Advances in Animal and Veterinary Sciences
Research Article
Comparative Assessment of Sensitivity and Specificity of some Diagnostic Procedures of Brucellosis using Different Approaches
H. I. Hosein1*, Amal M. Abdel-Raouf2, Bahaa S. Madkour2, Amira Mazeed3, Sherin R. Rouby1
1Department of Veterinary Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Department of Animal Medicine, Faculty of Veterinary Medicine, Aswan University, Aswan Egypt; 3Department of Brucella Researches, Animal Health Research Institute, Giza, Egypt.
Abstract | In Egypt, Brucella melitensis has been identified in both animals and humans with severe economic losses and public health risk. The Egyptian government still conducts the test and slaughter policy for the control of brucellosis based on sero-testing which is considered the corner stone of surveillance and control programs. However, none of the commonly used serological procedures has been revealed to be 100% consistent as an effective tool for diagnosis of brucellosis. Cattle populations employed in this study included 1738 lactating cows belonged to three dairy herds with history of brucellosis located at Damietta, Behira, and Sharkia Governorates, Egypt. The aim of this study was to assess the sensitivity and the specificity of the commonly used serological tests using different methods. Brucella melitensis biovar 3 could be recovered from the milk of 92 (46. 46 %) out of 198 lactating cows. Assessment of sensitivity, specificity, positive predictive value and negative predictive value of serological tests using Brucella isolation as the gold standard revealed 96.73%, 48.11%, 61.81% and 94.44% respectively. Parallel testing of 960 dairy cows suggests that using BAPAT, RBPT, cELISA and CFT in a parallel manner created the highest overall sensitivity and specificity when considered as their sum and increased the sensitivity of the screening. The combined sensitivity and specificity of RBPT and CFT of 580 dairy cows using a serial interpretation at individual animal level revealed 30 (5.17%) and 26 (4.48%) respectively. It was concluded that the solution to the problems of epidemiological investigation of Brucella outbreaks to ensure precise diagnosis will require employing a battery of tests possessing different tasks of the immune response. Predictive values were found to be more appropriate than are sensitivity and specificity during screening programs.
Keywords | Brucellosis, Predictive values, RBT, Sensitivity, Specificity
Received | July 22, 2021; Accepted | August 02, 2021; Published | November 01, 2021
*Correspondence | H. I. Hosein, Department of Veterinary Medicine, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: [email protected]
Citation | Hosein HI, Abdel-Raouf AM, Madkour BS, Mazeed A, Rouby SR (2021). Comparative assessment of sensitivity and specificity of some diagnostic procedures of brucellosis using different approaches. Adv. Anim. Vet. Sci. 9(12): 2176-2183.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.12.2176.2183
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hosein et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Brucellosis is a major constraint to livestock production in many developing countries worldwide. Brucellosis has been recognized in Egypt since 1939 as an important cause of reproductive failure in dairy cattle, causing serious economic losses due to drop in fertility, calf and milk losses, costs for programs of control and eradication and prohibition on trade. Major importance is attributed to its public health significance due to its high transmissibility to humans causing acute disease that may lead to serious chronic complications as reported by the OIE (2018).
Following Brucella infection, placentitis develops in pregnant females with subsequent late abortion (OIE, 2018). Low fertility, long inter-calving interval, and retention of the placenta, are common sequelae (Nicoletti, 2010). As the portals of exit of Brucellae from their ruminant reservoirs include both the genital tract and udder, transmission of Brucella infection takes place when bacteria are shed after abortion or parturition in aborted materials as well as colostrum and milk (Rhyan et al., 2009).
As Brucellae are intracellular pathogens, major difficulties are encountered during their recovery from clinical samples (Alton et al., 1988). Also, culturing of Brucellae is laborious and hazardous. Therefore, immunodiagnostic methods which are capable to test mass animal population are employed for epizootiological investigation of brucellosis outbreaks. However, such methods suffer from certain limitations as a result of antigenic similarity among Brucellae and other related Gram negative bacteria leading to false diagnosis and interference with control programs as reported by Kittelberger et al. (1997).
