Advances in Animal and Veterinary Sciences
Research Article
Anatomical and Radiographical Studies on Heart of Red Fox (Vulpes vulpes) with Special References to Its Coronary Arteries
Samah H El-Bably, Yara S. Abouelela*
Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt.
Abstract | The present paper considered as a descriptive study applied on the heart of the red fox (Vulpes vulpes) as one of the wild animals, as well as we deal with clarifying valves, chambers, and coronary arteries of the heart in addition to coronary artery radiography. five red foxes of either sex with an average weight of 8-10 kg were selected for this study. The animals were scarified by sodium thiopental injection. Two animals were used for anatomical description as well as for sagittal and cross-section study. The other three animals were used for studying both arterial architectures and for radiographic imaging. The heart of the red fox fixed by the presence of the pericardiophrenic ligament in place of the pericardiosternal one and it was composed of four main champers as in all vertebrates while its internal features carrying some different aspects. The left ventricle was large, long enough in comparison to the right one as well as their papillary muscles gave special appearance unlike other mammals. The left coronary artery was the dominant branch supplying the cardiac muscles. Otherwise, the interventricular infrasinuosal artery emanates from the left circumflex artery instead of the right coronary artery like other higher vertebrates, while the right coronary artery was small and gave off small ramifications on the right ventricular wall.
Keywords | Heart, Red Fox, Coronary Arteries, Cardiac Valves, Radiography.
Received | February 02, 2021; Accepted | February 11, 2021; Published | March 30, 2021
*Correspondence | Yara S. Abouelela, Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt; Email: [email protected]
Citation | El-Bably SH, Abouelela YS (2021). Anatomical and radiographical studies on heart of red fox (vulpes vulpes) with special references to its coronary arteries. Adv. Anim. Vet. Sci. 9(5): 754-760.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.754.760
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 El-Bably and Abouelela. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Anatomy Continues as quite probably one of the most valuable branches of medicine. Over recent years, the anatomical dissection on the heart of different animals was increased (Perez and Lima, 2007). The presence of the descriptive anatomical genetic mutability within the different species allows the creation of complete different adaptations (Paredes and Martínez, 2019), which confirmed by using modern techniques as radiography (Jepsen-Grant et al., 2013) that helps in the surgical approaches, clinical practices as well as diagnosis of many diseases or abnormal cases (Hill and Iaizzo 2005).
One of the most vital aspects in heart dissection is the pattern of the coronary vessels (Oliveira et al., 2010) in a dog which essential to insight the pathology of coronary diseases (Genain et al., 2018) in pig, sheep and dog, and interpretation of imaging of coronary artery (Yatesa and Zamvarb, 2014). Coronary vessels have been discussed in detail in goat (Barszcz et al., 2019), and in dog (Oliveira et al., 2010; Chirilean, 2015). On the other hand, the anatomy of cardiac valves has great clinical importance so considered in detail through different mammals (Hutchison and Rea, 2015), in the heart of the ostrich (Alsafy et al, 2009), in ovine (Mathur et al., 2019) and in dog tricuspid valve (Paredes and Martínez, 2019). In addition to surgical modification of the mitral valve regurgitation in animals (Goetzenich et al., 2010) as well as the tricuspid valve in pig (Ross et al., 2020b) as a model in human medicine (Misfeld and Sievers, 2007; Ross et al., 2020 c).
The accessible literature on the heart anatomy of the fox is scanty, so further information on its anatomical features is required. In the present work, we intended to describe the anatomical details of the heart chambers as well as both atrioventricular cardiac valves by sagittal and transverse sections, in addition to angio-architures of the coronary arteries and its distribution over the cardiac chambers.
Materials and Methods
Animal Ethics
Animals used for the current study and euthanasia producers were ethically approved by Cairo University institutional animal care and use committee (CU-IACUC) with number (Vet CU24112020253). In the present study five healthy adult red fox breed with average weight 8-10kg and aged 3-4 years of either sex obtained from the Western Egyptian desert were used.
Anatomical inspection
All the red fox animals were euthanized by anesthetizing them with ketamine (20 mg/Kg body weight /IM) mixed with (1 mg/Kg body weight) of xylazine. Once relaxation of muscles occurred, they were sacrificed with intravenous injection of 2.5% sodium thiopental injection (60 mg/Kg body weight).
