Advances in Animal and Veterinary Sciences
Research Article
Latex Agglutination: A Rapid, Specific Immunoassay for Diagnosis of Ruminant Brucellosis
Cleopatra M. Roushdy2*, Abdel-Moneim M. Moustafa1, Mohamed G. Abdelwahab1, Faysal K. Ibrahim1, Essam M. El-bauomy2
1Faculty of Veterinary Medicine, Benha University, Egypt; 2Animal Health Research Institute, ARC, Dokki, Giza, Egypt. P.O. 12618.
Abstract | Latex agglutination immunoassay is widely applied in diagnosis favored by advances in nanotechnology. This study was designed to investigate an inexpensive approach for producing highly specific protein antigen. Soluble Brucella proteins (SBPs 50) fractions were extracted from reference strain of Brucella abortus (strain 99) by differential precipitation with 50% ammonium sulfate. Analysis of the attained proteins by electrophoresis (SDS-PAGE) resulted in predominant 37.6 kDa protein, a medium-sized 30.6 kDa, and low molecular weight protein of 17.2 kDa when compared with low-molecular-mass marker ranged from (14 kDa to 92 kDa). To sustain as an advanced antigen applied in diagnosis of ruminant brucellosis, covalent coupling of obtained SBPs 50 to carboxylated polystyrene microsphere latex beads (0.81 ± 0.15 µm diameter) was used to assess the potential diagnostic utility of latex beads coated with the obtained SBPs. The developed assay (latex agglutination test, LAT) scored high diagnostic specificity (DSp) in all examined species; cattle, buffaloes, sheep, and goats (92.3%, 99.0%, 94.1%, and 95.9%) respectively. The diagnostic sensitivity (DSe) of LAT recorded (97.2%, 95.0%, 91.7%, and 85.6%) for cattle, buffaloes, sheep and goats correspondingly which were relatively lower than other counterpart conventional screening immunoassays; buffered antigens (BAPA and RBT). Kappa agreement values (ƙ) between latex agglutination test (LAT) and complement fixation test (CFT), were (0.90, 0.95, 0.83 and 0.76) for cattle, buffaloes, sheep and goats correspondingly indicating almost perfect correlation in examined species except in goats which showed substantial correlation. Besides DSe, DSp and ƙ agreement, other diagnostic indicators were assessed. From the results of this study, Latex agglutination test coated with SBPs 50 was proved to be an advisable method for diagnosis of ruminant brucellosis due to its high specificity, simplicity, fast, promptness, easy to interpret technique and low cost.
Keywords | Brucella abortus, Soluble Brucella proteins (SBPs50), Carboxylated polystyrene latex beads, Latex agglut nation immunoassay, Ruminant brucellosis
Received | April 28, 2021; Accepted | May 27, 2021; Published | July 28, 2021
*Correspondence | Cleopatra M. Roushdy, Animal Health Research Institute, ARC, Dokki, Giza, Egypt. P.O. 12618; Email: [email protected]
Citation | Roushdy CM, Moustafa AMM, Abdelwahab MG, Ibrahim FK, El-bauomy EM (2021). Latex agglutination: a rapid, specific immunoassay for diagnosis of ruminant brucellosis. Adv. Anim. Vet. Sci. 9(9): 1292-1301.
DOI | https://dx.doi.org/10.17582/journal.aavs/2021/9.9.1292.1301
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Roushdy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Brucellosis is an infectious disease of several terrestrial and marine animals and humans caused by bacteria of the genus Brucella (Lounes et al., 2021). It is widespread in the Middle East and Mediterranean Basin, as well as in many tropical and subtropical geographical regions (Pappas et al., 2006; Barend et al., 2009). Brucellosis diagnostic tests fall into two categories: direct that demonstrate the presence of organisms and indirect (detect an immune response to their antigens) either humoral or cellular, (Saavedra et al., 2019). There are well established immunoassays used for serological diagnosis of brucellosis, Buffered Brucella antigen tests (BBAT) including RBT [Rose Bengal test] and BPAT [Buffered plate agglutination test]), fluorescence polarization assay (FPA), complement fixation test (CFT), and indirect/competitive enzyme-linked immunosorbent assay (I- or C-ELISA). All of these tests are recommended and prescribed for international trade (OIE, 2009) and must be associated with panel of screening and confirmatory immunoassays in parallel.
For the sake of effective control and eradication strategies of brucellosis in our developing countries, it is extremely important to diagnose it promptly and accurately through improving diagnostic sensitivity of immunoassays in animal and human (OIE, 2016).
