Advances in Animal and Veterinary Sciences
Research Article
Health Risk Assessment of Heavy Metals for Egyptian Population via Consumption of Poultry Edibles
Manal A. M. Mahmoud, Hosnia S. Abdel-Mohsein
Department of Animal Hygiene, Faculty of Veterinary Medicine, Assiut University, Assiut 71526 – Egypt.
Abstract | Exposure to heavy metals through poultry consumption may lead to health risks in Egypt, especially in areas with expanding industrial and agricultural activities. Lead (Pb), Cadmium (cd), Aluminum (Al) and Nickel (Ni) concentrations were determined in poultry muscle and liver using ZEEnit 700P Atomic Absorption Spectrophotometer with Graphite Furness. Mean concentrations of Pb and Cd in poultry muscle and liver exceeded the maximum tolerable limit set by the European communities. However, Al and Ni concentrations were below the guideline limits. According to the Egyptian standardization, only 33% and 66% of liver and muscle samples from Assiut exceeded the limit, respectively. The estimated weekly and daily intakes in the examined samples were below the FAO/WHO Guidelines limits. Meanwhile, Target Hazard Quotients (THQ) and Hazard Index (HI) were calculated to estimate the human health risk of Egyptian population. In this study, THQ and HI were more than 1 for Cd when compared with their reference dose (RfD) in poultry edibles from both Assiut and Qena. The study emphasizes the potential public health risk of Cd contamination from poultry consumption to the local inhabitants in Egypt.
Keywords | Health risk; Lead; Cadmium; Aluminum; Nickel, Poultry edibles
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 30, 2014; Revised | December 16, 2014; Accepted | December 18, 2014; Published | December 26, 2014
*Correspondence | Hosnia S. Abdel-Mohsein, Faculty of Veterinary Medicine, Assiut University, Assiut, Egypt; Email: [email protected]
Citation | Mahmoud MAM, Abdel-Mohsein HS (2015). Health risk assessment of heavy metals for Egyptian population via consumption of poultry edibles. Adv. Anim. Vet. Sci. 3(1): 58-70.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.1.58.70
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Mahmoud and Abdel-Mohsein. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Environmental pollution with heavy metals in Egypt derives from rapid industrial growth, advances in agriculture fertilizers and urban human activities. Pollution by the metal and its by-products dispersion during production, recycling and disposal impaired health of the population. According to State Information Service (SIS, 2013) in Egypt; Assiut and Qena are agricultural as well as industrial provinces. Assiut host big industries such as: fertilizers, pharmaceuticals, cement, and petrol. Qena is well known for Aluminium Complex Factory, which is one of the biggest industrial plants in the Middle East, in addition to paper, cement, natural gas, light tar, spinning and weaving, as well as non-woven fabric and plastics industries.
Heavy metal contamination is a major problem of our environment and they are also one of the major contaminating agents of our food supply (Gholizadeh et al., 2009; Khairy, 2009). Uncontrolled pollution levels particularly in developing countries have drawn more attention to the heavy metal problem. Metals of major interest in bioavailability studies, as listed by the U.S. Environmental Protection Agency are aluminium, arsenic, cadmium, chromium, copper, mercury, nickel, lead, selenium, and antimony (McKinney and Rogers, 1992). Lead, cadmium, aluminum and nickel were selected, in this study, because of their potential for human exposure and increased health risk. Lead and cadmium are among the main toxic and abundant metals which accumulate in food chain (Dermirezen and Uruç, 2006) and easily absorbed from atmospheric air and from the digestive tract (Krejpcio and Trojanowska, 2000). Exposure to lead may cause kidney and nervous system problems as well as inhibit heme synthesis (Berny et al., 1994). However, cadmium is known to be an endocrine disturbing substance and may lead to the development of prostate and breast cancer as well as kidney and skeletal damage in humans (Saha and Zaman, 2012; Nordberg et al., 2002).
Food is unquestionably the main source of aluminium intake. Exposure is unavoidable because of the wide use of this element in day-to-day life and in industry. For many years, aluminium was not considered a health threat because of its relatively low bioavailability. In 1965, animal experiments suggested a possible connection between aluminium and Alzheimer’s disease; the findings of Alfrey were the first to establish a connection between neurologic diseases of dialysis patients (Alfrey et al., 1976). In addition, Aluminum is well known as a neurotoxicant and has been shown to have deleterious effects on skeletal and hematopoietic systems of humans (Domingo, 1995). Nickel is a known hematotoxic, immunotoxic, hepatotoxic, pulmotoxic and nephrotoxic agent. Nickel confirms lethal if it surpasses the allowed amount in edibles (Nriagu and Pacyna, 1988).