The principal goal of diagnosis of an infectious disease is commonly to search for detection of infected cases maintaining false positive cases to a minimal level. Therefore, the ideal is to use battery of tests including tests with excellent positive predictivity value (PPV) to ensure the existence of the disease (Chachra et al., 2009), in addition to employing other tests with worthy negative predictivity value (NPV) to eliminate the possibility of infection.
Although, various serological tests are available, no sole test is suitable in all epizootiological studies due to variation of their sensitivity and specificity (Matope et al., 2011; Mert et al., 2003; OIE, 2018). Tests employing Acidified antigen such as Buffered Acidified Plate Antigen test (BAPAT) and Rose Bengal Plate test (RBPT) are highly sensitive, but still require validation with other serological procedures (OIE, 2018). The complement fixation test (CFT) identifies IgG immunoglobulins and is used as a confirmatory test owing to its high specificity, therefore it is prescribed by the OIE (2018) for international or intra-community trade. However, the test may classify S19 immunized cattle as positives, (Nielsen, 2002) and sometimes reveals anti-complementary activity (Searson, 1982). Enzyme linked immunosorbent assay (ELISA) is widely used as a standard assay for the diagnosis of brucellosis. The assay measures IgG, IgA and IgM antibodies allowing a better analysis during investigation of Brucella outbreaks as reported by Geresu and Kassa (2016). Moreover, Godfroid et al. (2002) recommended the serial use of two serological procedures to exploit the accurateness of sero-testing in epidemiological studies.
The current work was carried out for comparative assessment of sensitivity (Se) and specificity (Sp) of the commonly used brucellosis serological tests among cattle in different localities using different methods of estimation.
Material and methods
Ethics approval
All procedures were carried out according to the experimental standards approved by the Animal Research Ethics Committee at Faculty of Veterinary Medicine, Beni-Suef-University.
Area of study
The present study was carried out in three dairy cattle farms with history of brucellosis located at Damietta, Behira, and Sharkia Governorates, Egypt for a period of 18 months from January 2019 to June 2020. The history of the investigated dairy farms indicated that these farms have no vaccination history against the disease.
Animals and samples
Cattle populations employed in this study included 1738 lactating cows from dairy herds with history of brucellosis located at Damietta, Behira, and Sharkia Governorates, Egypt. About 10 mL of blood were collected in vacutainer tube from the jugular vein of each of cattle. The samples were allowed to clot for two hours at room temperature. Sera were centrifuged at 2500 rpm for 10 minutes and stored at -20 °C until use.
First group: (using Brucella isolation as the gold standard). A total of 198 lactating cows at Behira governorate were subjected to bacteriological examination of their milk. Milk samples or udder secretions were collected from every quarter of the udder twice daily and kept at – 20 °C. Milk samples were centrifuged (6000 x g) for 15 minutes and the cream and deposit were mixed. Blood sera of these animals were collected and subjected to BPAT, RBPT and CFT.
Second group: (Parallel testing): four tests were applied to each blood serum sample at the same time. A total of 960 lactating cows at Damietta governorate were serologically examined by RBPT, BAPAT, cELISA and CFT.
Third group: (Series testing): serological tests were conducted sequentially (serially), but only the individual blood serum sample that was positive to the initial test was retested. A total of 580 lactating cows at Sharkia governorate were serologically examined by RBPT, and then positive samples were further confirmed with CFT.
Serological tests
BPAT, RBT and CFT, (Warm micro technique) using complement fixation test antigen were conducted according to Alton et al. (1988) and OIE (2018). BAPAT and RBPT antigens were obtained from the Veterinary Sera and Vaccine Research Institute, (VSVRI) Abbassia, Cairo, Egypt. Complement fixation test antigen was obtained from the National Veterinary Services Laboratories (NVSL) Ames, Iowa state, USA.
Competitive ELISA (cELISA) using monoclonal Antibodies (MAb) was performed according to the manufacturer’s instructions and procedures using the commercial competitive ELISA kits for diagnosis of brucellosis (SVANOVIR Brucella- Ab c-ELISA), (Svanova Biotech, Uppsala, Sweden).