Diagnosis of brucellosis is mostly based on serological methods that have used antigens prepared from whole cell preparations, sonicated cell extracts or lipopolysaccharide (LPS) enriched fractions. Because all smooth species share common epitopes in the OPS, virtually all serological tests for an antibody to these bacteria use B. abortus antigen in the form of whole cells, SLPS or O-polysaccharide (OIE, 2009). By virtue of the cross reaction from other Gram-negative bacteria that have similar structures of sLPS, false positive reactions (FPRs) are generated, (Pajuaba et al., 2009). As specificity of these tests are low, alternative antigens have been characterized as potentially useful tools in diagnostic tests for brucellosis.
Seeking for new non-LPS antigen, a variety of proteins can act as antigens during brucellosis infection. Some soluble proteins such as 26-kDa cytosoluble protein (BP26), also known as (CP28), and lumazine synthase (18 kD protein) have been identified as an immunodominant antigens in infected cattle, sheep, goats, and humans. (Cloeckaert et al., 2001; Xin et al., 2013). BP26 protein and also cytoplasmic proteins, appear to be a good diagnostic antigen could be utilized in confirmatory tests (Cloeckaert et al., 2001; Gupta et al., 2007). Moreover many Brucella outer membrane proteins (OMPs) have been widely recognized and extensively characterized as potential immunogenic and protective antigens (Cassataro et al., 2005). Both of periplasmic and cytoplasmic antigens comprise water-soluble proteins (Ducrotoy et al., 2016).
Since 1956, polystyrene latex microspheres are widely used as solid support for biomolecules, mainly proteins, for immunologically-based assays in disease diagnosis and bacterial typing. (Marrero et al., 2016; Ortika et al., 2013). Polymeric particles as polystyrene microspheres conveniently sensitized with protein constitute a desirable immuno-reagent owing to their high specificity to promote agglutination reaction. These particles are referred to as immuno-microspheres (Rembaum et al., 1979). Microspheres may react by covalent binding with antigens or antibodies and have been extensively used in immunoassay technique (Covolan et al., 1997).
In principle, latex agglutination test means agglutination reactions between antigen and antibody via coating on polystyrene beads; as polymer colloids are used as carriers for antibodies or antigens. LAT may be ideal as it requires a minimum of expertise to perform and to read, does not depend on a cold chain for transportation, storage and does not require expensive equipment (Sheik-Mohamed and Velema, 1999).
Therefore, the study aimed to extract well identified immuno-dominant soluble Brucella proteins (SBPs 50) from B. abortus S99. Characterization of the gained proteins and covalent coupling of the extracted protein on blue carboxylated polystyrene microsphere latex beads as an antigen to be used in a well-developed latex agglutination assay (LAT). Finally evaluation of diagnostic performance of the newly developed LAT immunoassay in infected cattle, buffaloes, sheep and goats in parallel with other serological tests taking CFT as quantitative in lieu gold standard.
Material And Methods
Reagents and materials
All chemicals used in this study were of analytical grade. They were used without further purification. Sterile double distilled and deionized water was used in all solutions and buffers of prepared latex antigen. Trypticase soy broth, normal saline (0.85%), 5% saline, ammonium sulphate (50%), Coomassie brilliant blue G-250 and R-250 (Merck, Germany), bovine serum albumin (BSA, Sigma, USA) , sodium dodecyl sulfate, polyacrylamide (5% - 12.5%) 2-mercaptoethanol, TEMED (tetramethylethylenediamine), ammonium persulphate, glycerol, MES buffer (2-N-Morpholino-ethanesulfonic acid, Sigma, USA) Mol. wt. (195.24), 0.025 M, pH 6, glycine, sodium azide (NaN3), Phosphate buffer saline (PBS) , pH 7.3 and Tween 20. Blue carboxylated polystyrene latex beads (CAB800NM) 10% solids was purchased from Magsphere.INC., USA. Low -molecular-weight standard marker (14-94 kDa, Sigma, USA).
Bacterial strain: B. abortus strain 99 (NCTC Number 11363) was obtained from the culture collection of the Department of Brucellosis Research, Animal Health Research Institute, Egypt. The original seed was supplied by the Animal and Plant Health Agency (formerly, Central Veterinary Laboratory) Weybridge, UK.
