Advances in Animal and Veterinary Sciences
Research Article
The Potency of Lepidium sativum and Commiphora molmol Extracts on Trichinella spiralis Stages and Host Interaction
Gehan L. Abuelenain1*, Zeinab H. Fahmy1, Amal M. Elshennawy1, Azza M. Fahmy1, Eman M. Ali1, Olfat Hammam2, AbDel-Wanes A. Abdel-Aziz3
1Department of Immunology and Drug Evaluation, TBRI, Giza, Egypt; 2Department of Pathology, TBRI, Giza, Egypt; 3Department of Medicinal Chemistry, TBRI, Giza, Egypt, Affiliated with Fatima College of Health Sciences, Al Ain, United Arab Emirates.
Abstract | Trichinellosis is one of the zoonotic diseases that cause health and economic problems worldwide. Albendazole is the approved anti-parasite medicine but resisted by the larvae that reside in the muscle cells, transforming them into tumor-like nest cells. This study aimed to investigate the potential effect of two medicinal plants, Lepidium sativum (Garden cress) and Commiphora molmol (Myrrh), on trichinellosis because of their folk utilization in the Middle East and some other African countries. Male Swiss albino mice (n=106) were randomly placed into two groups postinfection with Trichinella spiralis larvae (3.0 x102). One group of infected mice received either Garden cress (500mg/kg), Myrrh (500mg/kg), or mono-combined with Albendazole (50mg/kg) on day zero for three consecutive days, and was decapitated on day seven post-infection (pi). The other group of mice received the same regimens on day 30 and were sacrificed on day 35 pi. Three control groups ran in parallel: Albendazole treated-infected mice, non-infected, and infected mice. The highest reduction of worm counts was due to Albendazole and its combinations with either Garden cress or Myrrh. However, the most significant impact on larvae was in favor of Myrrh alone or combined with Albendazole. The muscular tissues were ameliorated with Myrrh’s treatment protocols, which induced the nest cells’ larvae destruction. The biochemical tests indicated the engagement of vital proteins in the action mode of all treatments. Therefore, we concluded that Myrrh might be a valuable future antagonizing drug of trichinellosis. However, further studies on the administered concentrations of both Garden cress and Myrrh are vital to verify the optimum dose with the best action mode on the host responses.
Keywords | Trichinella spiralis, Trichinellosis, Drug discovery, Medicinal plants, Garden cress, Myrrh, Lepidium sativum, Commiphora molmol
Received | March 06, 2021; Accepted | June 25, 2021; Published | July 28, 2021
*Correspondence | Gehan L. Abuelenain, Department of Immunology and Drug Evaluation, TBRI, Giza, Egypt; Email: [email protected]
Citation | Abuelenain GL, Fahmy ZH, Elshennawy AM, Fahmy AM, Ali EM, Hammam O, Abdel-Aziz A-WA(2021). The potency of Lepidium sativum and Commiphora molmol extracts on Trichinella spiralis stages and host interaction. Adv. Anim. Vet. Sci. 9(9): 1376-1382.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.9.1376.1382
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abuelenain et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Trichinella spiralis, an encapsulated round, tissue-dwelling nematode (Shao et al., 2020). It is accountable for trichinosis disease, one of the global foodborne zoonotic diseases (Knopp et al., 2012), which is highly difficult to be diagnosed at early stages. When detected, larvae would be normal inhabitants of the muscles where symptoms grow more in-depth, and treatment becomes more challenging (Bruschi, 2014). Trichinosis is spread almost worldwide (Pozio and Zarlenga, 2013). The infection can yield serious health complications in patients; besides, it causes economic crisis due to the high financial costs of disease control at the farm and slaughterhouse level and meat inspections and freezing (Devleesschauwer et al., 2015). Recently, a Trichinella freeze-resistant species (oddball) is believed to be risky for people who live in icy countries (Sharma et al., 2020).
Unfortunately, the infection is a tough challenge because it cannot be manifested and detected at the disease’s onset (Dupouy-Camet et al., 2002; Wang et al., 2017). Consumption of undercooked or raw meat (mainly pork) contaminated with encysted larvae of Trichinella species can contribute to trichinellosis (Schantz et al., 1999; Rainova et al., 2016). The magnitude of the infection in humans is related to the number of larvae ingested (Teunis et al., 2012). Variable signs and symptoms characterize the disease and are related to the ubiquitous distribution of parasites in the body. The infection begins with penetrating the host intestinal epithelium by the infective larvae, which eventually form a collagen capsule inside the striated muscles, transforming the muscle cells into nurse cells (Wu et al., 2008) that protect the muscle larvae from being damaged by the humoral and cellular immune response (Xu et al., 2017).
