Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (6): 316 – 320Seroepidemiology of Infectious Laryngotracheitis (ILT) in the Commercial Layer Farms of Chittagong District, Bangladesh
Mohammad Inkeyas Uddin1*, Ashim Baran Sen2, Muhammad Shafiqul Islam3, Shubhagata Das3, Nasima Sultana4, Ripatun Nahar Ripa5, Abul Kashem5, Kazi Mohammad Kamaruddin1
- Poultry Research and Training Center, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4202, Bangladesh
- Department of Livestock Services, Begumganj, Noakhali, Bangladesh
- Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4202. Bangladesh
- Scientific officer(ex), CP. Bangladesh
- Department of Microbiology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong–4202. Bangladesh
*Corresponding author: [email protected]
ARTICLE CITATION:
Uddin MI, Sen AB, Islam MS, Das S, Sultana N, Ripa RN, Kashem A, Kamaruddin KM (2014). Seroepidemiology of infectious laryngotracheitis (ILT) in the commercial layer farms of Chittagong district, Bangladesh. Adv. Anim. Vet. Sci. 2 (6): 316 – 320.
Received: 2014–04–13, Revised: 2014–05–14, Accepted: 2014–05–15
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.6.316.320
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
This study was carried out to determine the seroprevalence of Infectious Laryngotracheitis virus (ILTV) in commercial layer farms of Chittagong district, Bangladesh. Antigen coated indirect ELISA was performed to determine the antibody titre against ILTV. The overall seroprevalence of ILTV was 17.33% in commercial layer farms in 5 selected Upazilas of Chittagong district. The highest seroprevalence was found in Anowara upazila (26.67%) followed by Rangunia (18.46%), Raozan (16.67%), Boalkhali (13.33%) with the lowest prevalence in Patia (10.90%). The seroprevalence of ILTV was found higher in winter (24%) season compared to rainy (16%) and summer (12%). Significantly higher seroprevalence of ILTV was observed in the birds of 10–35 weeks of age (23.24%) than the birds of 36–70 weeks of age (7.83%). Prevalence of ILT was found significantly higher (P < 0.05) in the farms maintaining lower biosecurity (biosecurity category 2) (22.78%) than in the farms maintaining higher biosecurity (biosecurity category 1) (9.17%) and the ILT was more predominant in the birds rearing in deep liter (23.48%) than in the cages (13.14%) which is statistically significant (P < 0.05) with χ2– value of 4.9144. These results denoted that wide seroprevalence of ILTV in commercial layer farms of Chittagong district of Bangladesh.
INTRODUCTION
Infectious laryngotracheitis (ILT) is an acute respiratory disease of chickens caused by avian herpesviridae Alphaherpesvirinae gallid Herpes virus 1 that infects the upper respiratory tract and ocular organ of poultry (Garba et al., 2012) and characterized by respiratory depression, gasping, and expectoration of bloody exudates (Yukihiro et al., 1988) with high rates of morbidity and mortality up to 70% in acute form of the infection and the mild form includes depression, reduced egg production up to 30 percent and weight gain, conjunctivitis, swelling of the infraorbital sinuses (almond shaped eyes), and nasal discharge (Shan–Chia and Joseph, 2012). All ages of chickens are affected, but chickens older than 3 weeks are most susceptible to ILTV. It also affects pheasants, partridges and peafowl (OIE, 2008). ILT can infect turkeys at about 100 days of age where dyspnea and