The five sacrificed red foxes were subjected to manual dissection in order to open the thorax cavity for exposing the heart. Two of these specimens were examined for external features and dissected by making sagittal sections to inspects the right and left atrioventricular cavities and its valves and measure its width using Vernier Caliber as well as four cross sections at different levels 0.5cm between the first two crosses and 1cm between the other two to show the thickness of the ventricular walls and their length, while the other three specimens were used for vascular architectures and radiographic imaging techniques.
Vascular architectures of coronary arteries
Two sacrificed red foxes were used for dissection of coronary arteries. Thoracic aorta cannulated and rinsed with warm saline solution NaCl 0.9% to eliminate any blood clotting from the arteries. Thoracic aorta was then infused with prepared red latex (red rotring ink combined with white latex neoprene) and then kept as such for 3-5 days in 10% formalin solution at room temperature till hardening (Aril et al., 2011). The heart was dissected to reveal the coronary arteries and their distribution (Chirilean, 2015). The photographs of coronary arteries were manipulated by photoshop program cc x64 to colour the veins with blue. Specimens photographs were snapped by digital camera. All nomenclatures are based on Nomina Anatomica Veterinaria (2017).
Radiographic images
One heart specimen was used for contrast X-ray images. The specimen was injected with latex neoprene mixed with lead oxide into both coronary ostium (left and right). The images were taken using the radiography machine. (Fisher imaging, Chicago, USA).
Results
Heart Chambers
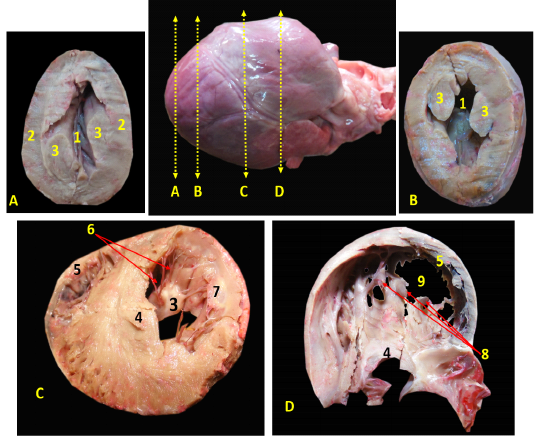
The heart of red fox was located within the thoracic cavity extended from the fourth to sixth intercostal space, caudoventral directed towards the diaphragm (Fig. 1/A). The heart was enclosed within the pericardium which extended to form the pericardiophrenic ligament between the apex of the heart and the diaphragm (Fig. 1/A). The pericardium carried the phrenic nerve which passed over the left atrium of the heart (Fig. 1/B).
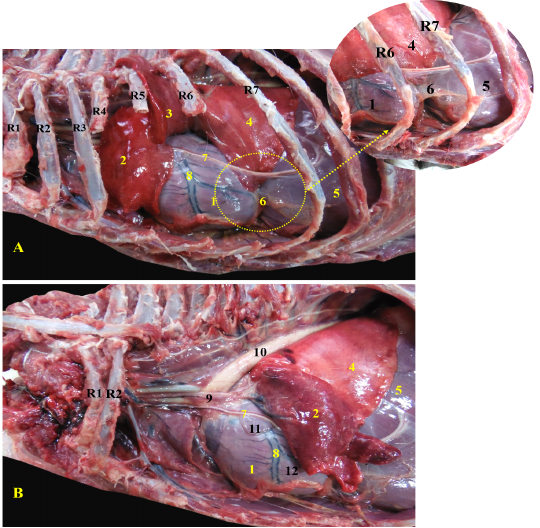
Figure 1: Showing the heart of red fox insitu A- showing the heart insitu after rib reflection, B - showing the heart after reflection of the left cranial lobe of lung.
