Advances in Animal and Veterinary Sciences
Review Article
Advances in Animal and Veterinary Sciences 2 (2): 73 – 77.An insight into the Recent Advances on the Physiology and Treatment of Retention of Fetal Membranes in Cattle
Muhammad Zubair*, Maqbool Ahmad ,
*Corresponding author: [email protected]
ARTICLE CITATION:
Zubair M, Ahmad M (2014). An insight into the recent advances on the physiology and treatment of retention of fetal membranes in cattle. Adv. Anim. Vet. Sci. 2 (2): 73 – 77.
Received: 2013–12–11, Revised: 2013–12–27, Accepted: 2013–12–29
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.2.73.77
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The fetal membranes which are commonly called placenta are the connections between dam and fetus for the normal circulation of nutrients to fetus. If the placenta is not expelled within normal time, it is called as retention of fetal membrane. Retention of fetal membrane is the common cause of reduction of fertility in cattle. For the management of this disease, it is necessary to understand the etiology and pathophsiology of this disease. The normal separation of fetal membranes consists of complex hormonal process that starts before parturition in cattle. The causes of retention of fetal membranes include immature placentomes due to infections, induction of parturition, difficult birth, hormonal imbalance and nutritional deficiencies. The treatments which are commonly used for the retention of fetal membranes do not show the significant effects. The use of antibiotics minimizes the growth of microorganism in retained fetal membrane but they have not shown improvement in the subsequent fertility of affected animals. Recently, new treatments like injection of collagenase enzyme into umbilical arteries and intrauterine use of ozone gas produces useful results but these therapies are costly. The objective of this review is to understand the physiological processes of fetal membranes expulsion, causes and treatments of retention of fetal membranes.
INTRODUCTION
The retention of fetal membrane can be defined as the failure of expulsion of fetal membranes (Drillich et al., 2006).The normal time for expulsion of fetal membrane varies from 8–48 hours after parturition (Lee et al., 1989). However, in one study 66% cattle expelled their fetal membranes 6 hours after parturition (Van et al., 1992). The adverse effects of RFM on reproductive performance of cattle are: delay in first service (Stevens et al., 1997), reduction of pregnancy rate (McDougall et al., 2001) increase in services per conception (Holt et al., 1989). The RFM also leads to endometritis, puperal metritis and mastitis (Bruun et al., 2002) and these diseases ultimately cause the reduction in the fertility and milk production of cattle (Laven et al., 1996). Number of factors like difficult birth, twinning of calves, abortion, and increase in the age of animal are responsible for delayed expulsion of fetal membranes (Han and Kim, 2005). Recently, some vitamins and minerals are also identified as cause of RFM (Akar and Yeldiz, 2005).
Inadequate supplementation of a ration with vitamins A and E, β–carotene, iodine, selenium, copper and zinc may also induce abortion in cows with increase incidence of RFM (Markiewicz et al., 2001). The exact cause of RFM is still not known and this hampers the search for preventive and therapeutic measures (Holt et al., 1989). Various prophylactic and therapeutic approaches have been postulated by many workers ranging from no treatment to hormonal, chemotherapeutic and manual removal (Majeed et al., 1991). Knowledge of the placental anatomy and physiology is helpful to understand causes of RFM and formulate treatment plans accordingly. The following review focuses on the normal placental detachment, causes and risk factors for RFM, and therapeutic options.
Physiology of Placental Maturation and Separation.
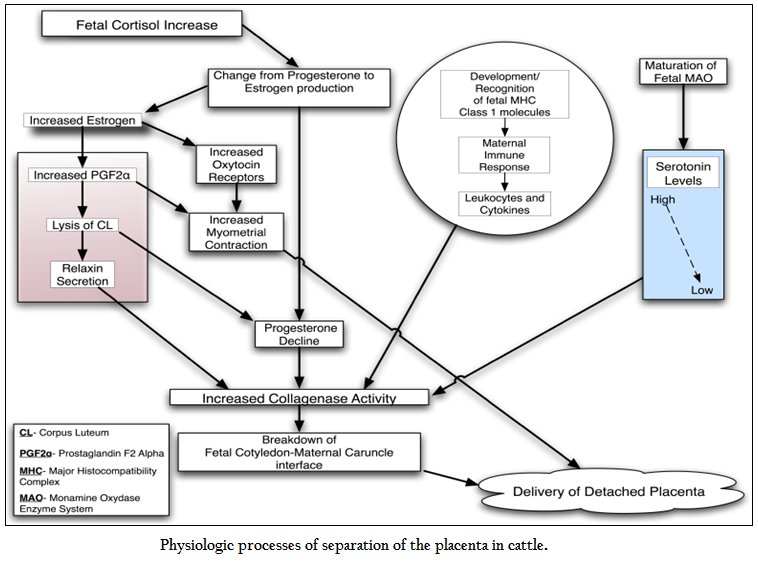
The type of placenta in cattle is cotyledonary which is formed by the fetal cotyledons and maternal cruncles making new structure called palcentomes. Many villi are present at this connection for the interaction between these two structures. The connection sites are enriched with collagens which play an important role for the separation of cruncles from cotyledons at the time of fetal expulsion (Eiler et al., 1993).The normal events of parturition are initiated by placental enzymes for the conversion of the progesterone into estrogen (Flint et al., 1979).The increased level of estrogen causes the sensitivity of receptors for oxytocin on myometrium as well as increased concentration of prostaglandin