Sampling and sample size: Six hundred and forty serum samples were taken kindly from Department of Brucellosis (Animal Health Research Institute, Egypt) randomly representing different governorates of Egypt (upper and lower Egypt) and including both sexes at different ages. A total of 160 sample of each species (cattle, buffaloes, sheep and goats) were selected. Sampled animals have no history of vaccination against brucellosis. Coded identified samples and control (positive and negative) samples were kept at -20oC till examination. Sample size was calculated online depending on prevalence of Brucellosis using Epitools Epitools-Epidemiological Calculators (Sergeant, 2018).
Diagnostic serological tests:Evaluation of new antigen encompass latex beads coated with SBPs 50 in Latex agglutination test (LAT) in comparison with other validated well established screening tests BAPA, RBT 8% and modified RBT (m RB 8%) for small ruminants were done. CFT confirmatory test was used as a gold standard reference immunoassay (Yohannes et al., 2012).
Buffered acidified plate antigen test (BAPA) was performed as Angus and Barton (1984), and m RB 8% in small ruminants was done according to Blasco et al. (1994). While RBT in large ruminants (8% cell) and CFT were performed as described by (Alton et al., 1988).
Antigens for these immunoassays were obtained from the (Veterinary Serum and Vaccine Research Institute, Abassia, Egypt), while antigen of CFT was imported from the APHA Scientific (formally AHVLA Scientific). Both hemolysin and complement prepared in laboratory (Department of Brucellosis, AHRI, Egypt) and results of CFT were converted to ICFTU/ml and interpreted as positive at > 20 ICFTU/ml according to the European Economic Community (EEC). Results of qualitative tests (BAPA and RBT) were recorded as scores of 1+ to 4+ according to the agglutination degree.
Extraction of soluble Brucella proteins (SBPs): As previously described by Zhan, (1993) harvested viable B. abortus strain 99 (5 ml of suspension of 1010 /ml) were added to 800 ml of sterile Trypticase soy broth and incubated at 37 o C on shaker incubator for 48 hours. The temperature modulated to 66 o C for one hour to kill bacteria, then washing the harvested bacteria once with saline. Hot saline extracts were obtained by suspending organisms in saline and autoclaved at 121o C for 20 minutes. Centrifugation of the autoclaved suspension at 12000 xg for 15 min, and the supernatant was collected and precipitated with ammonium sulphate (50% saturation). After centrifugation of the precipitant at 8000 xg for 15 min, the resultant pellets was dissolved in (0.01 M) PBS and dialyzed against PBS for 48 hours, this preparation was named SBPs50. Finally, the protein was centrifuged at 12000 xg and lyophilized.
Biochemical analysis of antigen: Quantification of protein before and after coating using Bradford assay (1976); a protein determination method which involves the binding of Coomassie brilliant blue (G-250) to protein and compared with standard curve of bovine serum albumin as a standard. The residual protein after coating with latex, was measured at A595 nm using Nanodrop ND-1000 (full UV-visible spectrum, 220-1000 nm) spectrophotometer which measure 1ul sample added on the end of fiber optic cable with high accuracy (SPECTRO star Nano -BMG Labtech).
Characterization of soluble Brucella proteins by using one-dimensional sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) protocol by (Sambrook and Russell, 2001) for determination of different pattern bands in SBPs 50. The stacking and separating gels for minigel consisted of 5 and 12.5% acrylamide, respectively. Samples were heated at 100°C for 90 seconds in 0.05 M Tris buffer (pH 6.5) containing 2% SDS, 10% 2-mercaptoethanol, and 10% glycerol. The gel were run in vertical slab gel apparatus at 15 V/cm for 3 hours and were visualized after being stained with Coomassie brilliant blue (R-250). The molecular weight of peptides were determined by comparing their relative mobility with that of low-molecular-weight standard marker (14-94 kDa). A computerized analysis of protein patterns was completed by using Gene Analyzer (SynGene, GeneTools).
Sensitization of latex microsphere: Super active blue carboxyl-modified polystyrene latex beads (10% solids) was cleaned by addition of sterile deionized water (1:4) to remove impurities and surfactant. Centrifugation at 6000 xg for 10 min and the deionized water was discarded.
Coating of soluble Brucella proteins (SBPs 50) antigen on latex beads: Covalent coupling of the proteins to carboxylated latex leads started with addition of 1ml (40 mg/ml) to 10 ml MES buffer (pH 6) then vortexed. The mixture was centrifuged at 6,000 xg for 10 min to sediment the particles, the supernatant formed was removed and the pellet dispersed in 10 ml MES buffer twice. Finally the pellet was suspended in 5 ml MES buffer to get 2% solids (20 mg/ml) for labeling beads with the calculated amount of the protein (600 ug/ml). Using a dilute microsphere suspension (≤1% solids) to ensure coating particles singly, so clumping during coating will be less likely. Equations described in the Bangs Laboratories protocols for adsorption to microspheres were used (Bangs laboratories Inc., 2018).