R= Rib, 1-Heart, 2- left cranial lobe of lung (Cr part), 3- left cranial lobe of lung (Cd part), 4- left caudal lobe of lung, 5- diaphragm, 6- pericardiophrenic ligament, 7- phrenic nerve, 8- left interventricular artery, 9-ascending aorta, 10- descending aorta, 11- left atrium, 12- left ventricle
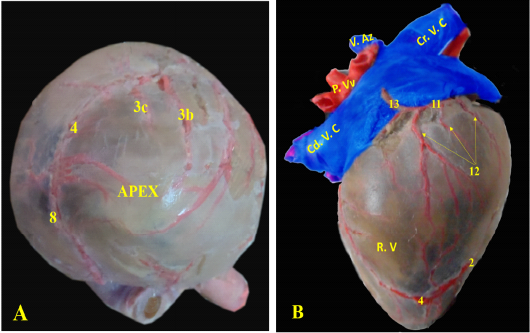
The heart of the red fox is composed of four Chambers; two ventricles, the right one faces cranially and the left one faces caudally toward the diaphragm (Fig. 2/A). Both ventricles were separated by the interventricular septum. The interatrial septum separated the two atria (Fig. 2/ B, C).
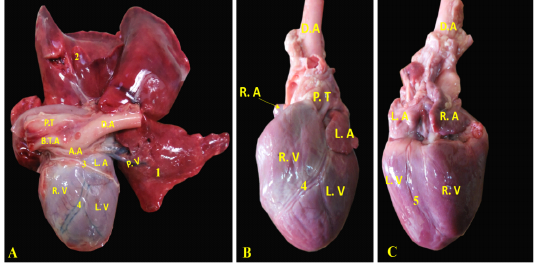
Figure 2: Showing the separated heart of red fox. A- showing the heart and lung separate with pericardium, B- showing the left side of the heart without pericardium, C- showing the right side of the heart without pericardium.
RA= Right atrium, RV= right ventricle, LA= left atrium, LV= left ventricle, AA- ascending Aorta, PT= pulmonary trunk, PVv= pulmonary vein, B.T.A = brachiocephalic trunk, DA= descending aorta.
1- Left cranial lobe of lung, 2- Right lung, 3- phrenic nerve, 4- interventricular paraconal artery,
5- interventricular infrasinuosal artery
Ventricular walls were thicker than the atrial walls (Fig. 3/A)., The right ventricular wall was 0.3- 0.5 cm thick (Fig. 3/B, C) while the left ventricle wall was 0.6-0.8 cm thick (Fig. 3/B, C). The thickness of interventricular septum was 0.7- 0.9 cm (Fig. 3/B, C). In contrary, the atrial walls were very thin (Fig. 3/C).
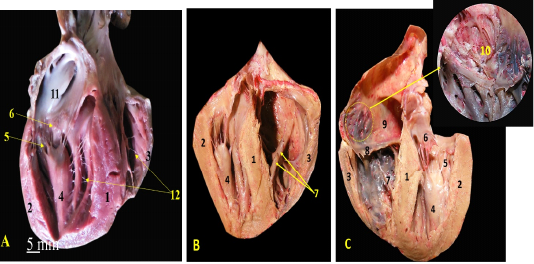
Figure 3: A, B, C showing the sagittal sections of the heart of red fox. With scale bar= 5mm, 1-Interventricular septum, 2- wall of left ventricle, 3- wall of right ventricle, 4- papillary muscle of left ventricle, 5- chorda tendineae of mitral valve, 6- valvular leaflet of mitral valve, 7- papillary muscle of right ventricle, 8- valvular leaflet of tricuspid valve, 9- right atrium, 10- pectinate muscle of right atrium, 11-left atrium, 12- trabeculae cornae
The left ventricle internally was longer than the right one and it was the only chamber which extended to the apex of the heart (Fig. 3/A, B) as well as it carried two very long and strong powerful papillary muscles arising from the lateral ventricular wall (Fig. 3/A, B) and taken its origin from the apex of the heart (Fig. 4/A, B) till reached near the atrioventricular junction to share the mitral valve foundation (Fig. 4/C). The right ventricle held internally three rod-like papillary muscles (Fig. 3/B, C) that attached to the lateral wall only and did not stretch to the apical end of the ventricle which was part of the tricuspid valve (Fig. 4/D). The apical ends of both ventricles have trabeculae carneae which considered a muscular ridges and columns that allows over stretching of the ventricular walls (Fig. 3/A, B). The left atrium (Fig. 2/B) internally carried the auricle which seemed like an earlike flap that filled with multiple pectinate muscles that appeared also on the lateral right atrial wall (Fig. 3/C).
A, B: successive cross sections at the apex of heart to show the left ventricular lumen only with interval 0,5 cm between them. C, D: the cross sections in the middle of the heart to show both ventricular lumens with interval 1 cm.