F2 alpha (Fuchs et al.,1999).This increased prostaglandin results in the contraction of myometrium and regression of corpus leuteum (CL) (Janszen et al., 1993).The luteolysis of CL results in the secretion of relaxin hormone along with a decline in the progesterone levels (Mush et al., 1987).These two conditions are responsible for the initiation. of collagenase activity. Relaxin causes the lysis of collagen, softening of the cervix and expansion of pelvic ligaments. On the other hand, progesterone during the pregnancy inhibits the myometial contraction and collagenase activity and reduction in the level of progesterone at parturition might allow the activity of enzymes which are necessary for the separation of placental membranes (Maj et al., 1997).The normal process of placental separation is multifactorial and starts before the process of parturition (Figure 1). It is also suggested that serotonin is necessary for the normal attachment of placental membranes (Fecteau, 2001). High level of fetal and placental serotonin might help for the maintenance of placental attachment and cell proliferation during the process of pregnancy (Fecteau et al., 2001) and inhibiting the activity of proteinase (Eiler et al., 1993).The increase in the activity of enzyme at the time of parturition causes the increase in metabolization and reduction of serotonin which might help in placental separation and expulsion (Fecteau et al., 2001). Besides the changes in the profile of hormones that cause breakdown of cotyledon–cruncle linkages through enzymes, the immune response of dam plays an important role for the breakdown of placenta. The chemotactic activity of leukocytes is increased in those cows which normally expel the placenta (Gunnink et al., 1983); (Kimura et al., 2002) and neutorphil are attracted by the cytokine interleukin–8 in cotyledons in the process of parturition (Kimura et al., 2002). The trophoblast cells activate the immune response through major histocompatibility proteins that help in the separation of placenta (Gunnink et al., 1983).The level of these proteins increase during the last trimester (Davies et al., 2004).
The labor is characterized by the high level of prostaglandin along oxytocin and creation of high mechanical contraction within the uterus which is necessary for normal expulsion of fetal membranes (Janszen et al., 1993) and (Lye et al., 1996). The contraction of uterus ends into the third stage of parturition which causes the expulsion of placenta (Laven et al., 1996). Still the role of contraction of uterus in the separation of fetal membranes is not clear. The release of fetus from the uterus results in the reduction of blood circulation within the placenta and subsequently shrinkage of villi takes place (Laven et al., 1996). The forces of uterine contraction might cause disconnection between cruncles and cotyledons, even though the absence of any harm to villi of fetal in normal parturition suggests the evidence that this process is not mechanical (Bjorkman et al., 1960). It is though currently that contraction of uterus is important for the expulsion of fetal membranes; the early lack of myometrial contraction is not the prerequisite of retained fetal membranes (Paisley et al., 1986; Grunert et al., 1986).
Causes of RFM
The number of factors which are responsible for the RFM are induction of parturition (Terblanche et al.,1976), short gestation period (Muller et al., 1974) , abortion of fetus (Joosten et al., 1987) ; (Roberts et al., 1986) twin fetus (Muller et al., 1974; Erb et al., 1958) difficult birth (Joosten et al., 1987; Rajala et al., 1988) fetotomy (Joosten et al., 1987; Wehrend et al., 2002), mineral and vitamin deficiency like vitamin E, selenium and vitamin A (Julien et al., 1976; Ronning et al., 1953). The RFM is also caused by infectious diseases like bovine viral diarrhea (Niskanen et al., 1995) and immunosuppression (Laven et al., 1996). Whereas the exact mechanism responsible for these factors is not completely known, the complex process of multiple hormones and biochemical events leads that disturbance in these events causes the RFM. The studies of physiological factors which are responsible for the detachment of fetal membranes help in the diagnosis of etiologies of RFM. The function of immunosuppression during the retention of fetal membrane is not entirely understood (Gunnink et al., 1984; Kimura et al., 2002; Peter et al., 1987). To maintain pregnancy, it is necessary to suppress the immune response to save the fetus from rejection and RFM may be the due to the result of failure of switching off this protective activity of immune system.
