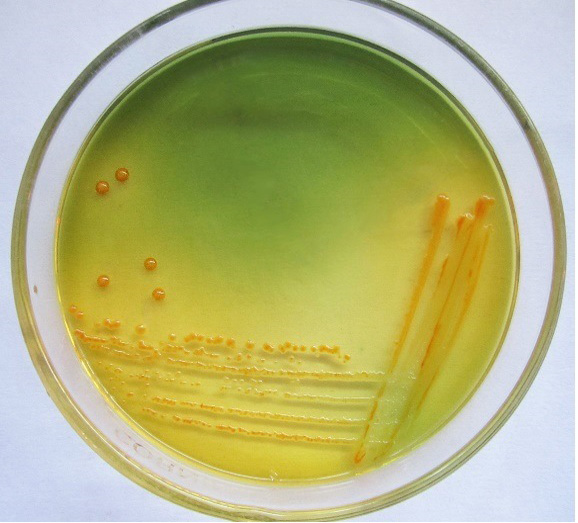
Only 65 samples (47 were from pneumonic and 18 from healthy camels) showed typical yellow dome shaped colonies (Figure 1) on Simmon’s citrate agar with 1% inositol (SCAI) from 163 nasal discharge samples of pneumonic and healthy camels and further these 65 isolates were confirmed with 16S-23S rDNA internal transcribed spacer (ITS) region based specific primers showing species-specific amplicon of 130 bp (Figure 2). In the present study, without any variation all isolates showed pink lactose fermenter mucoid colonies on MacConkey agar (MCA). Nonmetallic dark colored mucoid growth on EMB agar, non-hemolytic grey-white, mucoid colonies on sheep blood agar (Figure 3). And mucoviscosity observed in all other growth medium with large capsule. All isolates showed typical IMViC pattern (--++) and other biochemical characters (Table 1) as described in literature, except for six isolates from pneumonic camels that were found ability to produce indole. 100% isolates from pneumonic camels were urease producing and most of isolates of pneumonic camels were urease producers within first three days of observation while out of 18 isolates from healthy camels, three (16.66%) were urease negative and rest of the 15 isolates showed positive results after three days of incubation (Table 2). Only 25 (53.19%) isolates from pneumonic camels showed existence of virulent hypermucoviscosity trait (Figure 4) while none of the K. pneumoniae isolates from healthy camel exhibited hypermucoviscosity.
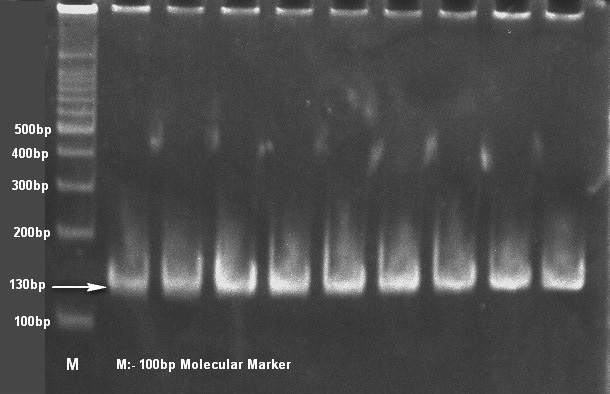
Figure 2: Klebsiella pneumonaie isolates showing typical 130bp size product with specific primers on 8% polyacrylamide gel
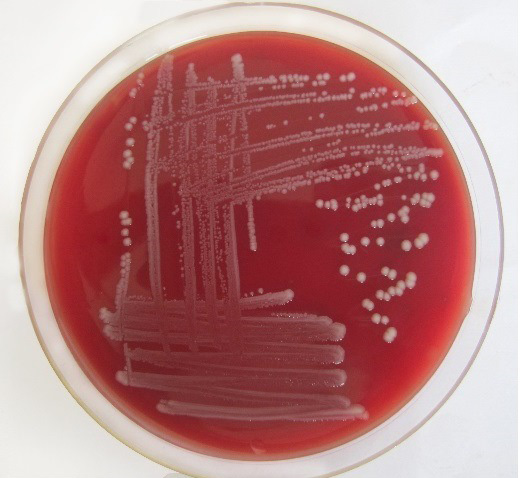
Figure 3: Klebsiella pneumoniae showing non-hemolytic grey-white, mucoid colonies on sheep blood agar
Table 1: Secondary biochemical tests of Klebsiella pneumoniae isolates from pneumonic and healthy camels
|
S. No. |
Test |
Reaction |
|
1 |
Growth on 10°C |
Negative |
|
2 |
Growth on 44.5°C |
Positive |
|
3 |
Growth on SCAI |
Yellow mucoid dome shaped colonies |
|
4 |
Growth on EMB agar |
Dark mucoid non-metallic colonies |
|
5 |
Growth on BCP agar |
Yellow mucoid colonies |
|
6 |
Growth on TSI agar |
Acid/Acid/No H2S (Y/Y/-) |
|
7 |
Gelatin Liquefaction |
Negative |
|
8 |
Aesculin Hydrolysis |
Positive |
|
9 |
Nitrate Reduction |
Positive |
|
10 |
Malonate Utilization |
Positive |
|
11 |
Arginine Hydrolysis |
Negative |
|
12 |
Lysine Decaboxylation |
Positive |
|
13 |
Phenylalanine Deamination |
Negative |
|
14 |
ONPG (o-Nitrophenyl-β-d Galactopyranoside) |
Positive |
Discussion
In the present study, we have examined the primary and secondary biochemical characters and virulence traits of Klebsiella pneumoniae isolates obtained from severe deep nasal discharge of pneumonic and healthy camels. Samples which were showing yellow dome shaped colonies on Simmon’s citrate agar with 1% inositol (SCAI) also found confirmed with K. pneumoniae species specific primers. Similar to present study, Sharma et al. (2013) has also reported specificity of these species specific primers for detection of K. pneumoniae from acute respiratory tract infections of camels. In addition, Liu et al. (2008) was also detected this organism with same primers and similar conditions from infant formula food sources. It proves genotypic specificity of this method for K. pneumoniae detection not only from food sources but also from animal origin. It also indicates the good discriminative capacity of SCAI agar therefore SCAI agar can be used as a selective medium for isolation of K. pneumoniae. Van Kregten et al. (1984) had also reported selectivity of SCAI agar for K. pneumoniae with similar results, who developed a medium based on the presence of two carbon sources, citrate and myo-inositol, without any inhibitor because K. pneumoniae has ability to utilize both citrate and myo-inositol (Dworkin et al., 2006).