The World Health Organization (WHO) reported that about a quarter of the diseases facing mankind today occured due to prolonged exposure to environmental pollution (UNEP, 2008). The number of Egyptian population with kidney failure, due to heavy metal exposure, is about 35000 and the incidence rose scary as it exceeding 4% at a rate of 500 patients per million populations (Abdalla, 2012). In addition, the prevalence of renal failure, liver cirrhosis, and chronic anaemia diseases was markedly increased among the Egyptian population in the last few years due to environmental pollution with high exposure to metal poisoning (Salem et al., 2000).
Poultry is a more efficient feed converter and has a shorter production cycle than red meat animals. Responding to increased demand, Egypt’s poultry meat production has trended upward over the last 40 years. Rising demand for poultry meat pushed poultry to 44.3 percent of total domestic production (Taha, 2003). Relatively low and competitive prices as well as dietary and nutritional properties, compared to other meats, are main factors explaining poultry meat’s attractiveness. The increasing demand of food safety has accelerated research regarding the risk associated with food consumption contaminated by heavy metals (Mansour et al., 2009). Estimation of the potential risks to human health through the target hazard quotient method (THQ) associated with intake of heavy metals via consumption of poultry is a must for Egypt ian population safety. Although the THQ -based risk assessment method does not provide a quantitative estimate of the probability of an exposed population experiencing an adverse health effect, it does provide an indication of the risk level associated with pollutant exposure. This method of risk estimation has recently been used by many researchers and has been shown to be valid and useful (Akoto et al., 2014; Saha and Zaman, 2012; Wang et al., 2005; Chien et al., 2002).
In Egypt, chicken muscle and liver are a major source of animal protein to the population and are widely consumed. Consequently, The Egyptian population face challenges related to food quality and safety. According to our best knowledge health risk assessment of heavy metals for Egyptian population via consumption of poultry edibles is not reported. Additionally, there is no available data about Al levels contaminating poultry muscles or liver. The present work aimed to determine the concentration of heavy metals (Pb, Cd, Al and Ni) in the liver and muscle of poultry collected from Assiut and Qena in Egypt. Although muscle tended to accumulate low concentrations of metals, it is important to compare their levels to the known guideline safe levels because muscle constitutes the greatest mass of the poultry that is consumed. It is also necessary to assess the potential health risk of heavy metals to local residents by poultry edibles consumption.
MATERIAL AND METHODS
Sample Collection and Preparation
Forty eight liver and thirty six muscle samples were collected from broiler poultry farms in Assiut and Qena Provinces. Assiut farms are private farms with capacity ranged from 3000 to 4000 birds. While, Qena farms are governmental farms with 15000-20000 birds’ capacity. The samples were collected from 35-42 days –old age birds. Collected samples were transported in an ice box to the laboratory then kept at -20°C until analysis.
All laboratory equipments and containers were washed with Nitric acid 10% solution prior to each use to ensure cleaning. About 5-6 gm of each tissue sample was cut into small pieces and dried in hot air oven at 80°C until reaching a constant weight. The dried tissues were homogenized and grinded to a powder. One gram of each dry sample was weighed, transferred into screw caped glass bottle and 3 ml of nitric acid high grade (68%) (Merck KGaA, 64271 Darmstadt, Germany) was added and the mixture was left overnight at room temperature. Digestion was completed in a water bath at 90°C for about 1-2 hour until the tissue completely dissolved and the solution become clear (Al-Weher, 2008). The digests was allowed to cool, filtered through Whatman (Ashless no. 42) filter paper, then transferred to 25ml volumetric flask and made up to mark with deionized water. Metal analysis (Pb, Cd, Al, and Ni) was carried out in the Central Laboratory of the Faculty of Veterinary Medicine; Assiut University using a ZEEnit 700P Atomic Absorption Spectrophotometer with Graphite Furness Unite (AASG).
Sample Analysis
Aqueous standard stock solutions were prepared for Pb, Cd, Al, and Ni using appropriate salts (Merck KGaA, 64271 Darmstadt, Germany). Working calibration standards of Pb, Cd, Al, and Ni were prepared by serial dilutions of concentrated stock solutions of 1000 mg/L. Four working standards were prepared in triplicate for each metal by serial dilution of the stock solution. These standards and blank solution were aspirated into AASG as described by the manufacturers to obtain the absorbance of each of the samples. A calibration curve for the absorbance versus concentration of the standard metal concentrations was prepared for each metal from which calibration graph for each of the metals in the sample was determined as described by Nnaji et al. (2007).