To date, the treatments for T. spiralis are limited all over the world. The broad-spectrum drugs, such as Albendazole and mebendazole (Cabie et al., 1996; Gottstein et al., 2009), have many disadvantages (Matadamas-Martínez et al., 2013), such as leaving patients helpless in some cases. Moreover, those drugs and other anti-helminthics are effective on the T. spiralis enteric stages, not the systemic ones (Sharma et al., 2015) due to the parasite’s resistance and low drug absorption. Since the discovery or development of anti-trichinosis therapy is necessary to eliminate the parasite intestinal forms (Kocięcka, 2000; Yr and Yf, 2015), medicinal plants have been lately tested either as mixtures of various biologically active ingredients or alone.
Garden cress (Lepidium sativum; Hab al Rashad) seeds are well known in traditional medicine to treat various human and animal ailments (Gedif and Hahn, 2003; Teklehaymanot et al., 2007). The herb is a medicinal plant that demonstrated effective anti-coccidian, bacterial, oxidant, inflammatory, and parasitic effects (Adamu and Boonkaewwan, 2014; Raish et al., 2016; Getahun et al., 2020). Another medicinal plant, known as Myrrh, Commiphora molmol (Atta and Alkofahi, 1998), is believed to be effective in treating various parasites (Dolara et al., 2000; Kimura et al., 2001), including T. spiralis in vivo (Basyoni and El-Sabaa, 2013) and in-vitro (Abdelrahman et al., 2020).
The present study was designed to examine the efficacy of either Garden cress or Myrrh alone, combined or mono combined with Albendazole, through evaluating the host-parasitic relationship under the treatment protocols. The effectiveness indicators included parasitological, serological, and histopathological tests.
MATERIALS AND METHODS
Ethics
Anesthetic procedures complied with the ethical guidelines approved by the Ethical Committee of the Federal Legislation and National Institutes of Health Guidelines in the USA and followed the rules of animal ethics that are globally applicable. All experimental groups were housed and held under ethical and standard conditions in the Parasitology Lab at Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
Experimental animal design
Parasite-free, male, Swiss albino mice (n=106, 25-30g each) were obtained from the Animal House at TBRI, Giza, Egypt. Mice were laboratory-bred for 6 to 8 weeks, then deployed into two batches after being infected with Trichinella spiralis larvae (3.0 x102 each). For three consecutive days pi, the first batch of mice received Garden cress (500mg/kg) and Commiphora Myrrh (500mg/kg) either alone or combined with Albendazole (50mg/kg), and those mice were sacrificed on day seven pi. The other batch of infected mice received the same regimens on day 30 and decapitated 35 pi. The control groups were maintained parallel with each cohort and included: Albendazole-treated-infected mice and non-infected and infected mice. Mice fed regularly on a diet containing 24% protein, 4% fat, and about 4% - 5% fiber and water ad-libitum at a temperature of 24 ˚C. In addition, intestines, diaphragm, and sera were collected for the parasitological, histological, and serological examinations.
Parasite inoculum preparation
Trichinella spiralis cycle is managed in the Parasitology Lab at TBRI. Larvae were recovered from the muscles of infected pigs and prepared as described previously (Guenther et al., 2008). The inoculum in saline containing 1.5% gelatin was adjusted after counting the larvae by hemocytometer several times. The experimental mice were starved for 12h before the oral administration of the inoculum. Each mouse had received an inoculum of 300 larvae.
Extraction of plants
Myrrh and Garden cress seeds were purchased from a local market in Egypt, identified, fermented, and extracted at the Medicinal Chemistry Department, TBRI, Giza, Egypt. Two hundred grams of Commiphora molmol (Myrrh) resin powder were soaked in one liter of 90% ethanol and kept on a shaker at 4oC in a refrigerator for three days. First, the ethanol extract was filtrated using a double layer of musk to get rid of debris. Then, a rotatory evaporator connected to a vacuum pump was used to concentrate the liquid extract at 50˚C under low pressure. That ethanol extract was kept in the refrigerator for further use as described (Shalaby and Hammouda, 2014). Next, the extraction of Lepidium sativum (Garden cress) was carried out by fermentation similar to the Commiphora; however, the plant powder was soaked in methanol (MeOH). Finally, all extracted samples were stored at 4°C until use.
