Advances in Animal and Veterinary Sciences
Research Article
Clinical, Ruminal, Hematobiochemical Alterations and Ultrasonographic Examination of Vagal Indigestion in Cow Calves
Noura El- Shahat Attia1*, Eslam Fouad Eisa2, Yasmin Hassan Bayoumi1
1Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt; 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | The aim of the present study was to throw light on the clinical characteristics of vagal indigestion of cow calves and its related ruminal, hematobiochemical alterations and ultrasonographic findings. A total of 35 (25 male and 10 non-pregnant females) cow calves with vagal indigestion have been studied. The calves examined were aged 6-18 months. The affected animals were admitted to the clinic of Faculty of Veterinary Medicine, Zagazig University, Egypt. Ten apparently healthy cow calves were also included as control. The typical clinical symptoms were progressive abdominal distension resulting into papple shaped abdomen, dehydration and scanty feces. Ruminal fluid samples, blood samples were collected from all animals to estimate the ruminal changes and the hematological picture besides some of the selected biochemical parameters. Acidic pH “5.5-6” and high chloride concentration “> 30meq/L” were recorded in ruminal fluid analysis. Laboratory findings revealed a significant decrease in hemoglobin (Hb.) concentration and RBCs count and a significant increase in PCV and WBCs count in affected animals. There was a significant decrease in serum total protein and albumin levels, with a significant rise in serum globulin and fibrinogen levels. A 3.5 MHz linear transducer was used to assess the frequency, amplitude, duration and speed of the reticular contractions per two minutes while the animals were unsedated and in standing position. Ultrasonographically, the reticular motility were increased in 18 animals (4-8/2 minutes), decreased in 9 animals (< 2 contractions/2 minutes) and complete reticular atony was recorded in 8 animals. In control animals and those with vagal indigestion, the position, the contour and the size of the reticulum and the region surrounding the reticulum were not significantly different. In conclusion, VI is associated with characteristic clinical signs besides ruminal, hematobiochemical and ultrasonographic changes.
Keywords | Vagal indigestion, Calves, Ruminal, Biochemical, Ultrasonography.
Received | May 14, 2021; Accepted | June 12, 2021; Published | July 28, 2021
*Correspondence | Noura El-Shahat Attia, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt; Email: [email protected]
Citation | Attia NS, Eisa EF, Bayoumi YH (2021). Clinical, ruminal, hematobiochemical alterations and ultrasonographic examination of vagal indigestion in cow calves. Adv. Anim. Vet. Sci. 9(9): 1400-1407.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1400.1407
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Attia and Bayoumi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A major clinical problem is digestive tract disorders in ruminants, especially cattle and buffaloes. One such illness is vagal indigestion (VI). In bovine practice, VI is not widespread yet gives the veterinarian a major diagnostic challenge under field conditions, with minimal diagnostic facilities. Both the vagus and the splanchic innervate the ruminant stomach, however, vagal nerve is responsible for the cyclic movements. Vagal indigestion in ruminants is a condition associated with functional stomach disorders, resulting in complete or partial damage, compression or inflammation of the vagus nerve.
Vagal indigestion is also known as Hoflund’s syndrome in cattle. It is characterized by impaired emptying of the abomasum, the fore stomach, or both (Dirksen, 2002; Radostits et al., 2007). Two distinct category were defined as VI, the first being proximal functional stenosis between the reticulum and omasum. Achalasia of the reticulo-omasal orifice tends to result in the delay or cessation of the passage of food through the omasum (Radostits et al., 2007). Hence, the term failure of omasal transport (OTF) (Whitlock 1980; Fubini et al., 1989). The second type is distal functional stenosis and is referred to as distal functional stenosis between the abomasum and duodenum. A group of gastrointestinal disorders in bovines arising from mechanical or functional obstruction of the fore stomach and/or abomasum outflow involves vagal indigestion syndrome (Rebhun et al., 1988).