Quality Control and Recovery Determination
The detection limit for each metal was calculated as double the standard deviation of a series of measurements of a solution, the concentration of which is distinctly detectable above, but close to blank absorbance measurement (USEPA, 1983). In addition, a recovery study of the total analytical procedure was carried out for metals in selected muscle samples by spiking analysed samples with different concentrations of aliquots of metal standards (a multi-element standard solution) and then reanalysed the samples. All determinations were replicated three times. In order to determine the reliability of instruments, a blank and known standard were run after every 10 samples. Acceptable recoveries for the metals were 104.3% for Pb; 89.8% for Cd; 85.3% for Al and 107.1% for Ni. Heavy metal concentrations are expressed on a dry tissue basis and given as µg/gm dry weight (µg/g d wt). Calculated moisture content of the muscle and liver samples were 73.6%± 261 and 74.7± 2.7%, respectively.
Determination of Target Hazard Quotients (THQ)
Health risk to Egyptian population from poultry intake was characterized by Target Hazard Quotient (THQ). This is the ratio between the exposure and the reference doses (RfD). Rfd represents reference oral dose that is an estimation of the daily exposure of a contaminant to which the human population may be continually exposed over a lifetime without an appreciable risk of harmful effects (Akoto et al., 2014). RfD value for Pb, Cd, Ni and Al is 0.004, 0.001, 0.02 and 1(µg/g bw/day) respectively (USEPA, 2006). The population will pose no risk if the ratio is less than 1 and if the ratio is equal or greater than 1 then population will experience health risk. The following equation is used (Chien et al., 2002):
THQ = (EF X ED X FIR X C / RfD X BW X AT) X 10-3
Where THQ is the target hazard quotient; EF is exposure frequency (365 days/year); ED is the exposure duration (70 years, average lifetime); FIR is the food ingestion rate (g/day); C is the heavy metal concentration in poultry (µg/g); RfD is the oral reference dose (mg/kg/ day); BW is the average adult body weight (70 kg); and AT is the averaging exposure time (365 days/ year × number of exposure years, assuming 70 years).
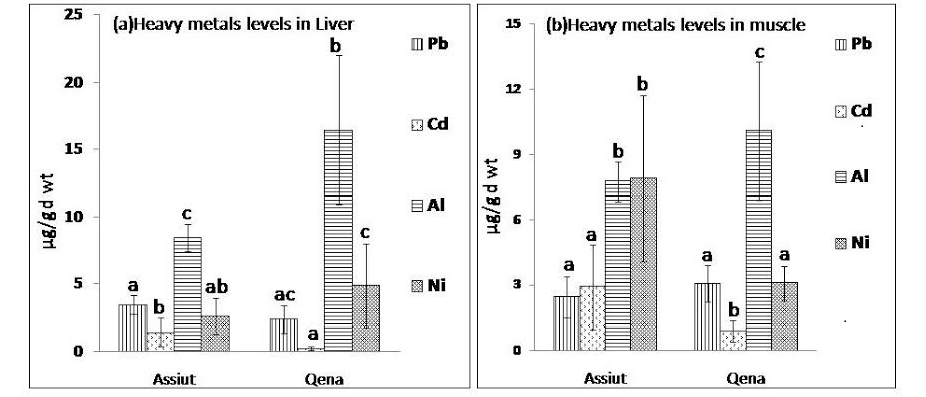
Figure 1: Mean concentration of heavy metals Pb, Cd, Al and Ni expressed as µg/g d wt in (a) liver samples and (b) muscle samples from Assiut and Qena broiler farms.
abc: means with different letters are significantly different (p<0.05)
Hazard Index (HI)
To evaluate the potential risk to human health through more than one heavy metal, the hazard index (HI) has been developed (USEPA, 1989). The hazard index is the sum of the hazard quotients as described in the following equation:
HI = Σ HQ = HQ Pb + HQ Cd + HQ Al + HQ Ni
Where Σ HQ is the summation of hazard quotients of metals and HQ Pb; HQ Cd; HQ Al and HQ Ni are the hazard quotients for lead, cadmium, aluminium and nickel, respectively. It is assumed that magnitude of adverse effect will be proportional to the sum of multiple metal Exposures. The hazard index for the toxic element Pb, Cd, Al and Ni has been calculated in the present study. When the hazard index exceeds 1.0, there is concern for potential health effect (Huang et al., 2008).
Statistical Analysis
The data was statistically analysed using SPSS version 19 statistical package programs. A one- way analysis of variance (ANOVA) was performed. Differences in mean values were accepted as being statistically significant if P< 0.05. The means were separated using Tukey’s test. The statistical significance of the correlation was reported at both P ≤ 0.01 and P ≤ 0.05levels.