depression is the observed clinical sign in infected turkeys (Portz et al., 2008). Other avian species are resistant to ILTV infection (Hayles et al., 1976). Usually the Infection is acquired via the upper respiratory tract and transmission occurs most readily from clinically infected birds, latent infected carriers, contaminated dust, litter, beetles, drinking water and fomites (Hughes et al., 1987). Other possible sources of transmission includ dog, crows, and cats (Kingsbury and Jungherr, 1958) whereas wind–borne transmission of the ILTV spread between farms is critical (Johnson et al., 2005). ILT virus can survive outside the host for several weeks and persist longer in cold environment (Jordan and Pattison, 1996) and annually causes significant economic losses in the poultry industry worldwide each year (Garba et al., 2012). In Bangladesh, Poultry industry is a growing industry and total population of poultry is around 228.47 million (DLS, 2009). It is suspected that Infectious Laryngotracheitis (ILT) which is a contagious respiratory disease is playing a vital role to decrease the egg production in layer birds causing severe economic losses of the farmers in our country Many techniques have been described for the detection of ILTV (Bauer et al., 1999). ELISA has been reported to have better sensitivity than Serum Neutralization (SN), Fluorescent Antibody (FA) and Agar Gel Immunodiffusion (AGID) for detection ILTV antibody in chicken sera (Adair et al., 1985). But, there is a limited study on ILTV infection in the local poultry in Bangladesh. Islam et al. (2012) conducted a research on the characterization of the field viruses by physico–chemical properties against pH, heat, ether and chloroform, serological test such as virus neutralization test (VNT) and passive haemagglutination (PHA) test in Gajipur district. To the best of our knowledge no study has been conducted yet on the seroepidemiology of ILTV in chickens in Chittagong district, Bangladesh. The present study was conducted for the investigation of seroprevalance of ILTV in layer chickens manifested the clinical signs characteristics of ILT in the commercial layer farms of Chittagong district with the identification of different risk factors responsible for the seroprevalence of ILT in Chittagong region. Considering the prevalence of the disease and losses to the commercial poultry farmers it is felt that there is a national need to control and to protect the chicken population against the disease.
MATERIALS AND METHODS
Study Area and Study Period
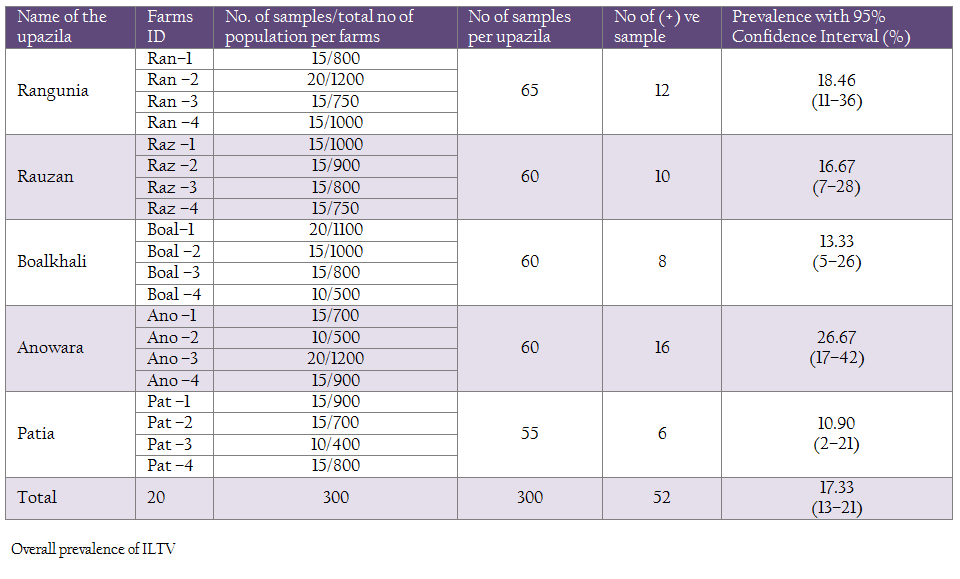
The study was carried out in Chittagong district which is situated geographically in the south–eastern part of Bangladesh during the period of October’ 2011 to September’ 2012 to determine the seroepidemiology of Infectious laryngotracheitis (ILT) from the five upazilas (Anowara, Rangunia , Raozan , Boalkhali and Patia).
Sampling Category
The samples were categorized on the basis of 4 criteria i.e. season of the year: Winter (mid October to February), summer (March to May) and Rainy (June to September); age group: (10–35 weeks and 36– 70 weeks); biosecurity category: (category1 i.e. farms with foot bath, spray, disinfection of utensils, restricted entrance, regular medication, proper disposal of carcass and category 2 i.e. farm with spray, open entrance, irregular medication and disinfection); and rearing system (Cage and deep liter system).