Clinical symptoms of VI syndrome may result from diseases causing injury, inflammation or vagus nerve strain. However, in most cases of VI, vagal nerve damage is not present and traumatic reticuloperitonitis sequalae is the most common cause. Abomasal diseases, lack of water access, pyloric stenosis/obstructions, and reduced abomasal emptying and or vagal neuritis are the key causes of VI in calves. Vagal nerve damage is caused by a variety of mechanical causes. Mechanical causes may be intraluminal or extra luminal. Indigestible materials such as sand gravel, hair balls, fabric and foreign bodies, coarse food, the presence of neoplasia such as lymphosarcoma or fibropapilloma adjacent to the stomach, cardiac fibromyxoma are the intraluminal triggers (Movassaghi et al., 2013; Hussain et al., 2014a). Vagal indigestion can occur as a postoperative complication of abomasum displacement or abomasum volvulus RDA/AVV (Kumper, 1995).
Gradual weight loss and gradual distension of the abdomen are the most noticeable clinical symptoms, creating the traditional “papple” (pear plus apple) contour as seen from behind. On transrectal palpation, the rumen is distended and L-shape is apparent. Affected animals become inappetant with rumination that is markedly impaired or absent. Ruminal motility may be static, hypomotile or full hypermotile. Recurrent tympany is a normal occurrence. Decreased fecal production occurs and undigested content can be found in the feces. In some instances, Melena can be noticed. Bradycardia and dehydration are other common findings. The rectal temperature is average or slightly below normal (Garry, 1996; Radostits et al., 2007). Depression, muscle weakness, apathy and indifference to normal stimuli, lowering of head, sunken eyes, dry muzzle, cold extremities and gradually weakness and recumbency are other symptoms (Behl et al., 1997; Radostits et al., 2007).
It is necessary to have a history and a general clinical image, but these provide no clear indication of the condition. The goals of this research were to identify particular clinical, ruminal, biochemical changes and ultrasonographic observations that may have diagnostic potential.
Material and methods
Ethical approval
The ethics approval of field study was complies with institutional, national, or international guidelines and the local ethics committee (Medical Research Ethics Committee of Zagazig University).
Animals
Thirty five (25 male and 10 not pregnant female) cow claves out of 300 clinical cases of gastrointestinal disorders (100 buffaloes and 200 cattle) were admitted to the clinic of Faculty of Veterinary Medicine, Zagazig University, Egypt; during the study period from September 2018 to August 2020. Animals aged from 6 months to 18 months weighting 100- 300kgs. The main presenting complaints were anorexia, distension of the abdomen from both sides, decrease fecal output. All the animals were registered with data relevant to the length of the illness. A full history including feed and water intake, rumination status, defecation, tympanic type and pain have been noted in each case. These animals were treated for varying periods by local veterinarians using conventional treatments before being referred to the Veterinary hospital but with no response. For comparison, ten apparently healthy cow calves of the same age were selected and served as a control group.
Clinical examinations
Thorough physical examinations of diseased as well as clinically healthy calves were carried out according to the standard methods described by Dirksen et al. (1990). Close up observations were conducted to evaluate the body condition, the abdominal circumference, color of visible mucous membrane, feeding activity, in addition to the pattern of urination and defecation. Vital signs including rectal temperature, heart and respiration rates were essentially recorded. The rectal temperature was measured using a commercially available mercury thermometer; while the heart and respiration rates were measured by the auscultation of the heart and lung respectively. Pain Tests were also employed for detection of reticular foreign bodies.
Ruminal fluid analysis
About 200 ml of rumen fluid samples collected using oro-ruminal “stomach tube” for ruminal fluid analysis (Kiro, 2017).
Physical examination of the rumen fluid sample
Physical examination of the rumen fluid samples including color, odor and consistency was applied. The color and odor of rumen fluid are assessed by visual inspection and smell immediately after collection. Rumen fluid consistency is assessed by slowly turning a glass or plastic tube half filled with rumen fluid 45- 60° left and right from an upright position. The assessment of the rumen fluid consistency should also assess for evidence of bubbles.