RESULTS
Heavy metals (Pb, Cd, Al, and Ni) were estimated in Liver and muscle samples from Assiut and Qena broiler farms. Figure 1 (a & b) shows the mean concentration and standard deviation of the analysed metals. Irrespective of the poultry source, the range in μg/g (dry weight) obtained for the heavy metals in liver samples analyzed were as follows: Al (13.6 ±5.9), Ni (4.1 ±2.8), Pb (2.75 ± 1) and Cd (0.66 ± 0.8). However, heavy metal concentrations (μg/g dry weight) from muscle samples (breast or leg muscle) analyzed were as follows: Al (9.27 ± 2.8), Ni (4.78 ± 3.3), Pb (2.8 ± 0.9) and Cd (1.6 ± 1.5). The mean concentration of Al in liver samples collected from both Qena and Assiut broiler farms was significantly higher (P<0.05) than the level of the other examined metals as observed in figure 1 (a). Additionally, it was clear that the Al concentration in liver samples collected from Qena (16.44 µg/g d wt) was significantly higher (P<0.05) than the concentration in liver samples collected from Assiut (8.44 µg/g d wt). On the other hand, Cd level in liver samples from Qena was 0.24 µg/g d wt and from Assiut was 1.41 µg/g d wt, which indicate that Cd levels were significantly lower than the levels of Pb, Al and Ni in both Assiut and Qena.
Data about the levels of Pb, Cd, Al, and Ni in muscle samples collected from Assiut and Qena broiler farms was presented in figure 1 (b). Among the tested metals, mean concentration of Al (10.1 µg/g d wt) was significantly higher (p<0.01) in muscle samples collected from Qena than the other examined elements. While in muscle samples collected from Assiut the mean concentration of Ni was the highest (7.9 µg/g d wt) followed by that of Al (7.74 µg/g d wt). However, in muscle samples from both Assiut and Qena the lowest concentration of the analysed metals was recorded for Cd (0.88 µg/g d wt and 2.44 µg/g d wt, respectively). Additionally, Al level was significantly higher (p<0.01) in liver samples (13.6 µg/g d wt) than in muscle samples (9.27 µg/g d wt), however, Cd levels were significantly higher (P<0.01) in muscles samples (1.6 µg/g d wt) than in liver samples (0.66 µg/g d wt).
Table 1: Estimated weekly and daily metal intakes (µg/week or µg/day w wt) for Egyptian population consuming poultry edibles
|
|
|
|
|
Assiut |
Qena |
||||
|
Metals |
PTWI |
PTWI 70 |
PTDI |
Muscle |
Liver |
Edible parts |
Muscle |
Liver |
Edible parts |
|
Pb EWI EDI |
25 |
1750 |
250
|
6.1 |
1.6 0.2 |
7.6 1.1 |
8.3 1.2 |
1.3 0.2 |
9.6 1.4 |
|
Cd EWI EDI |
7 |
490 |
70
|
7.4 1.1 |
0.7 0.1 |
8.0 1.1 |
2.4 0.3 |
0.1 0.02 |
2.5 0.4 |
|
Al EWI EDI |
1000 |
70000 |
10000
|
20.5 2.9 |
3.9 0.6 |
24.4 3.5 |
27.4 3.9 |
8.6 1.2 |
36 5.1 |
|
Ni EWI EDI |
35
|
2450
|
350
|
18.7 2.7 |
1.2 0.2 |
20.0 2.9 |
8.3 1.2 |
2.6 0.4 |
10.9 1.6 |
PTWI: Provisional permissible tolerable weekly intake (µg/kg body weight/week) according to JECFA 2004 and 2011; PTWI 70: PTWI for 70 kg person (µg/week); PTDI: Permissible tolerable daily intake for 70 kg person (µg/day); EWI: Estimated weekly intake (µg); EDI: Estimated daily intake (µg)
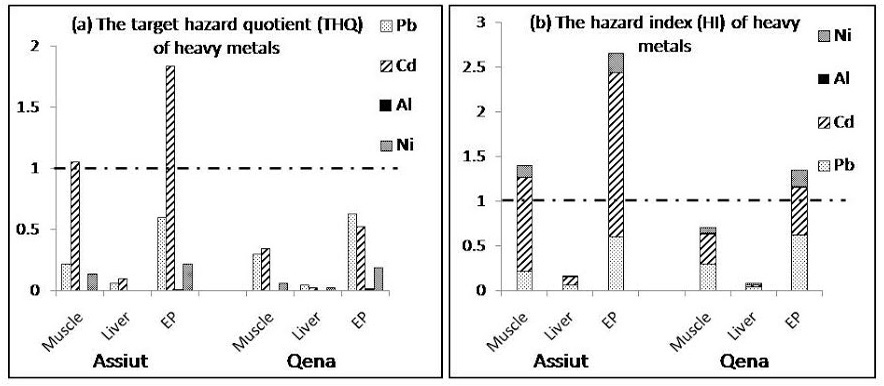
(a) Target hazard quotients (THQ) and (b) Hazard index for the Egyptian population in Assiut and Qena. The population will experience health risk if THQ ratio for individual metal or HI for the sum of metals is equal or greater than 1
EP: edible parts (liver and muscle)