Bacteriological examination
Milk cream-sediment mixtures were streaked on tryptose agar medium with antibiotics selective antibiotic supplement (Ewalt et al., 1983), (Oxoid). Isolation and identification of Brucella organisms were carried out according to the methods recommended by Alton et al. (1988); Ewalt et al. (2001); OIE (2018) guidelines. Identification was established using a Brucella genus specific conventional PCR targeting Immunodominant antigen, gene bp 26 while a Bruce-ladder multiplex PCR using five primers was used for molecular characterization of Brucella isolates at the species level as recommended by Garcia-Yoldi et al. (2006) (Table 1).
DNA extraction
Genomic DNA was extracted by heat inactivation of bacterial cells at 100°C for 10 minutes in sterile 1.5ml Eppendorf tubes. Tubes were centrifuged at 15,700 x g for 10 minutes and the supernatant crude DNA template was transferred into new sterile Eppendorf tubes.
PCR amplification
The PCR amplification was conducted using Labnet® Multigene Gradient thermal cycler, Catalog TC9600-G- 230V (Labnet International, Inc. Edison, NJ, USA). The thermal profile was initial denaturation at 95 °C for 4 min, 35 cycles of denaturation at 95 °C for 45 sec, annealing at 64 °C for 45 sec and extension at 72 °C for 45 sec (conventional PCR) or 3 min (multiplex PCR) and final extension at 72 °C for 7 min. PCR amplicons were electrophoresed in 1% agarose gel stained with ethidium bromide at 100 V for 40 min, visualized and photographed under UV illumination.
Diagnostic sensitivity and specificity
The diagnostic sensitivity was defined as percentage of true positive animals detected by the test with regard to the total true positives while, the diagnostic specificity was defined as percentage of true negative animals detected by the test with regard to the total true negatives.
Estimation of sensitivity and specificity using Brucella isolation as the gold standard:
Sensitivity (Se): It is the capacity of the test to detect diseased animals, when compared with the gold standard test
Sensitivity= True positive TP / True positive TP + false negative FN x 100
Specificity (Sp): It is the capacity of the test to detect non-diseased animals, when compared with the gold standard test
Specificity = True negative TN / True negative TN + false positive FP x 100
Positive predictive value (PPV) = TP / (TP + FP) x 100
Negative predictive value (NPV) = TN/ (TN+FN) x 100
Estimation of relative sensitivity and specificity after parallel testing:
Sensitivity, specificity, Positive predictive value and Negative predictive value were determined according to the method described by Trevethan (2017) using the following formulas:
Sensitivity = TP / (TP + FN) x 100
Specificity= TN / (TN+ FP) x 100
Positive predictive value = TP / (TP + FP) x 100
Negative predictive value= TN / (TN + FN) x 100
Where:
True positive or negative: Samples that were confirmed as being positive or negative by other two or more tests.
False positive or negative: Samples that were confirmed as being positive or negative by other one or non-tests.
Results
Serological tests
Results of estimation of Sensitivity, Specificity, Positive predictive value and Negative predictive value of serological tests under evaluation BPAT, RBPT as screening test and CFT as confirmatory test using Brucella isolation as the gold standard are illustrated in (Table 2). Positive serological cases were considered as those only were positive by the two screening tests and confirmed by the CFT.
Results of estimation of the relative Sensitivity, Specificity, Positive predictive value and Negative predictive value of serological tests under evaluation after parallel testing are shown in (Table 3).
Results of series testing for brucellosis using RBPT and CFT for detection of antibodies against Brucella species are shown in (Table 4). Out of the 580 cow’s sera that were tested by the RBPT as a screening test for brucellosis, 30 (5.17%) were positive. Retesting of the 30 RBPAT positive samples by the CFT as confirmatory test, revealed that 26 (4.48%) sera were positive. Samples were considered as positive for brucellosis, if they were positive for both RBPT and CFT.