Ruminal PH
The first 100 mL of the collected sample are usually contaminated with saliva “alkaline pH”. Therefore, to prevent misinterpretation of rumen pH, we should discard the first 50-100 mL of the collected rumen fluid sample to reduce the effect of alkaline saliva on pH (Kiro, 2017). Rumen fluid pH can be measured with a pH meter (Garry, 2000). Rumen fluid pH should be measured as soon as possible after collection of the sample (within minutes).
Ruminal fluid Chloride
For estimation of the chloride concentration, the rumen fluid was filtered through a double layer muslin cloth then centrifuged and the test should be carried out on the supernatant. Rumen chloride was detected using Bayer’s diagnostic kits.
Blood sampling
Blood samples were collected from all the examined calves through jugular vein puncture into tubes containing Dipotassium ethylene diaminetetraacetic acid (K2-EDTA) and plain tubes. The blood samples collected in the EDTA tubes were used for complete blood count “Hb., PCV, RBCs and WBCs”. For glucose estimation blood was collected in vials containing sodium fluoride. While those collected in plain tubes were left to coagulate to separate serum. Only clear non hemolyzed serum samples were collected after centrifugation at 3000 rpm, and kept frozen at −20° C until required for biochemical analyses (Kaneko et al., 1997).
Hemato- biochemical screening
Complete blood picture was determined using an automated blood cell analyzer (Sysmex XT-2000iV, Kobe, Japan). Blood glucose, serum total proteins (TP), albumin (Alb) was measured using commercial diagnostic kits obtained by Biomerieux, and Spin react, Spain. Serum globulins were calculated mathematically by subtracting albumin from total proteins. Fibrinogen (Fb) was estimated in plasma samples using commercially available bovine kits according to Orro et al. (2011).
Ultrasonography
As previously mentioned, the ultrasonographic examination was performed on standing, non-sedated animals using a 3.5 MHz linear transducer (Braun, 2003). The frequency of reticular contraction “reticular contraction per two minutes” was assessed. The size of reticulum and strength of contractions were evaluated. By positioning the transducer on the left ventral thoracic area, reticular motility was evaluated. The number, amplitude and velocity of reticular contractions and the length of the relaxation interval between two reticular biphasic contractions were evaluated. The outer contour of the reticulum was examined and any echogenic lesions were recorded if there were any.
Treatment and Follow-up
The conventional therapy was introduced. Evacuation of rumen contents through a stomach tube is an ineffective treatment and very few cases respond to this treatment. Besides offering fresh food and water, the following medications were given to affected animals: oral liquid paraffin (El Gomhorea Co. for Chemicals and Pharmaceuticals, Egypt) at a dose of 10 mL/kg daily for 3 days. Administration of fluids, broad spectrum antibiotics, calcium, potassium and chloride (Fubini et al., 1989). The main goal of treatment is to correct electrolyte imbalance “hypokalemic, hypochloremic, metabolic alkalosis” and to regulate the inflammatory and infectious processes (Braun et al.,1990; Hussain et al., 2014b).
At regular intervals (every 3 days to 45 days), all the owners were contacted by telephone. The owners were asked about the animals’ general health status; the animal’s response to treatment if the condition is worsening or the animal has improved.
Statistical analysis
The obtained data are presented as mean ± S.E. The data were analyzed using t-test to test for significant differences between control and animals with vagal indigestion. The differences in means were considered statistically significant at P < 0.05.