The results obtained in this study were compared with the maximum permissible limits for Pb, Cd and Ni. The Egyptian Organization for Standardization (EOS, 1993) suggested 2, 0.5 and 10 ug/g wet weights as guideline limits for Pb, Cd and Ni in food, respectively. The levels in this study were much below those limits from all samples, except for Cd, 33% and 66% of liver and muscle samples from Assiut exceeded the Egyptian limit, respectively. The concentrations of Pb and Cd from liver and muscle samples in both Qena and Assiut exceeded the permissible limits of 0.2 µg Pb/g wet weight and 0.05 µg Cd/g wet weight stipulated by the European Communities (EC, 2001), except liver samples in Qena, only 73% was higher than the standard guideline limit for cadmium. The permissible limit of Ni is 4μg/g (FNB, 2010). Our results revealed that both liver and muscle samples collected from Qena and Assiut showed nickel level below the permissible limit.
The total dietary exposure levels of heavy metals (Pb, Cd, Al, and Ni) determined in this study were compared with the provisional tolerable weekly intakes (PTWIs) to assess potential health risks faced by consumers as shown in table 1. The PTWI represents permissible human weekly exposure to those contaminants associated with the consumption of the contaminated organs. It can be observed that the estimated weekly intake of the examined metals in liver and muscle samples as well as edible parts (liver and muscle) either from Assiut or Qena was below the PTWI. The data presented in figure 2 (a & b)showed that the THQ and HI value for Cd in muscle samples and edible parts collected from Assiut are more than 1.0 and the highest value for HI was found in edible parts from Qena (1.346) and Assiut (2.662) indicating high potential risk to human health.
DISCUSSION
Concentrations of Heavy Metals in Poultry Edibles and Comparison with International Dietary Standard and Guidelines
Heavy metals, in general, are non-biodegradable, have long biological half-lives and have the potential for accumulation in the different body organs leading to acute as well as chronic toxic effects (Radwan and Salama, 2006). Negative effects of heavy metals are particularly dangerous to birds, whose metabolism is more rapid compared to other groups of animals. They are, therefore, exposed to a greater threat of accumulating heavy metals in their bodies (Felsmann, 1998).
The concentration average and standard deviation of heavy metals in poultry edibles analysed in Assiut and Qena are presented in figure 1 (a & b). Among the studied metals, Al concentration was the highest and Cd was the lowest in poultry edibles analysed, except for muscle samples from Assiut the highest metal wa nickel.
Metals have been shown to accumulate in poultry muscle than liver except for Al. Similar observation was recorded by Iwegbue et al. (2008) in Nigeria, which obtained higher levels of metals (Cd, Pb, Ni, Cu, and Zn) in chicken meats compared to gizzard. However, our result is not in agreement with some reports which tend to show that internal organs of poultry as liver and kidney accumulate Pb and Cd more than muscle (Zhuang et al., 2009; Falandysz et al., 1994; Oforka et al., 2012). In addition, the significantly higher Pb accumulation in parenchymatous organs was confirmed by Kramárová et al. (2005) who detected the highest Pb concentration in kidneys (0.115 to 0.780 µg/g) and in livers (0.177 to 1.904 µg/g) in wild animals. Moreover, gonads, liver, kidney and gills, were reported to be target organs for heavy metal accumulation in fish and can lead to pathological changes than in the muscle tissues (Yilmaz, 2003). According to Allen-Gil and Martynov (1995) the low levels of binding proteins in the fish muscle may account for their low concentrations of heavy metals. Distribution of pollutants among the various organs within an organism is not uniform but rather they accumulate in specific target organs (Terra et al., 2007). The amount of an element, which accumulates in the organs, depends on the interval of exposure, the quantity of ingested element, as well as animal age and breed (Massányi et al., 2000). However, element toxicity is affected by the route and form of ingestion as well as by the interaction between essential and toxic elements (Skalická et al., 2008).