The latex/protein mixture was incubated overnight with gentle mixing at room temperature. In the next day, the protein-labeled latex beads were centrifuged to separate particles from unbound protein. Then performing blocking step to fill unoccupied sites with protein, by using PBS
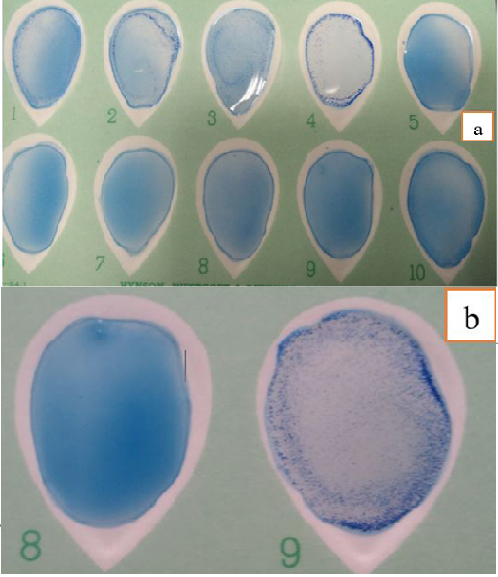
Figure 1: Different Degrees of Agglutination in Latex Agglutination Test using new SBPs Compared to Control Negative and Positive Sera (a) and (b).
and BSA 1 % with shaking for 30 min. at room temperature. The pellet was suspended in 10 ml washing buffer (PBS), and centrifuged at 6000 xg for 10 min to sediment the particles twice more for a total of 3 washes. Later on, coated latex beads were suspended in 10 ml storage buffer GBS-BSA (0.1% glycine, 1% BSA, 0.1% NaN3 in PBS) to a final concentration of 1% solids. Tween 20 (0.01%), a non-ionic surfactant was added to storage buffer (Passive adsorption protocol, n.d.). All reagents were stored at 4-8°C until used without freezing them. Hence, the concentration of adsorbed protein was estimated indirectly as the difference
between the initial total protein and the concentration of unbound.
Performance of agglutination assay: It was performed by addition of equal volumes (30 µl) of the sensitized latex antigen and sera sample on white card, mixed to be homogeneous by using a disposable plastic applicator with tilting motion gently rocked for 8 minutes. Finally agglutination was determined by direct visual examination macroscopically with unaided eye and expressed qualitatively as “+” (agglutination) or “-” (no agglutination) with uppermost illumination. A positive reaction was graded on a scale of + to 4+ on the basis of degree of agglutination present in relation to control serum samples (Figure 1 a and b).
Pretreatment of test sample to enhance the agglutination: Several dilutions of examined serum samples by addition of saline (5%) or normal saline to determine the effect of each dilution on agglutination reaction of LAT, and suitable dilution of serum in different species was applied in LAT.
Statistical analysis for validation of LAT: All the following analyses were performed using SPSS (Version 20, IBM), following the validation guideline described in (OIE, 2013).
Table 1: Electrophoretic Profile of SBPs 50 by SDS-PAGE: (Analysis by SynGene, GeneTools).
| Quantity calibration | Low molecular weight Marker | (S B Ps 50) | |
| Peak | Assigned MW (kDa) | Bands | Apparent Mol. weight (kDa) |
| 1 | 94 | 1 |
37.618 |
| 2 | 67 | 2 | 30.626 |
| 3 | 43 | 3 |
17.288 |
| 4 | 30 | ||
| 5 |
20.1 |
||
| 6 | 14 | ||
Performance Indicators of Serological Tests: These included the calculation of diagnostic sensitivity (DSe), diagnostic specificity (DSp) with a 95% confidence interval (CI), PPV (positive predictive value), NPV (negative predictive value), LR+ (likelihood ratio of a positive result), and LR- (likelihood ratio of negative result). Validation was measured according to (Greiner et al., 2000).
Estimation of area under the receiver operating characteristic (ROC) curve (AUC): Data were obtained from ROC curve was AUC which is a useful adjunct to DSe and DSp estimates for a quantitative diagnostic test (Greiner et al., 2000; Zweig and Campbell, 1993).
Kappa (ƙ) Agreement: Estimation of Kappa agreement (ƙ) between LAT and CFT in lieu the gold standard in different ruminant species was used to judge the matching of results at P< 0.05.