Sample Collection and Serum Preparation
A total of 300 blood samples were collected randomly and aseptically according to Islam et al. (2010). In brief, about 1 ml of blood was collected from a wing vein of each chicken using sterile 2–ml disposable plastic syringe without anticoagulant and were kept in ice box and transported to the molecular biology laboratory of the poultry research and training centre (PRTC) in Chittagong veterinary and animal sciences university (CVASU) where the serological test (ELISA) was performed. The blood was then centrifuged at 1,000 g for 10 minutes so as to clearly separate the serum from the blood. The sera was then collected in a sterile eppendorf tube and stored at –20°C until further study
Enzyme Linked Immunosorbent Assay (ELISA)
The collected serum samples were subjected to indirect ELISA by using commercial ILTV Antibody Test Kit (CK124, Biochek, Holland). Following manufacturer’s instructions each test sample was diluted at 1:500 with sample diluents supplied in the kit.100 μl of negative control was added to wells A1 & B1 and 100 μl of positive control was added to wells C1 & D1.Then 100 μl of diluted samples were added into the appropriate wells (antigen coated) and incubated at room temperature (22˚–27˚C) for 60 minutes by covering the plate with lid. After incubation, the content of the wells were aspirated and washed with approximately 200 μl of phosphate buffered wash solution for 4 times using an automated ELISA washer (Microplate Washer, 2600–C, J. P. SELECTA, Spain). Plate drying was avoided between plate washings and prior to the addition of conjugate. Then 100 μl of conjugate reagent (Chicken Anti Alkaline Phosphate) was added into each wells and incubated at room temperature (22˚–27˚C) for 60 minutes.
Following washing for 5 times with wash buffer, 100 μl of substrate reagent was added into appropriate each wells and incubated at room temperature (22–27˚C) for 30 minutes. Finally, 100 μl of stop solution was added to the each well to stop the reaction. Then the microtitre ELISA plate was placed in the ELISA reader (Microplate Reader, 2100–C, J.P. SELECTA, Spain) and the intensity of the colour produced from the ELISA test was measured photometrically at 405nm wavelength.
OD Value Measurement
The presence or absence of antibody to ILTV is determined by calculating the S/P ratio according to the methods provided by the manufacturer of the test kit. Samples with an S/P (Sample to Positive) of 0.50 or greater contain anti–ILT antibodies and are considered positive. If the S/P ratio is less than 0.50, the sample is classified as negative for ILT antibodies.
Data Analysis
Descriptive statistical analyses of various risk factor and dependent variables were done using Intercooled STATA 9.2 (Stata Corporation 2008). Chi–square was used for statistical analysis of the prevalence of antibodies in different categories of this study. Overall seroprevalence was expressed as percentage with 95% confidence interval and significance was determined at P < 0.05.
RESULTS
Overall prevalence of Infectious Laryngotracheitis (ILT)
In this study, a total of 300 samples from 20 commercial layer farms (Hisex Brown strain) were tested for antibodies against ILTV using ELISA technique. Fifty two out of 300 serum samples were found positive represents 17.33%. Where the highest prevalence was found in Anowara (26.67%) followed by Rangunia (18.46%), Raozan (16.67%), Boalkhali (13.33%) and lowest prevalence in Patia (10.90%) (Table 1).
Seasonal Prevalence of ILT
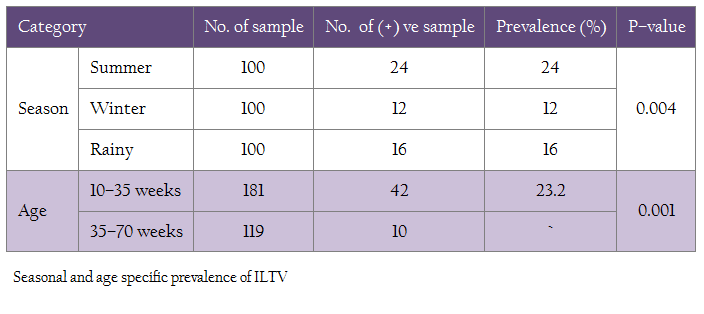
The 300 samples were collected covering 3 main seasons of the year (winter, summer and rainy) taking 100 samples for each. The highest prevalence of Infectious Laryngotracheitis (ILT) was found during winter (12%), followed by rainy (16%) and summer (24%). The difference of seasonal prevalence was found to be statistically significant (P<0.05) (Table 2).