Lead and Cadmium
Pb and Cd are classified among the most toxic heavy metals which have no known biochemical benefits to animals and humans (Akoto et al., 2014). The wide spread industrial production of perfumes, batteries, oils and fats, cement-making, quarrying (especially limestone), and brick-making, as well as, agricultural discharges, sewage effluents, high ways or motor boat traffic, mine and smelting operations, consider main sources of lead in the environment. Discarded manufactured materials to air, soil, water, and food have led to widespread human and animal intoxication (Humphreys, 1991; Mahaffey, 1977). The predominant sources exposure to Cd are through lubricating oils, diesel oil, fertilizers and rubber car tires as well as, the use of fertilizers and sludge containing Cd (Skalická et al., 2002). With inhalation and ingestion as exposure routes, animals in the environment have the potential to act as sentinels for lead and cadmium contamination of water, air, and soil (Ceruti et al., 2002; Bischoff et al., 2010).
It was obvious that there is a great variation between guideline limits from Egypt and the international limits. Levels of Cd and Pb were under the permissible limits of the Egyptian Organization Standardization (EOS, 1993) except for Cd in Assiut. However, Cd and Pb have concentrations greatly above the safe limits recommended by EC. This is of interest considering that Cd and Pb are toxic and their accumulation may lead to serious health issues. The absorption of heavy metals varies to certain extent depending on various factors. Diet, rich in carbohydrates but poor in proteins, calcium and phosphorus accelerates absorption of lead into blood. Moreover, Pb ingested during period of fasting gets absorbed to a much greater extent than lead ingested with food (WHO, 1992). Clinical signs of acute Pb poisoning in chickens include muscle weakness, ataxia, loss of appetite, marked weight loss, and eventual drop in egg production and/or severe anemia (Salisbury et al., 1958). Chronic exposures may also result in degeneration of motor nerves in the spinal cord and axonal loss in peripheral nerves, along with muscle atrophy and myodegeneration (Kaufer, 1979). Even trace levels of lead in the diet (1.0 mg/kg) can result in growth retardation (Bakalli et al., 1995). Moreover, ingested Pb resulted in elevated blood levels and deposition in bone, soft tissues and eggs (Trampel et al., 2003). In addition, the accumulation of trace amounts over time in humans, of particular concern is the potential for children to be exposed throw acute neurotoxic effects or abnormal neurodevelopment in chronic lead exposure (Canfield et al., 2003). Excess Pb is known to reduce the cognitive development and intellectual performance in children and to increase blood pressure and cardiovascular disease incidence in adults (EC, 2001).
Cadmium is an environmental contaminant unique among metals because of its diverse toxic effects, extremely protracted biological half-life, low rate of excretion from the body and predominant storage in soft tissue (Beňová et al., 2007). Tissue Cd concentrations in animals are closely related to Cd levels in feedstuffs and the duration of Cd load (Bokori et al., 1995). Absorption and accumulation of Cd in tissue seems to be determined by a wide range of factors: nutritional and vitamin status, such as iron status, age and sex (Torra et al., 1995; Flanagan et al., 1978). Cd Food is the most important source of cadmium exposure in the general non-smoking population in most countries (WHO, 1992). Cd exposure may cause kidney damage including a tubular dysfunction, evidenced by an increased excretion of low molecular weight proteins or enzymes. Skeletal damage, first reported from Japan in the 1950s, called the itai-itai (a combination of osteomalacia and osteoporosis) which may occur in relatively low cadmium exposure, evidenced by low bone mineral density (osteoporosis) and fractures (Staessen et al., 1999; Alfven et al., 2000; Nordberg et al., 2002). Animal experiments have suggested that cadmium may be a risk factor for cardiovascular disease (Jarup et al., 1998; Nishijo et al., 1995). The International Agency for Research on Cancer has classified Cd as a human carcinogen (group I), for prostate and kidney cancer, on the basis of sufficient evidence in both humans and experimental animals (IARC, 1993).
It can be illustrated that Pb and Cd content of muscle and liver exceeded the permissible limits and higher than that reported by other researchers. Comparing our results with the previously reported metals data in poultry, it can be noticed that Cd and Pb levels were higher than those reported by Oforka et al. (2012) for liver and muscle in Nigeria. Cd level in poultry muscle reported by Falandysz et al. (1994) in Poland, Mariam et al. (2004) in Pakistan, Gonzalez-Weller et al. (2006) in Spain and Badis et al. (2014) in Algeria was lower than those detected in our study.