Table 1: Primer sets for conventional and Bruce ladder multiplex PCR
| Primer | Sequence (5'–3') | Amplicon size (bp) |
|
BMEI0535f BMEI0535r |
GCG-CAT-TCT-TCG-GTT-ATG-AA CGC-AGG-CGA-AAA-CAG-CTA-TAA |
450A |
|
BMEI0998f BMEI0997r |
ATC-CTA-TTG-CCC-CGA-TAA-GG GCT-TCG-CAT-TTT-CAC-TGT-AGC |
1682 |
|
BMEII0843f BMEII0844r |
TTT-ACA-CAG-GCA-ATC-CAG-CA GCG-TCC-AGT-TGT-TGT-TGA-TG |
1071 |
|
BMEII0428f BMEII0428r |
GCC-GCT-ATT-ATG-TGG-ACT-GG AAT-GAC-TTC-ACG-GTC-GTT-CG |
587 |
|
BR0953f BR0953r |
GGA-ACA-CTA-CGC-CAC-CTT-GT GAT-GGA-GCA-AAC-GCT-GAA-G |
272 |
|
BMEI0752f BMEI0752r |
CAG-GCA-AAC-CCT-CAG-AAG-C GAT-GTG-GTA-ACG-CAC-ACC-AA |
218
|
A: Primer sets for conventional PCR
Table 2: Sensitivity, Specificity, Positive predictive value and Negative predictive value of BPAT, RBPT and CFT using Brucella isolation as the gold standard.
|
Sero testing outcome |
Infection status as determined by the gold standard (B. isolation) |
Total |
|||
| Positive | Negative | ||||
|
Result |
Positive |
89 (TP) | 55 (FP) |
144 (TP + FP) (Manifestation prevalence) |
PPV TP / (TP + FP) 61.81% |
| Negative | 3 (FN) | 51 (TN) |
54 TN+FN |
NPV TN / (TN + FN) 94.44% |
|
|
Total results |
92 TP+FN (Actual prevalence) |
106 TN+FP |
198 Total number of samples processed from both tests |
||
| Sensitivity and specificity |
Se=TP/(TP+FN) 96.73% |
Sp=TN/(TN+FP) 48.11% |
|||
Table 3: Relative Sensitivity, specificity, Positive predictive value and Negative predictive of RBPT, BAPAT, cELISA and CFT parallel testing.
| No. of animals (960) | BPAT | RBPT | cELISA | CFT |
| 59 | + | + | + | + |
|
3 |
+ | + | + | - |
|
1 |
+ | + | - | - |
|
2 |
+ | + | - | + |
|
1 |
- | - | + | - |
|
894 |
- | - | - | - |
|
Total test positive |
65 | 65 | 63 | 61 |
|
TP |
64 | 64 | 62 |
61 |
| TN | 895 | 895 | 894 | 895 |
|
FP |
1 | 1 | 1 | 0 |
|
FN |
0 | 0 | 3 | 4 |
|
Relative sensitivity TP / (TP + FN) x 100 |
100 % | 100 % | 95.38 % | 93.85 % |
|
Relative specificity TN / (TN+ FP) x 100 |
99.89% | 99.89% | 99.89 | 100 % |
|
Positive predictive value TP / (TP + FP) x 100 |
98.46 % | 98.46 % | 98.41 | 100 % |
|
Negative predictive value TN / (TN + FN) x 100 |
100 % | 100% | 99.67 |
99.56% |
Table 4: Series testing for brucellosis using RBPT and CFT for detection of antibodies against Brucella species.
| Test | Examined animals | Positive |
|
RBPT (Initial test) |
580 | 30 (5.17%) |
|
CFT (Retesting of RBPT positive samples) |
30 | 26 (4.48%) |
Table 5: Phenotypic characteristics of Brucella isolates recovered from milk samples of cows.