Results
Clinical examination
All animals under investigation were anorexic with marked distension of the abdomen from both sides giving L- shaped abdomen “ papple shaped” apple plus pear shape when viewed from behind as in (Figure 1b) with sever distension of the whole left side as in (Figure 1a). There was decrease in the body weight but it is sometimes unnoticed due to abdomen distension which hides the fact that the muscle mass of the animal is diminishing. Pain test (pinching of wither) evidenced a negative response to pain. Over time, the animal develops a rough hair coat, loses condition, and becomes weak (in some cases to the point of recumbency), with marked clinical signs of dehydration “the eyes were sunken and skin elasticity was lost”. Depression with decrease the fecal output which sometimes contains indigestible feed particle. Mucous membrane was congested with congested blood capillaries (Figure 1c). The sounds of the rumen contractions are often reduced or almost absent in spite of hyperactivity because the rumen contents are
Table 1: Illustrate the result of vital signs monitoring in both groups
| Parameter | Control group | Vagal indigestion | No. of animals affected group |
| Rectal temperature °C | 38.4±0.2 |
38< T< 39.0 25 39.0<T< 39.5 7 T>39.5 3 |
|
| Heart rate bpm | 73.6±8 |
HR<55 8 56<HR<80 15 80 < HR < 100 7 HR > 100 5 |
|
| Respiratory rate brpm | 16.8±3 |
16< RR <30 23 RR > 30 12 |
|
| Rumen motility | 2-3/2 minutes |
Hypomotile< 2 contr/2 min 9 Hypermotile: > 4 contr/2 min 18 Absent: 0 contr/ 2 min 8 |
|
| Fecal appearance | Normal "semisolid, greenish and circumscribed" |
Dry or mucous or doughy 23 Absent 0 Diarrhea 5 Normal 7 |
|
| Foreign body tests | -ve |
-ve in all animals |
|
bpm - beats per minute; brpm - breaths per minute; contr -contractions
Table 2: Illustrate the result of ruminal findings of the animals under investigation
| Ruminal parameter | Control group | Vagal indigestion affected group | No. of animals |
| Color | olive-green to greenish-brown according to the feed |
Greenish-brown Blackish-green |
17 18 |
| Odor | Sweet and fermentative smell " Aromatic " |
Fetid smell in complete ruminostasis. The smell of abomasal contents in pyloric outflow obstruction |
8
20 |
| Consistency | Slightly viscous. | Frothy | 35 |
| PH | 6.0 to 7.2. |
Normal ranged from 6.5-7.0 in animal with proximal obstruction. Acidic 5.5-6 in animal with distal obstruction. |
15
20 |
| Rumen fluid chloride |
<30mEq/L.
|
<30mEq/L. in animal with proximal obstruction. >30mEq/l in animal with distal obstruction. |
15 20 |
Table 3: Illustrate the result of hematobiochemical indices of the animals under investigation
| Parameter | Control group | Vagal indigestion affected group |
P-value |
| Hb (g/dL) | 9.720 ± 0.09 | 8.070 ± 0.16 | < 0.001 |
| PCV (%) | 35.75 ± 0.63 | 41.70 ± 0.70 | < 0.001 |
|
RBCs (106/mL) |
5.410 ± 0.12 | 3.800 ± 0.13 | < 0.001 |
|
WBCS (103/ mL) |
8.150 ± 0.17 | 13.05 ± 0.12 | < 0.001 |
| Fb (mg/dl) | 230.6 ± 3.86 | 339.5 ± 7.94 | < 0.001 |
| Glucose (mg/dl) | 68.41 ± 1.25 | 44.54 ± 0.87 | < 0.001 |
| TP (g/dL) | 7.090 ± 0.08 | 6.090 ± 0.07 | < 0.001 |
| Albumin (g/dL) | 3.890 ± 0.05 | 2.400 ± 0.06 | < 0.001 |
| Globulin (g/dL) | 3.200 ± 0.11 | 3.690 ± 0.08 |
0.002 |
N.B P is significant at < 0.05
pasty and frothy as illustrated in (Table 1). Fluid-splashing sounds may also be audible on ballottement of the left and right flanks if the rumen is distended with excessive quantities of fluid.
Rectal findings
Scanty, pasty faces were present in the rectum. The rumen dorsal sac was packed with fluids and gas that gave it a mushy consistency. The rumen, covering the entire left abdomen, is distended, forcing the left kidney to the right of the midline. The ventral sac of the rumen is distended and palpable to the right of the midline (the characteristic “L-shaped” rumen).

Figure 1: Notice distension of left side of the abdomen (a), Bilateral distension of the abdomen with left ventral and dorsal distension and right ventral distension (apple/pear) (b). Congested eye capillaries and sunken eye (c).
Ruminal findings
Ruminal fluid findings were explained in (Table 2) showing change in color, odor and consistency “greenish- brown to blackish-green”, “ foul, fetid” and “pasty consistency containing a large number of small bubbles”. Acidic PH “5.5-6” and high chloride concentration “> 30mEq/L” were recorded.