1-cavity of left ventricle 2- wall of left ventricle, 3- papillary muscles of mitral valve, 4-Interventricular septum, 5- wall of right ventricle, 6- chorda tendineae, 7- valvular leaflet, 8- papillary muscle of tricuspid valve, 9- cavity of left ventricle.
The Valves
Both atria were connected to the ventricles through two atrioventricular valves, the left one is called mitral valve while the right one is called tricuspid valve.
The atrioventricular valves were formed from fibrous leaflets linked to the ventricular papillary muscles via the Chordae tendineae (Fig. 3).
The mitral valve was composed of two papillary muscles (Fig. 3/A, B, C) each one carrying 7-9 Chordae tendineae to connect with each corresponding cusp, anterior (aortic) and posterior (mural) (Fig. 4/C). The tricuspid valve has three papillary muscles (Figs. 3/B, C & 4/D) as well as three cusps; anterior, posterior and septal, all hanged to its corresponding papillary muscles through 7-9 Chordae tendineae per muscle (Fig. 3/C).
Coronary Arteries
A. coronaria sinistra, the left coronary artery was major branch supplying the heart, it was emanated from the left aortic sinus (Fig. 5/A, B). It was short and strong vessel about 0.2 to 0.5 cm in length (Fig. 5/B), which marked at the coronary groove and it was stuck among the pulmonary trunk as well as the left atrium. Then, it was split into a ramus interventricularis paraconalis as well as a ramus circumflexus (Fig. 5/B).
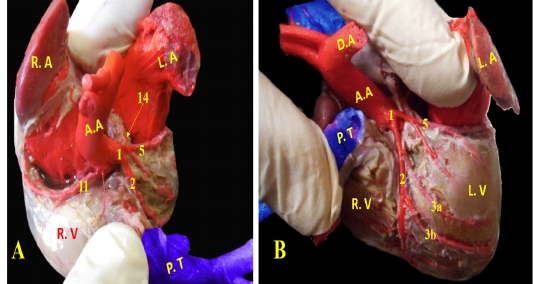
Figure 5: Showing coronary arteries, A- showing the origin of coronary arteries, B- showing the main branches of left coronary.
Ramus interventricularis paraconalis, the paraconal interventricular branch (Fig. 6/A) run at the cardiac auricular surface within the paraconal groove detaching 9-12 collateral branches; 4 to 6 of them called proximal (Fig. 6/A/3a), intermediate (Fig. 6/A, B/3b), and distal (Fig. 6/A, B/3c) collateral branches according to their distribution along the heart wall. The distal collateral branches were large and long vessels that instructed to nourish the left ventricular wall while the other collateral branches were minute twigs (Fig. 6/B) arborized on both ventricles then ended near the apex of the heart and released terminal marginal arterioles (Fig. 6/B, C) for the apex of the heart to anastomose with the interventricular infrasinuosal twig of the left circumflex artery.
Ramus circumflexus, the circumflex branch (Figs. 6/C, D & 7/A) at its origin it gave off a small septal artery which directed caudally and embedded into the cardiac septum. After that, the circumflex branch curved between both left ventricle and atrium till reached the right cardiac aspect then gave off three strong vessels supplying the left ventricle called intermediate collateral branches of the left ventricle (Fig. 6/C) and other minute twigs (deep collateral) toward the left atrium. Finally, the circumflex branch terminated by two major branches; last intermediate collateral branch in addition to the infrasinuosal branch (Fig. 6/D).
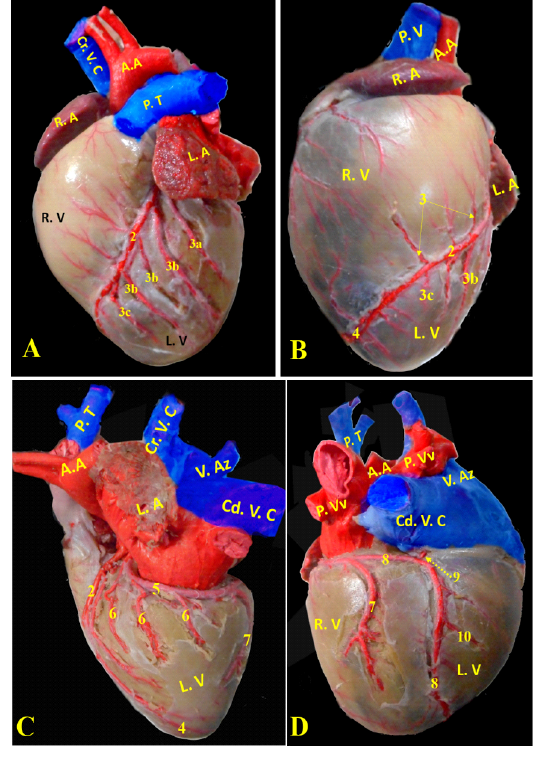
Figure 6: Showing division of left coronary arteries, A, B- showing the branches of left paraconal artery. C, D- showing the branches of left circumflex artery.