Pb concentration from poultry muscle was higher than those reported by Gonzales- Weller et al. (2006), Iwegbue et al. (2008) and Zhuang et al. (2009) and lower than the level obtained by Mariam et al. (2004) and Badis et al. ( 2014).
Aluminum
Al is the third most abundant elements in the earth crust and biological system probably evolved in the presence of appreciable concentrations (El-Rahman, 2003). Exposure is unavoidable because of the wide use of this element in day-to-day life and in industry (Bohrer et al., 2008). Al is used to make beverage cans, pots and pans, airplanes, siding and roofing, and foil. Aluminum compounds are used in many diverse and important industrial applications such as alums (aluminum sulfate) in water-treatment and alumina in abrasives and furnace linings. Widespread uses of the metal have stimulated considerable interest in their toxicity. Contaminated food is unquestionably the main source of Al intake (FAO/WHO, 1989).
Al has been shown to have deleterious effects on the central nervous, skeletal, and hematopoietic systems of humans (Domingo, 1995). The neurotoxicity of Al to patients with chronic renal disease is well established (Daugirdas, 1994), and its presence in the bloodstream leads to Al accumulation in bone and brain causing an encephalopathy called dementia dialytica. Several studies have pointed to Al as an exogen factor responsible for developed anemia signs both in experimental animals (Zaman et al., 1993; Fulton and Jeffery, 1994) and patients on hemodialysis (Parkinson et al., 1981). Al exposure increased delta-aminolevulinic acid dehydratase (delta-ALA-D) activity in bone marrow which promotes alterations on erythrocyte parameters in rats, probably as a consequence of alterations in the iron status (Farinaa et al., 2005). Al is well known as a neurotoxicant resulted from accumulated Al in brain and altered amino acid neurotransmitters, and its accumulation has been suggested to be an associated phenomenon in various neurological disorders as dementia, senile dementia, and Alzheimer disease (El-Rahman, 2003). Increased Al exposure can be compensated for by excretion via intestines and normal, healthy kidneys. Kidney insufficiency was shown to result in increased Al concentrations in the kidneys of dialysis patients (Krüger et al., 1984). In a more recent study, Al is also discussed as an endocrine disruptor in female Nile Tilapia (Oreochromis niloticus) (Correia et al., 2010).
Within the literature of specialty, we have not found any reference values of the concentration of Al in the tissues and organs taken from poultry, only from fish and plants. Müller et al. (1998) determined Al content of 128 items out of 12 food categories in Germany. Al was detected in Fish as well as meat and meat products at concentration of 3.2 and 5.4 µg/g fresh weight, respectively. In Spain, The more elevated Al concentrations were detected in food with a greater content of spices and aromatic herbs, pasta, certain vegetables and mushroom (López et al., 2002).
The permissible Al dose for an adult is quite high (60 mg/ day) (WHO, 1989). There is no information about maximum Al levels in poultry samples in Egyptian standards. Meanwhile, the concentrations of Al in different poultry parts in our study were below the WHO limit.
Nickel
Major man-made sources of nickel release are the combustion of coal and heavy fuel oil. Emissions from refineries and from refinery products (including road tar) are particularly important because of the large amount of refinery fuel oil and residues burnt which contain nickel from the original crude oil. Other sources include emissions from mining and refining operations, municipal waste incineration, and windblown dust. Nickel is found naturally in the earth’s crust (in various forms such as nickel sulphides and oxides), and is present in small quantities in soils, aquatic environments, and vegetation (USEPA, 2000).
There is little evidence that nickel compounds accumulate in the food chain. Nickel is not a cumulative toxin in animals or in humans. Almost all cases of acute nickel toxicity result from exposure to nickel carbonyl (Barceloux and Barceloux, 1999). The vast industrial use of nickel has led to environmental pollution by the metal and its by-products during production, recycling and disposal. Nickel is known hematotoxic, immunotoxic, hepatotoxic, pulmotoxic and nephrotoxic agent. Allergic skin reactions are common in individuals who are sensitive to nickel (Das and Buchner, 2007). Systemic contact dermatitis from nickel has been reported from a number of sources including medical devices and following experimental oral exposure (De Medeiros et al., 2008). Nickel can cause respiratory problems and it is carcinogenic (ATSDR, 2004). Nickel compounds are carcinogenic to human and are potent inducers of kidney and lung tumors in experimental animals (Lee et al., 2001) and induce genotoxicity and oxidative stress through the generation of reactive oxygen species (Lee, 2006). Ni level, in our study, was much higher than those reported by Oforka et al. (2012) for poultry liver and muscle in Nigeria.