Hemtobiochemical analysis
Hematological analysis revealed a significant decrease in Hb. concentration and RBCs count and a significant increase in PCV and WBCs count in diseased animals. There was a significant decrease in serum total protein and albumin levels, with a significant rise in serum globulin and fibrinogen levels as illustrated in (Table 3).
Ultrasonographic examination
In both classes, reticulum appears normally. It appears as a half-moon-shaped, echogenic structure. In the control group, the reticulum contract at regular intervals and is placed immediately adjacent to the diaphragm and ventral portion of the abdominal wall when relaxed, as shown in (Figure 2a). The reticulum was immediately adjacent to the ventral abdominal wall in 29 calves while in 6 calves it was displaced from the abdominal wall. The reticular contour was smooth in in all cases. Fibrinous adhesions were seen on the reticulum in eight calves.
Reticulum motility was assessed ultrasonographically. In control group, it was 2-3 contractions/ 2minutes. In the diseased animals, reticulum motility was increased 4-8 contractions/2 minutes in 18 cases. Reticulum motility was decreased < 2 contractions/2 minutes in 9 cases. Complete reticulum cessessation 0 contraction/ 2 minutes in 8 cases. The ruminal wall appears echogenic. Reticular contractions were biphasic and complete in 19 calves, biphasic and incomplete in 6 calves, monophasic and incomplete in two calves. Reverberation artifacts running parallel to the ruminal wall are seen in the region of the dorsal sac. The ingesta are located in the middle of the rumen and appear echogenic with gaseous inclusions. The fluid in the ventral aspect of the rumen is hypo- echogenic. In eight cases hyper-echogenic fibrin thread were located in the reticular region as in (Figure 2b). While the area surrounding the reticulum appeared normal in the other 27 calves.
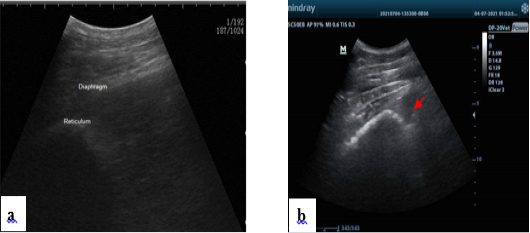
Figure 2: Normal ultrasonogram of the reticulum, notice half-moon shape (a). Presence of hypoechogenic fluid notice fibrin thread “arrow” in the reticular area in some diseased conditions (b).
Therapeutic trails
Just 12 out of 35 cases referred to medical therapies, including the evacuation of the contents of the rumen through a stomach tube. High oral dose of liquid paraffin, nerve tonic. “Fluid therapy for electrolyte correction “hypokalemic hypochloremic metabolic alkalosis”.
Discussion
Vagal indigestion has been used in veterinary medicine to refer to a clinical syndrome. This condition is associated with various etiologies (Whitlock, 1998). Vagal Indigestion has been considered to be caused by the vagal nerve damage. In fact, any case of chronic indigestion with clinical signs similar to traumatic reticuloperitonitis and did not respond to routine care was identified as VI. Calves are more vulnerable to abomasal dilatation and abomasal torsion, VI may occur as a sequelae for these disorders.