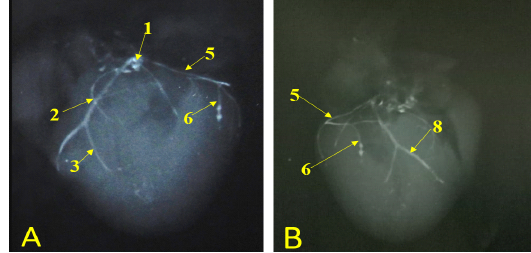
Figure 7: Showing radiographic images for division of the left coronary arteries, A- showing the left coronary artery. B- showing the branches of left circumflex artery.
The last intermediate collateral branch was large, deep in the wall but did not extend to the apex (Fig. 6/D). The infrasinuosal branch released the terminal branch that entered toward the interatrial septum as well as the base of the right ventricle. After that descended in the subsinuosal groove (Figs. 6/D, 7/B) that nourished the ventricular wall via small collateral twigs then lengthened till reached the apex of the heart to make anastomoses with the left interventricular paraconal artery (Fig. 8/A).
(Legend of figures 5, 6, 7, 8):
RA= Right atrium, RV= right ventricle, LA= left atrium, LV= left ventricle, AA- Aorta, PT= pulmonary trunk, PVv= pulmonary vein, V. Az = vena azygos.
1-left coronary artery, 2- left interventricular paraconal A, 3- collateral br of paraconal, 3a- proximal collateral br of paraconal, 3b intermediate collateral br of paraconal, 3c- distal collateral br of paraconal, 4- terminal br of paraconal, 5- left circumflex artery, 6-intermediate collateral branches, 7- last intermediate collateral branch, 8- the infrasinuosal artery, 9- the terminal branch, 10- collateral twigs of infrasinuosal artery, 11- right coronary, 12- ventral collateral branches of right coronary 13- dorsal collateral branches of right coronary, 14- septal artery.
A. coronaria dextra, the right coronary artery (Fig. 8/B) was a smaller branch compared with the left one, it emerged from the aortic sinus at the right semilunar leaflet. The right coronary branch lodged between both right auricle and ventricle till achieved the right ventricular border then gave off small ramifications; 5-8 ventral collateral branches (Fig. 8/B) to the right ventricular wall and 3-4 dorsal collateral branches (Fig. 8/B) to the right atrium.
Discussion
In the current study, the red fox heart positioned from the fourth intercostal space till achieved the sixth one within the thoracic cavity, on the other hand, Paredes and Martínez (2019) asserted that in dog the heart placed from the third rib till reached the caudal border of the sixth rib. The heart constituted from four chambers upper two atria and lower two ventricles which coincided by Ross et al. (2020 a) in the pig and in bovine (Emam and Abugherin, 2019).
In the present work, the left ventricular wall was thicker than the right one with narrower lumen due to intensive cardiac muscles besides the huge two papillary muscles that was explained by Smerup et al. (2016) in Giraffe how the heart produced such massive high arterial pressures. While in the ostrich heart (Alsafy et al., 2009) there were three small papillary muscles within the left ventricle.
The wall of right ventricle has three papillary muscles which share the tricuspid valve origin which confirmed by Mathur et al. (2019) in ovine and (Pokutta-Paskaleva et al., 2019) in porcine while (Perez et al., 2008) in Giraffe reported that there were two papillary muscles only in the right atrioventricular valve, but there was only one triangular papillary muscle within the right ventricular wall laterally in the ostrich (Alsafy et al., 2009). Although Paredes and Martínez, (2019) stated that in dog there were only two cusps in this valve, so term tricuspid was false. While in pig (Jett et al., 2018: Ross et al., 2020a, b) confirmed the presence of three cusps in the right atrioventricular valve.