| Brucella isolates | CO2 | H2S | Urease | Growth on dyes | Lysis by phage | Monospecific sera | Conclusion | |||||||
| Thionin | Fuchsin | Tp | Iz1 | A | M | R | ||||||||
| a | b | a | b | RTD | RTD 104 | RTD | ||||||||
| 92 isolates | - | - | + in 20 hrs | + | + | + | + | - | - | + | + | + | - | B. melitensis 3 |
| B. melitensis Ether | - | - | + in 18-24 hr. | + | + | + | + | - | - | + | + | + | - | B. melitensis 3 |
| B. abortus544 | - | + | + in 2 hrs | - | - | + | + | + | + | + | + | - | - | B. abortus 1 |
| B. suis1330 | - | +++ | ++ in < 15min. | + | + | - | - | - | + | + | + | - | - | B. Suis 1 |
RTD: routine test dilution; Tp : Tbilisi (Tb); Iz1 : Izatnagar (Iz1); a: 1:50000, b: 1:100000; A: anti Brucella abortus, M: anti Brucella melitensis, R: rough brucella antiserum
 Figure 1: Detection of Brucella in cultures using universal PCR assay Lane 1: Brucella melitensis (control positive), Lane 2: Rev1, Lane 3: Brucella abortus S19 (control positive), Lanes 4: Brucella abortus S99 (control positive), Lane 5: Brucella suis, Lanes 6: 9; field Brucella isolates, L: 100bpDNA ladder
Figure 1: Detection of Brucella in cultures using universal PCR assay Lane 1: Brucella melitensis (control positive), Lane 2: Rev1, Lane 3: Brucella abortus S19 (control positive), Lanes 4: Brucella abortus S99 (control positive), Lane 5: Brucella suis, Lanes 6: 9; field Brucella isolates, L: 100bpDNA ladder
Bacteriological examination
Bacteriological examination of milk of 198 dairy cows revealed isolation of 92 (46.46%) Brucella isolates that showed the distinctive features for the genus Brucella. Phenotypic characters, CO2 requirements, H2S production, urease, growth in presence of bacteriostatic dyes, lysis by Tbilisi phage and agglutination with A, M and R anti-sera) suggested identification of all Brucella cultures as Brucella melitensis biovar 3, (Table 5) and confirmed by PCR, (Figures 1 and 2).
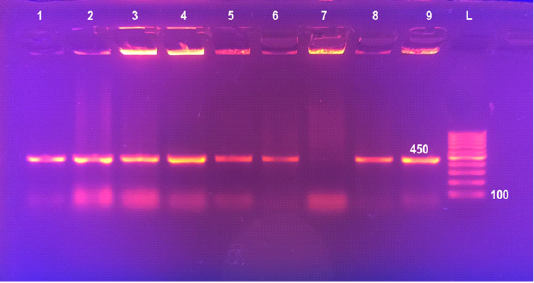 Figure 2: Detection of Brucella in cultures by Multiplex PCR Lane 1: 100bpDNA ladder, Lane 2: Brucella melitensis (control positive), Lane 3: Rev1, Lane 4: Brucella abortus S19 (control positive), Lanes 5: Brucella abortus S99 (control positive), Lane 6: Brucella suis, Lanes 7&8: field Brucella isolates. Lane 9: Brucella abortus 554 (control positive)
Figure 2: Detection of Brucella in cultures by Multiplex PCR Lane 1: 100bpDNA ladder, Lane 2: Brucella melitensis (control positive), Lane 3: Rev1, Lane 4: Brucella abortus S19 (control positive), Lanes 5: Brucella abortus S99 (control positive), Lane 6: Brucella suis, Lanes 7&8: field Brucella isolates. Lane 9: Brucella abortus 554 (control positive)
PCR assays
Universal PCR targeting immunodominant antigen, gene bp26 has amplified the amplicon 450 bp, characteristic for the presence of Brucella, lanes 6-9 (Figure 1). Using Multiplex PCR, all the 92 Brucella isolates recovered from milk of lactating cows (lanes 7 and 8) yielded three amplicons of 587 bp, 1071 bp and 1682 bp sizes characteristic for B. melitensis (Figure 2).