RESULTS AND DISCUSSION
Immunochemical analysis of sbps 50 antigen and determination of concentration of protein antigen before and after coating
Analysis of peptides bands of SBPs 50 by SDS-PAGE (12.5%) in Table 1 reveals three distinctive peptide bands with apparent molecular weights 37.6, 30.6 and 17.2 kDa when compared with low molecular weight marker ranging from 14 to 94 kDa.
In our experiment, concentration of the attained protein was 1.7 mg /ml using Bradford assay. Suitable amount of adsorbed protein to microsphere was calculated, and 10x excess protein was added. We found that half of the added protein remained unabsorbed after coating.
Diagnostic performance of serological immunoassays
A total of 640 serum samples were tested by BAPA, RBT, CFT and LAT using protein antigen SBPs 50 to judge the efficacy of the new antigen. Statistical evaluation of the obtained results was illustrated in (Table 2 and 3) for both large and small ruminants. Using two-by-two contingency table with groups of subjects divided according to a gold standard in rows and the used examined test in columns. In large ruminants (Table 2), LAT attained lower
Table 2: Diagnostic Performance of LAT in Relation to other Serological Immunoassays used in Large Ruminant.
| Parameter | Cattle | Buffaloes | ||||||||||||
| BAPA | RBT | LAT | BAPA | RBT | LAT | |||||||||
| T+ | T- | T+ | T- | T+ | T- | T+ | T- | T+ | T- | T+ | T- | |||
| D+ 108 |
107 a |
1 |
106 a |
2 |
105 a |
3 | D+ 60 |
59 a |
1 |
59 a |
1 |
57 a |
3 | |
| D- 52 | 9 |
43 b |
7 |
45 b |
4 |
48 b |
D- 100 | 5 |
95 b |
2 |
98 b |
1 |
99 b |
|
| DSe | 99.1% | 98.1% | 97.2% | 98.3% | 98.3% | 95% | ||||||||
| DSp | 82.7% | 86.5% | 92.3% | 95.0% | 98.0% | 99% | ||||||||
| PPV | 92.1 | 93 | 96.3 | 92.1 | 96.7 | 98.2 | ||||||||
| NPV | 98.9 | 95.7 | 94.1 | 98.9 | 98.9 | 97 | ||||||||
| LR+ | 5.7 | 7.3 | 12.6 | 19.6 | 49.2 | 95 | ||||||||
| LR- | 0.01 | 0.02 | 0.03 | 0.02 | 0.02 | 0.05 | ||||||||
| AUC | 0.91 | 0.92 | 0.95 | 0.97 | 0.98 | 0.97 | ||||||||
Where; D+: Positive CFT; D-: Negative CFT; a: True positive (TP); b: True negative (TN); T+: Test positive; T-: Test negative; FN: (D+&T-); FP: (D-&T+); DSe: diagnostic sensitivity (True positive rate) = TP/(TP+FN); DSp: diagnostic specificity (True negative rate) =TN/(TN+FP); PPV= TP/(TP+FP) proportion of diseased among subjects with a positive test result; NPV= TN/(TN+FN) proportion of non-diseased among subjects with a negative test. LR+ = sensitivity/(1-Sp) (the probability of an animal who has the disease testing positive divided by the probability of an animal who does not have the disease testing positive). LR- = (1-Se )/ specificity (the probability of an animal who has the disease testing negative divided by the probability of an animal who does not have the disease testing negative). Interpretation of LR- : <0.1 (very useful test), 0.1-0.2 (often useful test), 0.21-0.5 (sometimes useful tests) and 0.51-1 (rarely useful test). If the results in LR+ were >10 (very useful test), 5-10 (often useful test), 2-4.9 (sometimes useful tests) and 1-1.9 (rarely useful test). AUC: Area under the ROC curve.
Table 3: Diagnostic Performance of LAT in Relation to Other Serological Immunoassays used in Small Ruminant.