Our observation in the valvular chordae tendineae number that revealed 7-9 cords for corresponding papillary muscle in each mitral as well as tricuspid valve while Hutchison and Rea (2015) gave the number of chordae tendinea for tricuspid valve in ovine and bovine as 6-8 while the mitral valve has the half number of chordae tendinea for each papillary muscle, while pig has the same number in both valves that reached 8-12 chordae tendinea. This matched with Jett et al. (2018) as the porcine valves have more chordae than the ovine ones.
Our results confirmed that the left coronary artery was the major arterial supply for the cardiac muscles which in line with Perez et al. (2008) who found the same anatomical feature in Giraffe. Even though, the left coronary artery was predominated vessel in the heart of ruminants and domestic carnivores so-called left type (Barone, 1996). While the right coronary was dominant (Oliveira et al., 2011) in human.
In our study, the left coronary artery branched into two large vessels; the interventricular paraconal plus the left circumflex one which verified in dog (Oliveira et al, 2010) who suggested the presence of the diagonal interventricular branches in 13.3% of examined dogs. On the other hand, our work revealed that the septal branch arose from the left circumflex artery, while in cat, Barszcz et al. (2017) suspect the presence of it and its origin from the left coronary artery. Although in goat heart, it was asserted that the septal artery derived from the paraconal branch (Barszcz et al, 2019).
In our work, the paraconal vessel was terminated near the apex of the heart by giving off terminal marginal arterioles, but in dog, it ended before reaching the apex of the heart in 10.0% or at the apex in 56.7% as well as may extend beyond the apex to lodge within the subsinuosal interventricular sulcus in 33.3% (Oliveira et al., 2010). Thus, the arteries presented at the apex of the heart were branches from the left coronary artery only that was similar to that found in the dog (Oliveira et al., 2011).
The left circumflex artery which passed through the coronary groove till reached the right aspect of the heart to provide collateral branches of the left ventricle which in line with Oliveira et al. (2010) in dogs. Also, our findings agreed with (Perez and Lima, 2007) in tiger and (Chirilean, 2015) in dog as mentioned that the final branch of the left circumflex continued as the interventricular subsinuosal artery as in (Perez et al., 2008) in Giraffe and (Oliveira et al., 2011) in dog. The latter artery stretched till reached the apex of the heart to anastomose with the left interventricular paraconal artery. In contrast, Oliveira et al. (2010) stated that in dogs, the interventricular subsinuosal artery gave an ending at various sites.
The right coronary artery passed between the right auricle and the right ventricle on the other hand in goat (Barszcz et al., 2019) and in pig (Sahni et al., 2008) we reported that it situated between the right auricle and the pulmonary trunk.
The right coronary artery released small dorsal and ventral branches to the right ventricular wall and the right atrium while in goat, Barszcz et al. (2019) reported that it may be ramified on the atrial surface only of the heart or lengthened in the subsinuosal groove. In addition, Sahni et al. (2008) stated that in pig the right coronary artery in its third segment descended in the posterior interventricular groove as a posterior interventricular artery.
Conclusion
Our work focused on the heart of red fox to reveal the most particular aspects of similarity and dissimilarity with other domestic animals, as there were not any available literatures discuss the heart of red fox in details. Unexpectedly, the presence of the pericardiophrenic ligament was in place of the pericardiosternal one. The papillary muscles in both ventricles gave special appearance unlike other mammals. Additionally, the interventricular infrasinuosal artery arose from the left circumflex artery instead of the right coronary artery like other higher vertebrates.
Acknowledgments
The authors sincere acknowledgement intended to Assistant Prof. Dr. Ahmed Abdelgalil Assistant Professor of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine for his invaluable support to achieve the radiographic imaging in this study. Special acknowledgment to all technicians in Veterinary Teaching Hospital, Cairo University, for their selfless help during the work.
Authors contribution
Elbably SH and Abouelela YS designed the research work, performed the anatomical description of the heart and described the anatomical architecture of the coronary arteries and revised the manuscript draft. All authors reviewed and approved the last version of the manuscript.
Conflict of interest
There are no conflicts of interest to declare.
Funding
The authors research incorporated in this article did not obtain any particular grant from funding organizations in the public, or not-for-profit sectors.
References