The permissible limit of Nickel is 4 μg/g and 10 μg/g in food according to Food and Nutrition Board (FNB) and Egyptian Organization for Standardization (FNB, 2010 and EOS 1993). None of poultry samples was found to contain Ni concentration above the permissible limit recommended by standard guidelines.
Estimated Human Weekly/Daily Toxic Elements Intake from Poultry
However, different metals show the toxicity at different concentration and can potentially toxic at sufficient high concentrations; certain metals exhibit toxicity even at relatively low concentration. Provisional tolerable weekly intake (PTWI) depends on the amount, consumption period and contamination level of consumed food. The FAO/WHO in 2004 established PTWI of 7 and 25 µg/kg body weight/ week for humans, equalling 490, 1750 µg/week for 70 kg person (the average body weight in Egypt), respectively (JECFA, 2004). In addition, according to FAO/WHO in 2011 guidelines, the PTWI are 35 and 1000 µg/kg body weight/ week for Ni and Al, equalling 2450 and 70000 µg/week for 70 kg person, respectively (JECFA, 2011). The estimated weekly intake (EWI) values were calculated by assuming that a 70 kg individual will consume 100 g and 20 g of poultry muscle and liver serving/day, respectively. Thus, the EWI values of trace elements by an adult (mg/kg body weight) consuming poultry muscle and/ or liver were calculated using the averages posted in figure1 (i.e., EWI in mg/kg body weight = Mean concentration of element (µg /g wet weight) multiplied by the amount of poultry consumed/week (kg) and divided by the average weight of an individual (70 kg) (Table 1).
Hence, the total dietary exposure levels of heavy metals determined in this study were compared with PTWIs by the FAO/WHO (JECFA, 2004 and 2011) to assess potential health risks faced by consumers.
The result from table 1 suggests that the EWI of Pb, Cd, Al, and Ni by a 70 kg adult consuming 820 g of poultry edibles/week in Assiut and Qena were all below the respective PTWIs recommended standard guidelines (JECFA, 2004 and 2011).
Health Threat from Consuming Poultry in Egypt
Figure 2 (a & b) shows the estimated Target Hazard Quotients (THQ) for individual metals and Hazard Index (HI) from consumption of poultry in Assiut and Qena in Egypt. The THQ of Cd through the consumption of poultry muscle and edibles were more than 1, indicating that there is a potential significant health risk associated with the consumption of poultry from Assiut. Although Cd has the lowest concentrations among the studied metals,but its health risk was highest. This may be due to lower reference dose in diet, which consequently increased the THQ. The THQ of Pb, Al and Ni is generally less than 1, suggesting that people would not experience significant health risks from the intake of individual metals through poultry consumption. However, Pb may consider one of the health risk contributors, accounted for 60% and 62% of the THQ from poultry edibles in Assiut and Qena, respectively. The potential health risk of Al was the lowest, which may be ascribed to its higher oral reference dose (Figure 2 a).
Health risk index (HI) for Cd was more than 1 for poultry muscle and edibles in Assiut as well as edibles in Qena and among them the highest value was found in edibles in Assiut (2.7) indicating high potential risk to the Egyptian consumers. When the hazard index exceeds 1.0, there is concern for potential health effect (Huang et al., 2008) as exposure to more than one contaminant may produce an additive effect on the consumer.
CONCLUSIONS
Different concentrations of heavy metals were present in poultry edibles at the studied locations in Egypt. The estimated daily and weekly intake for Pb, Cd, Al, and Ni were well below the established PTWI/PTDI values recommended by FAO/WHO guidelines. Heavy metal levels were below the Egyptian standardization limits in all samples except for Cd. On the other hand, Cd and Pb levels exceeded the permissible limits stipulated by the European Communities nearly on all poultry samples. In addition, Cd has a THQ more than one for poultry edibles suggesting a health threat from Cd and Pb to the Egyptian population in the studied locations in Egypt.
RECOMMENDATIONS
There is a need for a continuous monitoring of contamination level of heavy metals especially Pb and Cd since they can accumulate to toxic levels. Cd and Pb contents will be lowered by applying restrictions on the use of cement and limestone-making, fertilizers and sludge containing metals. Efforts towards decreasing predominant sources exposure through lubricating oils, diesel oil and rubber car tires for Cd as well as high ways or motor boat traffic, mine and smelting for Pb. It is recommended that more locations in Egypt be studied to determine their levels of contamination by heavy metals. Such studies should also look at contamination of poultry from more toxic elements like arsenic and mercury and organics like pesticides. This will help to provide a beneficial poultry contamination data base that could become a hazard to human safety in Egypt.
REFERENCES