Discussion
Speybroeck et al. (2013) described the performance of a diagnostic test by two measures, the sensitivity and the specificity, that describing the capability of the test to reflect the correct disease status. For screening the seroprevalence of an infectious disease, more than one test is often necessary each with dissimilar performance. These tests may be conducted at the same time (parallel testing) or sequentially (serial testing) as reported by Sheringham et al. (2014). In serial testing, additional testing is performed only if the result of the first screening test is positive. This will improve the specificity but at the cost of lower sensitivity. On the other hand, in parallel testing, more than one screening test is conducted at the same time. Positive result of any test of the battery of the employed tests will classify the animal as positive. This will result in higher sensitivity but lower specificity.
In this study, estimation of sensitivity, specificity, positive predictive value and negative predictive value of serological tests under evaluation using Brucella isolation as the gold standard was carried out. Brucella organisms could be isolated from 92 (46. 46 %) out of 198 lactating cows. Sensitivity, specificity, positive predictive value and negative predictive value of the sum of sero-testing were estimated as 96.73, 48.11, 61.81% and 94.44% respectively (Table 2). Isolation of Brucella organisms offers a definitive diagnosis of brucellosis as gold standard diagnostic technique (Bricker, 2002; Al Dahouk et al., 2003). Bacteriological examinations failed to identify 55 (38.19 %) out of 144 serologically positive cows. Lower sensitivity of the culture technique may be attributed to the fastidious nature of Brucella (Alton et al., 1988). Failure of isolation may occur due to low number of viable bacteria in the clinical sample as well as when the clinical sample is heavily contaminated (Seleem et al., 2010). Intermittent shedding of Brucella in milk is an additional contributing limiting factor (Wernery et al., 2007). Moreover, Wareth et al. (2014) reported that the isolation rate of Brucellae is usually very low. Interestingly, three cows out of the 198 investigated cows, whose milk yield positive culture results, (Table 2) were serologically negative. Cases of culture positivity with concurrent seronegative results employing different serological procedures have been reported by Avijgan et al. (2009); Araj (2010); Hajia et al. (2013). This may be attributed to chronic cases with very low immunological or irregular response and undetectable level of antibodies. Moreover, sero-diagnosis is considered to be untrustworthy 2 - 3 weeks before and after abortion or calving signifying false-negative results (Haileselassie et al., 2010). The obtained results clarify that the specificity of a serological test is usually difficult to be evaluated on the basis of the results of culture findings as some animals yielding negative cultures may be truly infected but yielding negative bacteriological findings.
All Brucella isolates recovered in this study were identified and typed as Brucella melitensis biovar 3 on the basis of bacteriological identification (Table 5) and Multiplex PCR (Figure 2). Detection of B. melitensis biovar 3 in lactating cattle highlights the substantial danger to public health. Isolation of B. melitensis biovar 3 comes in agreement with several previous reports that described this species as the most prevalent in Egypt (Samaha et al., 2008; Menshawy et al., 2014; Hosein et al., 2017; Hosein et al., 2018).
In this study, parallel testing of 960 dairy cows was carried out for detection of brucellosis seroprevalence, (Table 3). Relative sensitivity, relative specificity, positive predictive value and negative predictive value of BPAT were estimated as, 100 %, 99.89%%, 98.46 % and 100 % respectively. RBT revealed 100 %, 99.89 %, 98.46 % and 100% respectively. cELISA showed 95.38%, 99.89%, 98.41%, 99.67% respectively. Concerning CFT, the relative sensitivity, relative specificity, positive predictive value and negative predictive were 93.85 %, 100 %, 100 % and 99.56 %, respectively. The result of the evaluation of serological tests in this study suggests that using BAPAT. RBPT, cELISA and CFT in parallel manner produced the highest overall sensitivity and specificity when considered as their sum. It is obvious that the parallel testing increases the sensitivity of the screening. Accordingly, it is recommended in the circumstances of herds with brucellosis suspected infection aiming at accelerating the eradication of the disease in infected herds. CFT proved to have the highest rate of specificity 100%% and positive predictive value 100%, and showed the least false positives, 0 cases (0%), Table 3. This indicates that the BPAT and RBT positive results should be confirmed by CFT. Al Dahouk et al. (2003) thought out that CFT should be used only as a confirmatory test. Importantly, the (OIE 2018) recommended the use of tests employing acidified antigens such as BAPAT and the RBPT as screening tests, and CFT as the confirmatory reference test for international trade of animals. Although CFT was found to be the highest specific test in this study, it is a complex method to perform necessitating good laboratory services and skilled staff.