Age Specific Prevalence of ILT
In this investigation, the prevalence of ILT was significantly higher (P=0.001) in the birds of 10–35 weeks of age (23.20%) than in the birds of 35–70 weeks of age (8.4%). The χ2– value was 11.7637(Table 2)
Prevalence of ILT on the Basis of Biosecurity
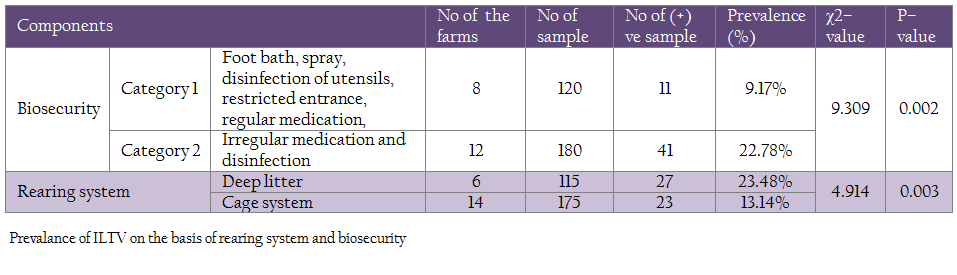
In the present study, the prevalence of ILT was significantly higher (P < 0.05) in the farms maintaining biosecurity category 2 (22.78%) than in the farms maintaining biosecurity category 1 (9.17%) (Table 3).
Prevalence of ILT on the Basis of Rearing System
In this study, it was observed that ILTV was more prevalent (23.48%) in the farms where birds were reared in deep liter system than those farms where birds were reared in cage system (13.14%). This difference was found to be statistically significant (P < 0.05) with χ2– value of 4.9144. (Table 3)
DISCUSSIONS
Seroprevalence of ILTV was undertaken with the commercial layer farms of Chittagong district where there was no record of conducting vaccination programme against ILT. In order to detect the ILTV antibodies ELISA test was chosen because ELISA was reported to have better sensitivity than Serum Neutralization (SN), Fluorescent Antibody (FA) and Agar Gel Immunodiffusion (AGID) (Adair et al., 1985). The overall seroprevalence of ILTV in the Chittagong district in the present study was 17.33% which was in consistent with the study of Verma et al. (1980) who conducted a serological study of 1230 serum samples of chicken at Uttar Pradesh, Andhra Pradesh and Madras in India and found Prevalence as 19.6%, 15.8% and 21.3% respectively. This study also revealed that among the 5 Upazilas of Chittagong district the prevalence of ILT was found highest in Anowara Upazilla (26.67%) compared to Rangunia (18.46%), Raozan (16.67%), Boalkhali (13.33%) and Patia (10.90%). The causes for this variation might be due to location of the farms. In Anowara most of the studied farms were situated in the settlement areas with backyard poultry farming. It was observed that prevalence of Infectious Laryngotracheitis (ILT) was significantly higher (P < 0.05) in winter season (24%) than rainy (16%) and summer (12%) season. It might be due to in winter when it is cooler and the virus lasts longer and rapidly transmits (Robertson and Egerton, 1981).The present study was partially consistent with the investigation of Bagust and Johnson (1995) and Pattison and Jordan (1996) who found that the prevalence of ILT was 26% and 29.4% respectively in winter or cold season and 14.3% and 15% in summer season respectively. Fulton et al. (2000) found higher seroprevalence rate that was 48.5%, 27% and 20% in winter, rainy and summer season respectively in the Silva state of Panama. This variation may be due to the differences in the status of maternal antibody, efficacy of the vaccination, biosecurity, mechanical carriers, host factor etc (Key et al., 1994). In this investigation, the samples were categorized into two age groups (10–35 weeks of age and 36–70 weeks of age). In this study, the prevalence of ILT was found to be 23.24% in the birds of 10–35 weeks of age which was partially consistent with the observation of Bauer et al. (1999) who found a seroprevalence rate of 25% in the birds of 20–30 weeks of age but it was lower than the findings of Fahey et al. (1990) who recorded a prevalence of ILT antibody to be 31% in the birds of 10–30 weeks of age and 37.1% in the birds of 15–35 weeks of age respectively. ILT antibody was found to be 7.83% in 36–70 weeks of age which was similar to the report of Davidson et al. (1988). However, for the same age group of birds, Fahey et al. (1990) recorded a 16% seroprevalence rate.