|
Parameter |
Sheep | Goats | |||||||||||
| BAPA | mRBT | LAT | BAPA | mRBT | LAT | ||||||||
| T+ | T- | T+ | T- | T+ | T- | T+ | T- | T+ | T- | T+ | T- | ||
| D+ 109 |
107 a |
2 |
106 a |
3 |
100 a |
9 | D+ 111 |
110 a |
1 |
109 a |
2 |
95 a |
16 |
| D- 51 | 8 |
43 b |
7 |
44 b |
3 |
48 b |
D- 49 | 9 |
40 b |
8 |
41 b |
2 |
47 b |
| Sensitivity | 98.2% | 97.2% | 91.7% | 99.1% | 98.2% | 85.6% | |||||||
| Specificity | 84.3% | 86.3% | 94.1% | 81.6% | 83.7% | 95.9 % | |||||||
| PPV | 93 | 93.8 | 97 | 92.4 | 93.1 | 97.9 | |||||||
| NPV | 95.5 | 93.6 | 84.2 | 97.5 | 95.3 | 74.6 | |||||||
| LR+ | 6.25 | 7.09 | 15.5 | 5.3 | 6 | 20.8 | |||||||
| LR- | 0.02 | 0.03 | 0.08 | 0.01 | 0.02 | 0.15 | |||||||
| AUC | 0.91 | 0.92 | 0.93 | 0.90 | 0.91 | 0.91 | |||||||
Table 4: Agreement Between CFT and Latex Agglutination Test using SBPs Antigen in Examined Sera of Ruminant Species.
| Animal species | CFT versus LAT with protein | |||
| Both tests negative | Both tests positive | Disagreement | Kappa Agreement | |
| Cattle | 48 (30%) | 105 (65.6%) | 7( 4.4% ) | 0.90±0.037 (almost perfect) |
| Buffaloes | 99 (61.9%) | 57 (35.6%) | 4 (2.5% ) | 0.95±0.026 (almost perfect) |
| Sheep | 48 (30%) | 100 (62.5%) | 12 (7.5% ) | 0.83±0.046 (almost perfect) |
| Goats | 47 (29.3%) | 95 (59.4%) | 18 (11.3%) | 0.76±0.053 (substantial) |
DSe than all other used comparative tests. While DSp of LAT was one of the highest values. Results obtained in small ruminants (Table 3) declared higher DSp and lower DSe in comparison to BAPA and mRBT. The obtained DSe values in cattle were (99.1, 98.1 and 97.2%), in buffaloes (98.3, 98.3 and 95 %), in sheep (98.2, 97.2 and 91.7%), and in goats (99.1, 98.2 and 85.6%) respectively. While our upshots of DSp were (82.7, 86.5, and 92.3%), (95, 98 and 99%), (84.3, 86.3 and 94.1), (81.6, 83.7 and 95.9 %) for BAPA, BCT and LAT in cattle, buffaloes, sheep and goats correspondingly.
In regards to predictive values; positive (PPV) and negative (NPV); LAT was of the highest PPV and the lowest NPV among all species. Estimated PPV and NPV of LAT were (96.3, 94.1%), (98.2, 97%), (97, 84.2%) (97.9, 74.6%) in cattle, buffaloes, sheep and goats respectively. Respecting, likelihood ratio both positive (LR+) and negative (LR-), LAT remains the highest value in LR+ and the lowest in (LR-) in Tables 2 and 3.
In calculation of area under ROC curve (AUC), LAT with SBPs 50 recorded higher values than BAPA and RBT in cattle (0.95) and sheep (0.93). While attained values were similar to BAPA in buffaloes (0.97) and to RBT in goats (0.91) as shown in Tables 2 and 3.
Matching Kappa agreement values (κ) between LAT and CFT (the reference standard) and interpretation of values was tabulated in Table 4. Values of κ were (0.9, 0.95, 0.83 and 0.76) in cattle, buffaloes, sheep and goats respectively.
Serological diagnosis of Brucellosis has advanced considerably in the last decades with very rapid screening sensitive and highly specific confirmatory tests (Nielsen, 2002). Most of the used confirmatory tests are expensive, complicated and not cost-effective for routine use in developing countries. So seeking of an ideal diagnostic test that fulfil high degree of validation concerning sensitivity and specificity, simplicity, quickness, in-expensiveness, repeatability and applicability to` a large number of individuals is the target (Nielsen et al., 2008). Hence, the development of specific antigens non- LPS group have been required to be a possible strategy for minimal or no-cross reaction in the diagnosis of brucellosis (Ko et al., 2012). Identification of immunogenic proteins is a step forward to understand the humoral immune response during Brucella infection (Xin et al., 2013). The use of a multiprotein diagnostic reagent in serological tests may open up new prospects for the improvement of serological diagnosis of brucellosis. (Bulashev et al., 2019).
By extraction of SBPs 50 (Zhan et al., 1993) using a common and inexpensive antichaotropic salts such as ammonium sulphate, which is common used for large scale precipitations. It can be used to precipitate proteins from the impure mixture through alteration the physicohemical properties of the protein causing it to fall out of solution.