In accordance with the results obtained in this study, cELISA shared both RBPT and BAPAT performance in term of specificity (99. 89%) but was the least sensitive compared with the other employed serological tests. The test is skilled of differentiating vaccine antibody response from natural infections with sensitivity varies from 92 to 100%, and specificity ranges from 90 and 99% as reported by Godfroid et al. (2010). The C-ELISA using MAb specific for one of the epitopes of the Brucella sp. has been shown to have similar specificity but lower sensitivity than the BAPAT and RBPT. This agrees with the findings reported by Muňoz et al. (2005). Therefore, the OIE (2018) recommended that positive c-ELISA reactions should be subjected to suitable confirmatory test. The test also excludes some but not all false positive serological reactions due to cross-reacting bacteria as well as reduces but not fully eliminates the reactions due to vaccination as discussed by Muňoz et al. (2005). Additionally ELISA is inexpensive and easy to perform and yield quantitative test results. Moreover, MacMillan (1990) found that ELISA’s specificity to IgG1immunoglobuuline detection is parallel to that of CFT and it can be used as a screening or confirmatory tool.
Serotesting using acidified antigens such as BAPAT and RBPT are extensively used for diagnosis of brucellosis (Nielsen, 2002; Praud et al., 2012). Antigen acidification inhibits IgM biding, preferring agglutination with IgG, increasing the test specificity (Poester et al., 2010). Therefore, BAPAT and RBPT are used as screening tools and positive samples should be subjected to a confirmatory method. RBPT and BAPAT continue to be the preferred screening tools owing to their speedy result and cost availability. In this study both tests showed the highest relative sensitivity 100%. However, their drawbacks are the possibility of producing false positive results as proved in this study which are commonly explained as a result of cross reactivity with different types of Gram-negative bacteria especially Yersinia enterocolitica O:9 (Emmerzaal et al., 2002).
In the present work, the combined sensitivity and specificity of RBPT and CFT of 580 dairy cows using a serial interpretation at individual animal level was estimated as 30 (5.17%) and 26 (4.48%) respectively. The RBPT was conducted as screening test and positive sera were then retested using CFT. Samples were considered positive, when reacted positive for both RBPT and CFT and the total overall seroprevalence was calculated by dividing the number of RBPT and CFT positive animals by the total tested animals. In epidemiological studies, the serial use of two tests is highly recommended to exploit the accuracy of the employed tests as reported by Godfroid et al. (2002). This increases the diagnostic specificity of screening, and is suggested for brucellosis testing especially in pre-movement of animals in brucellosis free herds to avoid false positive results. Interestingly, such combination of RBPT and CFT is considered the most extensively used serial testing system all over the world. The amalgamation of RBPT and CFT in seroprevalence investigations could therefore maximize the truthfulness of the expected results. The RBPT is generally considered to be a sensitive test (OIE, 2018) and CFT is documented as the most dependable diagnostic test for confirmatory purposes and seems to achieve the purpose of diagnosis that search for detection of infected animals while keeping false positives to a negligible level as confirmed in this investigation.
Conclusion
The enhancement and validation of available serological tests are required to enhance the specificity and sensitivity of the employed tests. Predictive values are more appropriate than are sensitivity and specificity during screening programs. Probably, the solution to the problems of epidemiological investigation of Brucella outbreaks with precise diagnosis will require employing of multiple tests possessing different tasks of the immune response.
Conflict of interest
The authors have declared no conflict of interest.
authors contribution
HIH designed the study and reviewed the manuscript. AMAR, BSM, AM performed the field and laboratory works, SRR carried out the PCR and drafted the manuscript.
References