Similar clinical signs were obtained previously by Smith (2002); Radostitis et al. (2007) and Braun et al. (2009). The explanation for the distension is the accumulation of gas and fluid in the rumeno-reticulum sacs. When the flow of the ruminoreticular contents into the omasum and abomasum was halted, the increased rumen volume result. The abdominal distension usually covers the whole left side of abdomen and right ventral quadrant of abdomen, giving “apple shaped” appearance to left side and “pear shaped” appearance to the right side, jointly giving the papple shaped when viewed from behind (Radostits et al., 2007; Braun et al., 2009). The fecal output is steadily reduced and feces can often be completely stopped (Braun et al., 2009; Hussain et al., 2014a). With an increase in undigested particles, feces consistency is usually scant and sticky (Behl et al., 1997; Radostits et al., 2007). Melena can be detected due to abomasum ulceration in some cases (Rebhun et al., 1988). Due to sustained inappetence rather than fluid sequestration and osmotic fluid drawing within the fore stomach the affected animals may be dehydrated. The lack of fluids to pass into the small intestine outside the abomasum for absorption may also be the potential cause of varying degrees of dehydration (Radostits et al., 2007). Normal, decreased or increased rumeno-reticular motility may be recorded (Braun et al., 2009). The strength of rumen contractions is reduced. Rumen contraction sounds are not audible as the ruminal content has become frothy, due to the prolonged contractions and failure of rumen empty. Increased ruminal motility is due to activation of “stretch receptors” of low threshold tension receptors (LTHTR) present in the reticulum. These receptors induce rumino-reticular motility and are triggered in the initial stages of VI by mild to moderate abdominal distension. In response to this distension, the rumen continuously senses this distension as a recent meal, and increases the rate of primary contractions. As “severe stretch” increases with rumeno-reticular distension, motility is decreased in frequency and amplitude due to stimulation of high threshold tension receptors (HTHTR). For rumeno-reticular moltility, these HTHTRs are inhibitory. When distension becomes more extreme, movements cease, presumably due to over-distension of the ruminal wall, resulting in a greater degree of HTHTRR activation (Constable et al., 2017).
Regarding the vital signs, our results were in harmony with Behl et al. (1997); Radostitis et al. (2007) and Braun et al. (2009) which in most cases reported normal rectal temperature, but also subnormal temperature were recorded in some cases. In most cases, respiration remains unaffected, but due to extreme abdominal distension, it may be increased.
Bradycardia is a characteristic sign of VI but is not always recorded (Rebhun et al., 1988; Radostitis et al., 2007; Braun et al., 2009). Bradycardia is due to the decreased feed intake rather than the increased a direct parasympathetic tone from the injured vagus nerve. Tachycardia develops as the distension progresses. Most parts of the gastrointestinal tract, such as the pharynx, oesophagus, and stomach, except the terminal portions of the colon, receive parasympathetic innervation through the vagus nerve. Therefore, the vagus nerve has a regulatory effect on the functions of the digestive system (Cunningham, 2002; Qian and Danielsson, 1996). Thus, damage of the vagus nerve or its branches causes disruptions in the functions of the digestive system and heart rate.
The examination of rumen fluid is an important technique used, in particular in digestive disorders and ruminoreticular dysfunction. The rumen functions as a fermentation vat where the rumen microbes that operate in a dynamic equilibrium state in a complex ecosystem cause efficient digestion of plant materials, and therefore rumen microbial health is essential in maintaining ruminants health (Cockcroft and Jackson, 2004). Abnormality in color and odor of ruminal fluid is attributed to initial hypermotility followed by hypomotility or total cessation and reticular emptying failure into the omasum. Due to inactive ruminal protozoa and the existence of large quantities of gases, pasty consistency containing a large number of tiny bubbles found in affected animals. Acidic ruminal pH is caused by the reflux of abomasal secretion into the rumen (Braun et al., 2011). However, White (2004) reported an alkaline pH and this may be due to obstruction of the forestomachs that prevented the onward transport of food with continuous swallowing of alkaline saliva. The gastric acid was sequestrated and its reflux into the rumen possibly decreased the ruminal pH and elevated ruminal chloride (Braun et al., 2012).