In current experiment, we found that SBPs 50 extracted from B. abortus S99 acquired antigenic criteria for detecting humoral immunity of diseased animals. Since the use of subsurface periplasmic protein (PPP) antigen of the same strain of Brucella in (IELISA-PPP) demonstrate higher figure of specificity (91.6%) of this immunoassay rather than (IELISA-LPS) that recorded (89.5%) (Abdel Hamid et al., 2012). As predicted, the high specificity of this antigen has proven to be away from surface LPS antigen incriminated for non-specific reaction. This result in concordance with other relative studies on SBPs of B. melitensis (Ismael et al., 2016).
To gain an objective specification of the apparent molecular weights of extracted proteins, SDS-PAGE was performed. Three distinctive bands of proteins (37.6 kDa, 30.6 kDa, and 17.2 kDa) were determined, as first step to calculate the amount of representative protein required in coating to achieve optimum surface saturation.
As commercial latex particles supplied in medium containing detergents, removal of impurities and surfactant is necessary prior coating so washing step by deionized water was applied. Using MES buffer in sensitization of latex to increase the protein density on the particle surface, it is known as a Good’s buffer, active in the pH range 5.5-6.7.
Sensitization of latex microsphere to develop protein antigen of LAT was by selecting suitable latex beads size (0.81 um) for coating to achieve universal distribution of antigen on latex. In respect to a particle size of 0.8 µm, about 105 latex particles is required to make one visible aggregate, and about 107 particles is needed to determine agglutination in a given test (Molina -Bolivar and Galisteo-Gonzalez, 2005). The microsphere beads of around 0.8 µm are the most frequently used in agglutination tests (Gella et al., 1991). The most important parameter influencing the production of a visible test is the concentration of added protein to get the optimum saturation of surface. About 23-40% of total protein added bound to carboxylate latex particles in best suitable concentration (Inzana, 1995). Following the recommendation by adding a 3X–10X excess of protein to achieve a monolayer distribution of protein over the microspheres (Bangs laboratories Inc., 2013), 10X excess of the calculated protein was added. To overcome the non-specific reaction which may develop during preparation of antigen, 0.1% BSA and 0.01%Tween-20 were used in storage buffer.
Another essential parameter in LAT is the pretreatment of serum samples, we found that dilution of serum samples by addition of saline (5%) enhance the agglutination reaction rather than normal saline especially for buffaloes and goats. The dilution factor to optimize the agglutination varied according to the examined species from 1:3 to 1:8. Highest dilution was used in buffaloes and goats samples. As dilution of serum reduce nonspecific interferences in LAT (Molina -Bolivar and Galisteo-Gonzalez, 2005). And also latex agglutination tests are subjected to a “prozone phenomena “wherein excess antibody over coat binding sites. Generating a falsely negative result and inhibition of agglutination if serum is not adequately diluted (Peaper and Landry, 2014; Sykes and Rankin, 2014) .Using of a 5.0% solution of sodium chloride as a serum diluent in agglutination tests reduced or eliminated the prozone effect (Gallenson, 1946).
Validation includes estimates of the analytical and diagnostic performance characteristics of a test (OIE, 2013). Diagnostic performance of applied immunoassays in this work is mentioned in Table 2 and 3. DSp relates to the aspect of diagnostic accuracy that describes the test ability to recognize healthy animals. As anticipated, higher values of DSp were measured by LAT in all examined species owing to its specific protein antigen (SBPs). In this study, we developed protein antigen to circumvent drawback of LPS and whole antigen which mainly causes false positive reactions (Pajuaba et al., 2009). Those are attributed to Gram-negative microorganisms sharing the LPS of cell wall such as Y. enterocolitica O:9, V. cholerae, S. typhimurium, and E. coli O157 (Nielsen et al., 2004)
Perhaps one of the most striking point of validation of novel antigen in LAT is diagnostic sensitivity (DSe) which is the probability of getting a positive test result in diseased animal. Our prepared antigen scored lower values of sensitivity than other comparable screening tests in detection of diseased animals. Otherwise, the highest recorded values were in cattle and the lowest DSe estimated in goats even after dilution of serum samples with 5% saline by a dilution factor 1/8. Our results are in agreement with that of Abdoel and Smits (2007) who recorded sensitivity (88.9%) and specificity (98.2%) of LAT although they used LPS coated latex in diagnosis of brucellosis in human. Also, Ismael et al. (2016) recorded DSe (99.33%) and DSp (99.88%) in ovine brucellosis using hot saline extract of soluble periplasmic proteins SBPPs of B. melitensis in LAT. Similar results of higher specificity and sensitivity in LAT were estimated in clinically diseased cattle with Brucellosis using latex that chemical-linked with B. melitensis 16M and extracted with 5% NaCl (Lu et al., 1995). While latex agglutination coated with recombinant outer membrane protein (rOMP28) of B. abortus by (Lim et al., 2012) revealed lower values of sensitivity (77%) and specificity (80.6%) for bovine brucellosis.