Assessment protocol
Worm count in the intestines on day 7 post-infection
Adult worms were recovered from the intestines of mice following the protocol of Denham (1965). Briefly, the intestine was opened longitudinally, washed, and then incubated in 10 ml saline at 37˚C for 2h to allow worms to leave the intestine to the container. Washing was done several times till the fluid become clear, then it was collected and centrifuged at 1,500 rpm for 5min. Finally, the sediment was reconstituted in a few drops of saline and examined under the dissecting microscope for counting the worms.
Larvae count in the muscles on day 35 post-infection
The diaphragm of each mouse was dissected and examined using the trichinoscopy or compressorium technique to count the number of larvae as previously described (Webster et al., 2006).
Biochemical tests
Blood sera were collected (Gamble et al., 1997) on day 35 pi to evaluate the renal proteins (urea and creatinine), liver enzymes (AST and ALT), albumin, and total protein. Commercial research kits were purchased from Egyptian companies: BioMed (total protein and albumin), Spectrum Co. (liver enzymes), and Egypt Biodiagnostic’s Co (kidney proteins). Tests were run according to the manufacturer’s guidelines.
Histopathological examination
The muscular tissue specimen of each mice was fixed in 10% formol-saline for 24h, washed in water for another 12h, and dehydrated in ascending grades of alcohols. Next, xylene removed the traces of alcohol, and then the tissue was embedded in paraffin blocks. Finally, 10μm thick sections were mounted on glass slides and stained with hematoxylin and eosin for the microscopic examination (Drury and Wallington, 1980).
Statistical analysis
Data were calculated and analyzed using the One-Way ANOVA test followed by the Bonferroni method for multiple pairwise comparisons. A probability of less than 0.05 was considered significant.
RESULTS AND DISCUSSION
On day 7pi, the most significant (p<0.001) effect on worm counts was attained by Albendazole combined with Myrrh (99.89%). Yet that reduction percentage was comparable to that of Albendazole-Garden cress combination (99.16%), or Albendazole alone (98.16%) as indicated by (Table 1 and Figure 1). The treatment with Myrrh alone or combined with Albendazole had the most significant (p<0.001) reduction percentage of the larvae numbers (93.00% vs. 93.56%) recovered from muscles 35 days pi, yet they are comparable. Interestingly, the treatment with Garden cress extract alone recorded the slightest reduction percentage of the larvae and worms numbers (70.67% and 58.07 %; respectively) compared to the other treatment protocols (Table 1 and Figure 2).
Table 1: The percentage of reduction of worm and larvae counts in all the experimental groups 7- and 35-days pi, respectively.
| Groups | % Reduction of worm counts | % Reduction of Larvae counts |
| Infected mice | - | - |
| Alb | 98.16% | 84.00% |
| Myrrh | 68.39% | 93.00% |
| Alb+Myrrh | 99.89% | 93.56% |
| Garden cress | 58.07% | 70.67% |
| Alb+ Garden cress | 99.16% | 88.22% |
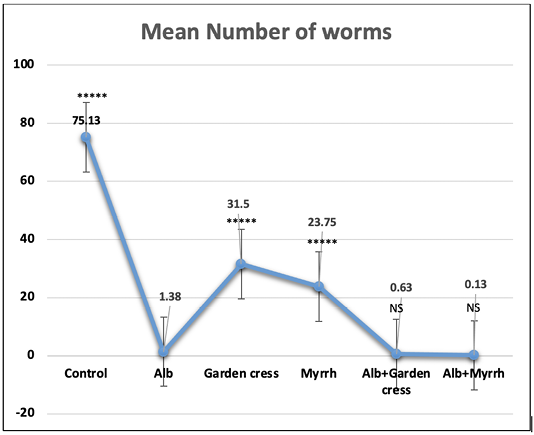
Figure 1: The worm count recovered from the intestines of control and experimental mice 7 days pi. The results are represented by the means±SE and Alb refers to Albendazole. All experimental groups are compared to Albendazole treated infected mic. ****P<0.0001; NS No significance.
The biochemical tests showed that the albumin and total protein levels of Albendazole-treated mice were comparable to those of non-infected ones and significantly increased (p<0.05) in the other treatment regimens. However, the infected control mice had unprecedented (p<0.001) lower albumin levels than non-infected ones. On the one hand, the creatinine levels increased in the sera of Albendazole-treated mice and decreased in the other treatment protocols (p<0.01, each). Yet, the urea levels were neither affected by T. spiralis infection nor the various treatments in all groups. On the other hand, the liver proteins, AST, and ALT levels increased remarkably in the infected mice, Albendazole-treated ones (p<0.0001), and the other treatment protocols (p<0.05) when compared to the non-infected mice (Figure 3).