The hematological picture offers an indicator of the animal’s general health and chronicity of the disease (Kaneko et al., 1997). Blood picture is a valuable method for explaining the inflammatory processes that can affect the vagal nerve. Our hematological findings were in consistence with Braun et al. (1990). The changes in the packed cells volume (PCV) and hemoglobin depend on the disease’s chronicity and the degree of dehydration that may occur. Usually, the PCV increases due to the more acute nature of the disease and the higher degree of dehydration and consequent blood concentration (Braun et al., 1990). However, Whitlock (1980) and Fubini et al. (1985) found that PCV may stay within the normal range or may slightly increase. A substantial reduction in Hb. concentration suggests anemia that can be attributed to the prolonged period of anorexia Aref and Abdel- Hakiem (2013). Increase in the total number of white blood cells reported in most animals, especially in cases which occur as a sequelea to TRP (Kuiper and Breukink, 1986; Fubini et al., 1989). However, in acute pyloric functional stenosis in cattle and buffaloes with cranial or caudal functional disorder, the normal range of leukocytic count was also identified (Braun et al., 1990). Normal, increased, decreased WBCs were recorded in animals with VI. If an inflammatory condition such as peritonitis is present, a neutrophilia may be present. Leukopenia may be recorded with diffuse peritonitis. In ruminants, fibrinogen is widely measured as it is the best predictor of inflammation since concentrations of fibrinogen frequently increase before changes in leucocytes (Latimer et al., 2003; Jones and Allison, 2007; Attia, 2016). Compared with control, slightly elevated serum fibrinogen is similar to that obtained by Wittek et al. (2005). Increased fibrinogen level may be due to the inflammatory response and adhesion of certain internal compartments, extensive para-reticular inflammatory adhesions (Radostits et al., 2007). Hematological outcomes were consistent with an inflammatory process due to an inflammatory response to gastrointestinal stasis leading to toxin absorption into the bloodstream (Sahoo et al., 2019). The observed hypoglycemia, hypoproteinemia, hypoalbuminemia can be due to malnutrition after anorexia and chronic starvation, and the inability of the liver to synthesize sufficient protein quantities. In response to inflammation, stress, or dehydration, there is an increased in concentration of globulin (Kaneko, 2008).
Ultrasonography is an ideal, easy noninvasive diagnostic tool for the investigation of bovine gastrointestinal disorders. The reticulum normally contracts once per minute in a biphasic manner, in which the first contraction is incomplete (Braun and Götz, 1994). Reticulum motility is initiated and controlled by the vagus nerve, which in turn is under the influence of the gastric center in the medulla oblongata (Constable et al., 1990). A number of factors, including eating, rumination and stress may affect reticulum motility (Braun and Rauch, 2008). The number of reticulum contractions is highest during eating (approximately 1.5 contraction/ min) and lowest when the animal is stressed (less than 1.0 contraction/ min). Animals with VI, the number of reticulum contractions may be reduced, normal or increased (Braun et al., 2009). Animals with reticulo-omasal stenosis had significantly more reticular contractions than animals with functional stenosis of the pylorus. Visualization of the reticulum at the 5th intercostal space in cases of VI due to extreme pressure of the distended abdomen on the diaphragm was recorded by Athar et al. (2010). Ultrasonographic image of the surrounding area of the reticulum is normal in most animals. However in some animals there is hyperechogenic fibrin threads which indicate some adhesions of some internal compartments which is confirmed by fibrinogen content. Overall, the ultrasonographic findings were non-specific and did not provide evidence of VI.
The literature in calves regarding VI is scarce. Thus, symptomatic therapy, primarily to mitigate inflammatory reaction and abdominal distension and to correct the electrolyte deficit, has been controlled in animals in the present research. In order to prevent complications associated with hypokalemia, hypochloremia, and hypocalcaemia, the electrolyte imbalances were evaluated. Medicinal treatments with broad spectrum antibiotics, replacement fluids and anti- fermentative medications, nerve tonics failed to improve the condition of the animal except in seven cases and for a little reduction in abdominal distension in other cases.
Conclusion
In conclusion, calves with VI were associated with marked clinical, ruminal and biochemical changes should be taken into consideration that could aid in an accurate diagnosis of such disorders. Ultrasonographic findings were non-specific and did not provide an accurate evidence of VI.. Normal reticular motility does not rule out vagal indigestion. However, reticular hypermotility and characteristic clinical signs support a tentative diagnosis of vagal indigestion.
Acknowledgments
The authors are grateful to the animals’ owner for their contributions and to the Director of Veterinary Teaching Hospital at Zagazig University for his support during this study.
Conflict of interest
The authors declare that they have no competing interests.
Authors Contribution
All authors has planned and conducted the study. NEA&EFE collect the sample YHB analyzed the data. All authors wrote the manuscript, discussed the results and contributed to the final version of the manuscript.
References