The prevalence of ILT was 22.78% in the farms maintaining lower level of biosecurity (biosecurity category 2) and 9.17% in the farms maintaining higher level of biosecurity (biosecurity category 1). The difference was in consistence with the survey of Sander et al. (1997) who found in Bastos region of Brazil a higher rate (25.14%) of infection of ILT and other infectious diseases in those farms that maintained lower level of biosecurity and less evidence of ILT (11%) and other diseases in the farms those followed strong biosecurity. Sellers et al. (2004) also found nearly similar result with lower and higher level of biosecurity who recorded 29% and 12% prevalence of ILT respectively in southeast Australia. This study was also supported by the Andreasen et al. (1990) who stated that biosecurity plays an important role in the spread and severity of ILT. If bio–security of the farm is not maintained properly or there is lower level of bio–security in the farms, the prevalence of different disease in the flocks is increased. The prevalence of ILT in cage rearing system was found significantly lower (13.51%) than the deep liter system (23.48%) rearing which was consistent with the observation of Guy and Bagust (2003) who recorded 15.2% and 25.4% in cage and deep liter rearing system respectively in North Carolina State. The study of Bauer et al. (1999) also revealed more or less similar results with a prevalence rate of 14.3% and 26.1% respectively in a serological investigation of 120 commercial layer farms of West Africa. Lohr and Saywell (1976) and Davidson et al. (1988) found 27.8% and 29% in New Zealand and in Pennsylvania respectively in cage rearing which were higher than the findings of present study. Although the same serological technique (ELISA) was used, this variation could be attributed to the different climatic conditions that exist in the different zones of respective research areas. The prevalence of ILT is therefore higher in the flocks rearing in the deep liter system than in the flocks rearing in the cage system (Robertson and Egerton, 1981) which goes with the findings of current study.
REFERENCES
Adair BM, Todd D, McKillop ER, Burns K (1985). Comparison of serological tests for detection of antibodies to infectious laryngotracheitis virus. Avian Path. 14: 461 – 469.
http://dx.doi.org/10.1080/03079458508436249
PMid:18766940
Andreasen JR, Glisson JR, Goodwin MA, Resurreccion RS, Villegas P, Brown J (1990). Studies of infectious laryngotracheitis vaccines: Immunity in layers. Avian Dis. 33: 524 – 530.
http://dx.doi.org/10.2307/1591116
Bagust TJ, Johnson MA (1995). Avian infectious laryngotracheitis: Virus–host interactions in relation to prospects for eradication. Avian Path. 24: 373 – 391.
http://dx.doi.org/10.1080/03079459508419079
PMid:18645796
Bauer B, Lohr JE, Kaleta EF (1999). Comparison of commercial ELISA kits from Australia and the USA with the serum neutralization test in cell cultures for the detection of antibodies to the infectious laryngotracheitis virus of chickens. Avian Dis. 28: 65 – 72.
Bauer B, Lohr JE, Kaleta EF (1999). The early history of Infectious laryngotracheitis. Avian Dis. 40(3): 494 – 500.
DLS (2009). Livestock and poultry profile of Bangladesh, Ministry of Fisheries and Livestock. DLS. 3 – 4.
Fahey KJ, Brown J, York JJ (1990). The role of mucosal antibody in immunity to infectious laryngotracheitis virus in chickens. J. Gen. Virol. 71: 2401 – 2405.
http://dx.doi.org/10.1099/0022-1317-71-10-2401
PMid:2172453
Fulton RM, Schrader DL, Will M (2000). Effect of route of vaccination and season in the occurrence of infectious laryngotracheitis in commercial egg–laying chickens. Avian Dis. 44: 8 – 16.
http://dx.doi.org/10.2307/1592502
PMid:10737639
Garba J, Faleke OO, Junaidu AU, Nwankwo IO (2012). Avian Infectious Laryngotrachietis Infection Virus in Some Domestic and Captured Wild Birds in Yobe State, Nigeria. J. Vet. Adv. 2(4): 173 – 177.
Guy JS, Bagust TJ (2003). Prevalence and comparison of serological diagnosis of Infectious Laringotracheitis in North Carolina. Avian Dis. 25: 331 –337.