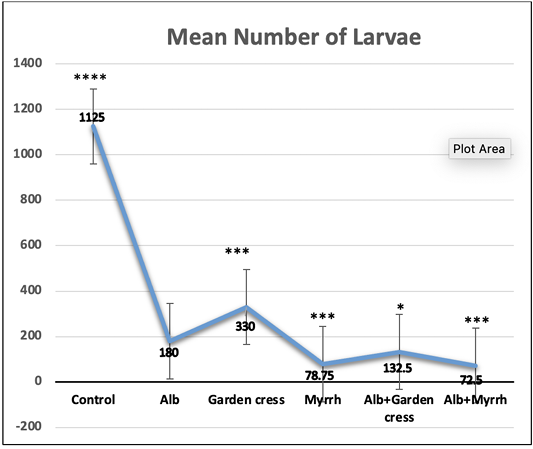
Figure 2: The larvae count recovered from control and experimental mice’s muscles on day 35 pi. The results are represented by the means±SE and Alb refers to Albendazole. All experimental groups are compared to Alb treated infected mic. ****P<0.0001; ***p<0.001; p<0.01; NS No significance.
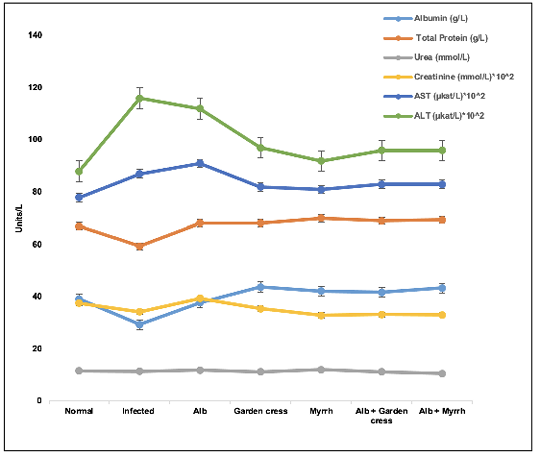
Figure 3: Liver and kidney proteins levels in the various treated groups on day 35 pi. The results are represented by the means±SE and Alb refers to Albendazole.
The histopathological changes showed infiltration of the immune cells around the encysted larvae in the muscles of all experimental mice. However, the least cellular infiltration favored Myrrh-treated mice. Moreover, larvae capsules’ prominent degeneration and vacuolation were due to the mono-combination of either Alb with Myrrh or Garden cress (Figure 4).
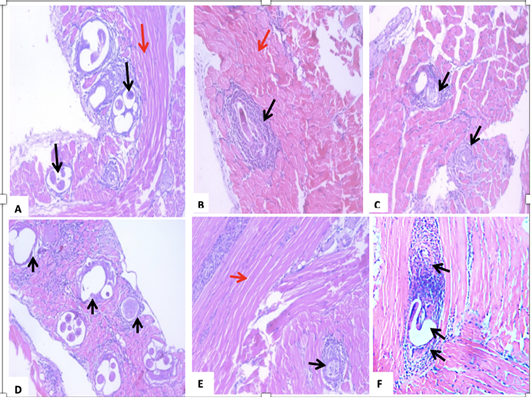
Figure 4: A section of skeletal muscle (red arrow) of a T. spiralis-infected mouse on the 35th-day pi (A) encysted larvae surrounded by intense inflammatory cellular infiltrate, which are indicated by black arrows. (B) treatment with Albendazole, showing Trichinella spiralis encysted larvae surrounded by intact capsules and severe inflammatory cellular infiltrate of mononuclear cells, mainly lymphocytes, and macrophages (black arrows). (C) treatment with Myrrh, showing a reduction in the number of larvae with degeneration of the larvae surrounded by mild inflammatory (black arrows). (D) treatment with Garden cress showing fragmentation and vacuolation of the larva with an invasion by inflammatory cells, mainly lymphocytes, and macrophages. Black arrows indicate vacuolated capsules. (E) combined Albendazole and Myrrh’s treatment showing a reduction in the number and a degeneration of the larvae, which were surrounded by mild inflammation (black arrows). (F) combined Albendazole and Garden cress treatment showing vacuolation of the nymph with intense invasion by the inflammatory cells consisting mainly of lymphocytes and macrophages (black arrows).