Regarding PPV which is an expression of true positive status of animal, whereas NPV is the probability of negative diagnosis when the test is negative (true negative). The PPV of LAT was higher than BAPA, RBT and mRBT in all examined species. The highest values of PPV indicate high diagnostic efficacy of LAT in detection of the true positive status of diseased animals. That gives LAT a superior advantage as a confirmatory tool in diagnosis of brucellosis. On the other hand, the lowest value of NPV in LAT indicates adequate detection of negative cases. Once again the obtained results are in concordance with previous results of Ismael et al. (2016) who found high PPV (98.68) and no significant difference in attained NPV (99.94) between other examined serological tests in ovine brucellosis.
Likelihood ratios are alternative statistics for summarizing diagnostic accuracy, which have several particularly powerful properties that make them more useful clinically than other statistics (Sackett et al., 2000). Good diagnostic tests have LR+ > 10 and their positive result has a significant contribution to the diagnosis and the bigger the number, the more convincingly the disease suggested. New immunoassay LAT resulted higher values of LR+ (very useful test) in comparison to other conventional screening tests in all examined ruminant samples. Contemplated good diagnostic tests have LR- < 0.1 (very useful test). In respect to our study, we found that LR- < 0.1 in all applied immunoassays including LAT in all species, unfortunately in goats LAT rated 0.15 (often useful test).
Furthermore, a complete description of accuracy is given by the area under the ROC curve (AUC) which is a global measure of diagnostic accuracy (indicator of the goodness of the test). The greater the AUC, the more discriminatory test that correctly classify those with and without the disease. The test of AUC 1.00 is a perfectly discriminatory test (Zweig and Campbell, 1993). At first glance to the abovementioned results in Table 2 and 3, it is noticed that LAT is a perfect discriminatory test in diagnosis of ruminant brucellosis. By reason of recorded AUC values of LAT (0.95, 0.97, 0.93 and 0.91) were > comparable values of AUC in screening tests. While estimated accuracy (true positive plus true negative divided by total number of animals) of LAT using rOMP28 in bovine was 78.5% (Lim et al., 2012)
Regarding to Kappa agreement values (κ) between LAT and CFT (reference standard) in lieu, and interpretation according to Landis and Koch (1977) was quantified in (Table 4). Results reflecting highest (κ) values in the range of (0.81–0.99) declared that LAT is almost perfect immunoassay in cattle, buffaloes and sheep otherwise in goats it is substantial test as κ value was 0.76±0.053 which lie in the range of (0.61–0.80).
From the aforementioned results and data obtained in this study, it is advisable to promote the diagnosis of brucellosis by LAT using new protein antigen owing to its high specificity and commercial potential as a low-cost alternative method for serological diagnosis of ruminant brucellosis.
CONCLUSION
We developed and evaluated a sensitive, highly specific and visually interpretable latex agglutination assay with SBPs 50 antigen for the qualitative diagnosis of ruminant brucellosis. Covalent attachment of carboxyl-functionalized microspheres latex beads is permanent, thus increasing shelf life of antigen. In-house development of LAT antigen would be time effective, stable, simple, needs no special equipment and require no technical skill. Worthy of note, it is portable, rapid, efficient, and useful under even the most primitive conditions.
ACKNOWLEDGEMENT
We are most grateful to all members of Brucella department (AHRI) for their kind support. And also express our great thanks to D. Mogeda K. Mansour (head of Electrophoresis unit) Biochemistry department (AHRI) for her kind help and contribution of Biochemical analysis of antigen. Our deep gratitude and sincere appreciation to D. Asharf Sayour (Senior Researcher, AHRI) for his guidance and unlimited help.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHORS CONTRIBUTION
Cleopatra M. Roushdy: Methodology ,Investigation , Formal analysis, Reviewing and Editing. Abdel-Moneim M. Moustafa: Conceptualization, Supervision, Reviewing and Editing. Mohamed G. Abdelwahab: Reviewing and Editing. Faysal K. Ibrahim: Supervision, Reviewing and Editing. Essam M. El-bauomy: Methodology, Writing–original draft, Validation, Visualization, Reviewing and Editing.
REFERENCES