Hayles LB, Macdonald KR, Newby WC, Wood CW, Gilchrist EW, MacNeill AC (1976). Epizootiology of infectious laryngotracheitis in British Columbia 1971–1973. Can. Vet. J. 17: 101 – 108
PMid:177165 PMCid:PMC1697213
Huges CS, Jones RC, Gaskell RM, Jordan FTW, Bradbury (1987). Demonstration in live chickens of the carrier state in infectious laryngotracheitis virus. Res. Vet. Sci. 42: 407 – 410.
Islam MS, Khan MSR, Islam MA, Hassan J, Affroze S, Islam MA (2010). Isolation and characterization of Infectious Laryngotracheitis virus in layer chickens. Bang. J. Vet. Med. 8(2): 123 – 130.
Johnson YJ, Colby MM, Tablante NL, Salem M, Gedamu N (2004). Application of Commercial and Backyard Poultry Geographic Information System Databases for the Identification of Risk Factors for Clinical Infectious Laryngotracheitis in a Cluster of Cases on the Delmarva Peninsula, Virginia–Maryland Regional College of Veterinary Medicine, University of Maryland, College Park. 8: 19 – 25.
Johnson YJ, Gedamu N, Colby MM, Myint MS, Steele SE, Salem M, Tablante NL (2005). Wind–borne transmission of infectious laryngotracheitis between commercial poultry operations. Int. J. Poult. Sci.4: 263 – 267
http://dx.doi.org/10.3923/ijps.2005.263.267
Kingsbury FW, Jungherr EL (1958). Indirect transmission of infectious laryngotracheitis in chickens. Avian Dis. 2: 54 – 63
http://dx.doi.org/10.2307/1587512
Lohr JE, Saywell DP (1976). Prevalence of antibodies to Infectious Laryngotracheitis Virus in poultry of New Zealand. N. Z. Vet. J. 24: 153 – 156.
http://dx.doi.org/10.1080/00480169.1976.34306
PMid:190570
OIE (2008). Avian Infectious laryngotracheitis. Manual of standard for diagnostic tests and vaccines for terrestrial animals. Office Internationale des Epizootics Paris. Pp, 1 – 35.
Pattison TF, Jordan KS (1996). Observations and evaluation of epidemiology of the infectious laryngotracheitis of poultry. J. Comp. Path. 55: 213 –244.
Portz C, Beltrão N, Furian TQ, Júnior AB, Macagnan M, Griebeler J, Lima Rosa CA, Colodel EM, Driemeier D, Back A, Barth Schatzmayr OM, Canal CW (2008). Natural infection of turkeys by infectious laryngotracheitis virus. Vet. Microb. 131: 57 – 64
http://dx.doi.org/10.1016/j.vetmic.2008.02.029
PMid:18436397
Robertson GM, Egerton JR (1981). Replication of infectious laryngotracheitis virus in chickens following vaccination. Aus. Vet. J. 57: 119 – 123.
http://dx.doi.org/10.1111/j.1751-0813.1981.tb00472.x
PMid:6266383
Sander JE, Savage CE, Thayer SG (1997). An outbreak and evaluation of ELISA titers to Infectious Laryngotracheitis in layer in Bastos region of Brazil. Avian Dis. 41(2): 426 – 432.
http://dx.doi.org/10.2307/1592199
Sellers H, Garcia M, Glisson J, Brown T (2004). Mild Infectious Laryngotracheitis in broiler in southeast Australia. Avian Dis. 48(2): 430 – 436.
http://dx.doi.org/10.1637/7129
PMid:15283433
Shan–Chia Ou, Joseph J Giambrone (2012). Infectious laryngotracheitis virus in chickens. World J .Virol. 12: 1(5): 142 – 149
Verma S, Singh SB, Prasad K (1980). Prevalence and its impacts of Infectious laryngotracheitis in commercial layer birds. Ind. Vet. J. 43: 128.
Yukihiro O, Kenji SA, Tsuguo M A, Takashima I (1988). Labeled Avidin–Biotin Enzyme–Linked Immunosorbent Assay for Detecting Antibody to Infectious Laryngotracheitis Virus in Chickens. Avian Dis. 32:24 – 31.
http://dx.doi.org/10.2307/1590944