In agreement with present study, Smith and Ngui-Yen (1980) were also found non hemolytic K. pneumoniae while Albesa et al. (1980) reported hemolytic K. pneumoniae in rabbit blood agar. In support of present study, indole production in K. pneumoniae has been also reported by Brown and Seidler (1973), Rennie and Duncan (1974) and Edwards and Ewing (1972) with 28%, 16% and 6% frequency respectively. None of the significant difference was observed in hemolytic pattern of K. pneumoniae isolates obtained from pneumonic and healthy camels in present study but variations were observed in indole production. This ability may contribute in virulence and antibiotic resistance since indole producing ability can act as a signalling molecule to regulate the expression of adhesion and biofilm-promoting factors (Martino et al., 2003; Nishida et al., 1978). Other secondary biochemical characters in present study were found similar as described earlier literature (Eguchi et al., 1987; Rennie and Duncan, 1974). These similar biochemical characters may explain through previous studies, who reported similar biochemical properties of K. pneumoniae from various sources without significant variations (Bouvet et al., 1989; Munoz et al., 2007). Thus it may also conclude that source of bacterial isolation not too much affects primary and secondary biochemical reaction of K. pneumoniae isolates.
Table 2: Urease production in Klebsiella pneumoniae isolates from pneumonic and healthy camels
|
Observation day |
Pneumonic camels |
Healthy camels |
||
|
Positive isolates |
Negative isolates |
Positive isolates |
Negative isolates |
|
|
First |
37 (78.72%) |
- |
- |
3 (16.66%) |
|
Second |
4 (8.51%) |
- |
||
|
Third |
3 (6.38%) |
- |
||
|
Fourth |
1 (2.12%) |
4 (22.22%) |
||
|
Fifth |
2 (4.25%) |
7 (38.55%) |
||
|
Sixth |
- |
3 (16.66%) |
||
|
Seventh |
- |
1 (5.55%) |
||
|
Total |
47 (100.0%) |
- |
15 (83.33%) |
3 (16.66%) |
In accordance to present study, Mobley and Warren (1996) and Wilson and Miles (1975) also reported that clinical isolates of K. pneumoniae were 100% urease producing. And present investigation also found that K. pneumoniae isolates from pneumonic camels were faster urease producing as compared to isolates from healthy camels. Urease production enables the bacteria to grow in acidic environment (Mobley and Warren, 1996)). Thus it has significant role in colonization, survival and pathogenesis of several bacterial species including K. pneumoniae (Burne and Chen, 2000; Maroncle et al., 2006).
Results of hypermucoviscosity trait in this study are almost comparable with finding of previous studies viz., Yu et al. (2006) reported 38% prevalence of virulent hypermucoviscosity trait in clinical isolates of K. pneumoniae. And Whitehouse et al. (2010) reported absence of virulent hypermucoviscosity trait in captive (apparently healthy) vervets. Thus the absence of virulent hypermucoviscosity trait among isolates from healthy may underset. In support of present study, Edwards (1928) also reported that extensive capsule production is necessary to produce metritis in mares and Simoons-Smit et al. (1986) established the relationship between the size of capsule of K. pneumoniae and pathogenicity in experimental lobar pneumonia. Since virulent hypermucoviscosity trait has results from overproduction of extra capsular polysaccharide mucoviscous web, it has significant role in virulence because these are resistant to phagocytosis by neutrophils and from serum killing by complement system and are commonly associated with community acquired pneumonia, bacteremia and distinct invasive syndromes such as primary liver abscesses, meningitis, and endophthalmitis (Vila et al., 2011; Lin et al., 2010).
In the present study, we found some differences regarding indole production, urease production and virulent hypermucoviscosity trait of isolates from pneumonic and healthy camels. It may indicates the differences in virulence and suggest further genotypic characterization of isolates to find genotypic mechanisms of virulence of Klebsiella pneumoniae to make proper diseases diagnosis and prevention.
Acknowledgments
We acknowledge the support and facilities provide by Head of department of veterinary microbiology and biotechnology, Dean of college of veterinary and animal science, Bikaner and Dean of Post Graduate Institute of Veterinary Education and Research- Jaipur for this study.
References