The potential actions of trichinellosis treatments were demonstrated for the early T. spiralis intestinal stages, not the circulating stages (larvae). This resistance to the current drugs (Rosenthal, 2021) is a motivation to investigate the potentiality of natural products extracted from medicinal plants, either alone or in association with current safe drugs, in eradicating the larval stages and the intestinal ones. In this study, we detected the Myrrh and Garden cress in vivo effectiveness, either alone or in combination with Albendazole, in antagonizing the diseases while focusing on the larvae stages and the concurrent changes 35 days pi.
CONCLUSIONS AND RECOMMENDATIONS
The data collected from Garden cress or Myrrh treatment protocols demonstrated a significant reduction of the intestinal worm and muscle larva counts on days 7 and 35 pi, in favor of Myrrh. Yet, Albendazole’s administration alone had the most significant impact on the worms, and the least on larvae, for the same schedule. These results indicate that the combination regimens may sustain the action of those individual drugs on the different parasite stages. These findings agree with the studies that proved the poor solubility and absorption of Albendazole by the intestinal cells, making it non-effective in damaging the muscle larvae stage (Despommier, 1998; Garcia Rodriguez et al., 2011). Although the Garden cress seeds were used widely and safely in nutrition (Shawle et al., 2016), there was a lack of physicochemical analyses, but its pharmaceutical action mode was reported (Dixit et al., 2020; Ibrahim et al., 2020). Moreover, we did not find any study on the effect of Garden cress on trichinellosis. Nevertheless, our treatment protocol was inspired by a previous study on Myrrh and Ivermectin’s efficacy on the T. spiralis model (Basyoni and El-Sabaa, 2013), which had similarities with our data on Myrrh.
To understand the dynamics of the candidate plant extracts on the disease, we measured some essential proteins associated with liver and kidney functions. Those proteins are vital parameters of medical diagnosis and treatment. The urea levels were affected neither by the treatment protocols nor the disease itself. However, creatinine levels of all treatment protocols except for Albendazole were less than those of the normal mice and infected controls. This finding indicates that Myrrh and Garden cress may accelerate the rate of creatinine clearance from the blood. Mainly it was verified that Garden cress has a diuretic activity in a rat model approximately one decade ago by (Patel et al., 2009).
The liver function measurements demonstrated high AST and ALT levels of the infected mice and Albendazole-treated ones due to the liver’s involvement in combating the disease and detoxifying the drug and the metabolites of the parasitic stages. These enzymes’ elevation may be due to the prolonged, high-dose treatment, and it could be reversible after a while (Weber, 2017). Both enzyme levels were less with the other experimental therapeutic agents (Myrrh and Garden cress), which may indicate lower toxicity of Myrrh and Garden cress than Albendazole.
Interestingly, the sera Albumin levels were approximately normal in the mice treated with Albendazole and Garden cress. At the same time, the other experimental groups had higher levels than the infected and normal mice. This finding may be the reason for increasing the total protein levels, which had a similar pattern of albumin in this protocol. Albumin is the protein that carries drugs and helps its absorption through the plasma membrane (Noorani et al., 2015), facilitating their entry into the cells, which might be needed at high levels due to the high concentration of Myrrh and or the combined treatments. This assumption can be verified in a future study when lower concentrations of Myrrh and combined regimens will be used to plot a correlation between those drugs and albumin levels in the sera.
The muscular tissues of the infected treated and untreated mice revealed the best effect at day 35 pi in a reduced number and diminished size of larvae. Their lesions are due to Myrrh, either alone or combined with any of the other drugs. The destruction of the muscle larvae was noticed in the groups treated with the Garden cress, even though their number and lesions were more significant than those treated with Myrrh’s regimens. The antispasmodic effect of Myrrh by blocking the secondary active transport-dependent Calcium ion channels of the plasma membrane of parasites (Gilani et al., 2010; Vissiennon et al., 2015) may enable the plant extract to penetrate the nest cells and degenerate the calcified shield of the muscular larvae. We also believe that the collagenase inhibitory effect of Myrrh extract (Leem, 2016) is the reason for regenerating the infected muscular tissues. More investigations are required to explain the biochemical action mode of Garden cress on the nest cells and the larvae. Therefore, we are currently studying the in vitro effects of Garden Cress and Myrrh on T. spiralis larvae and worms.
In conclusion, ethanol Myrrh extract had promising therapeutic effects on the muscle larvae and nest cells, dominating the Garden cress and Albendazole. However, the combination of Myrrh with Albendazole needs to be considered for further studies to elucidate the optimum and most efficient dose and schedule as anti-trichinellosis.
Novelty Statement
This is the first study to evaluate the anti-trichinellosis effects of Lepidium sativum (garden cress) alone and in combination with the reference drug albendazole
AUTHOR’S CONTRIBUTION
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