The cows which suffer from RFM subsequent to normal delivery found with decrease amount and activity of leukocytes prior to parturition process (Gunnink et al., 1984; Kimura et al., 2002). Particularly, the cows which suffer from RFM were found with reduced chemotaxis activity of neutrophils from one week prior to one week following parturition and reduced myeloperoxidase function from two weeks prior to two weeks subsequent parturition (Kimura et al., 2002). The interleukin–8 which has an important chemotactic activity for neutrophils was less in cows which suffer from RFM as compared to those cows which were normally expelled their placenta. These studies found that reduced activity of neutrophil might be the cause of retention of fetal membrane. Reduction of antioxidant enzyme activity is also found during the RFM (Wischral et al., 2001; Gupta et al., 2005). Less level of estrogen and superoxide dismutase was found in RFM (Wischral et al., 2001). It is also proposed that that there is an imbalance of antioxidant activity of placenta which results into decrease production of estrogen followed by the less level of PGF2a in the placenta (Wischral et al., 2001). The analysis of (44) studies by comparing the incidence of RFM in treated cows with vitamin E and cows without any treatment found that vitamin E reduced the incidence of RFM (Bourne et al., 2007). Vitamin E improves the antioxidant activity and also increases the leukocytosis and chemotaxis at placentomes which help in the normal expulsion of fetal membranes (Bourne et al., 2007). The differences in the activity of protease in retained and non–retained placenta (Gross et al., 1985; Eiler et al., 2007) indicate the changes in the activities of enzymes play the important roles in the cause of RFM. For instance, the collagenase is reduced but collagen of type III remains in normal level in cows with RFM. Disturbances in the normal profile of hormones within the uterus can stop the secretion of protease from the placentomes epithelium and inhibition of activity of leukocyte protease by immunosuppression. Either condition can cause the reduction of activity of protease. The initiation of third stage of parturition with the help of dexamethasone, alongwith or without prostaglandin is the major cause of retained placenta and the exact mechanism responsible for this cause is not known (Gross et al., 1985). The glucocorticoids could inhibit the activity of collagenase (Guerin et al., 2004). Dexamethasone also inhibits the secretion of PGF2a in the cells of cotyledons (Izhar et al., 1992) and injecting the PGF2a and dexamethasone could reduce occurrence of RFM but not completely (Gross et al., 1985; Gross et al., 1986). The induction of parturition causes the RFM but can be reduced if relaxin is injected along with dexamethasone. Various relations between the hypocalcemic level and RFM are made (Curtis et al., 1983; Melendez et al., 2004). The cows which suffer from RFM have lower level of plasma calcium as compared to cows without RFM (Melendez et al., 2004). Calcium is necessary for the collagenase action but reduced level of calcium found in retained placenta is not so low to exclude the activity of collagenase (Gross et al., 1985). In addition, only total calcium is observed in retained and without retained animals (Melendez et al., 2004). For instance, the level of calcium can be affected by the level of other minerals such as hypoalbuminemia. No reduction of RFM was found in cows which were fed calcium orally (Melendez et al., 2003). But the deficiency of calcium might predispose the cattle to dystocia (Correa et al., 1993) and loss of uterine contractions subsequent to the failure of fetal expulsion but the direct role of calcium in separation of fetal membranes is not understood. The risk factors which are responsible for RFM are injury to uterus, difficult birth and caesarian section. Injury can cause edema of villi which could interfere in separation of cruncles of dam from the cotyledons of fetus (Laven et al 1996); (Grunert et al., 1986). The normal disconnection process of placenta involves the separation of villi of cotyledons from the crypts of carucles. So the edema of villi could hinder the separation villi from crypts. In addition, the injury to uterus might results in increase secretion of heparin from the mast cells. Heparin causes the inhibition of collagenases (Au et al., 1992) and delay of involution of uterus and both these factors result into RFM (Eiler et al., 2007)
Treatments
Unfortunately, very fewer treatments are commonly used for the RFM and these treatments are based on traditional evidences. The manual handling of placenta is commonly used but no beneficial effects are reported on reproduction of animal or milk (Muller et al., 1974). The benefits of recent studies like manual removal and systemic antibiotics have not been reported on the reproductive performance of cattle when compared without treatment (Drillich et al 2006; Drillich et al., 2007). The manual removal can cause more uterine infections when compared to traditional treatments.
(Bolinder et al., 1988). Whereas, the evidences collected of current studies do not support to manual handling of fetal membranes (Peters et al., 1996). Certainly, the manual removal of placenta has more chances of injury to endometrium and reduces the phagocytic activity of leukocytes (Vandeplassche et al., 1982) and these two factors encourage the invasion of bacteria (Paisley et al., 1986; Peters et al., 1996). On the other hand, it is difficult to remove the whole placenta from the uterus, while some necrotic parts could be removed leading to bacterial infections (Paisley et al., 1986) or development of postpartum metritis.
Recently, new therapies have been introduced without the use of antibiotics. The use of ozone gas is also one of them. It has ability to inhibit the growth of microbes and fungi. The ozone gas has the ability of oxidative functions which is created by peroxides to destroy the microbes (Bocci et al., 2009; Travagli et al., 2009). Ozone gas does not develop the resistance as in antibiotics. Ozone gas is unstable and it dissociates into the powerful reactive molecules of oxygen under the usual pressure and temperature of atmosphere. It has properties of gas molecules and is combination of three oxygen atoms. It is a strong oxidant and can cause the activation of lymphocytes or monocytes to secrete many cytokines like interferon α, β and γ, tumor necrosis factor (TNF) α, interleukins (IL) 1, 2, 4, 6, 8 and 10, granulopoietins (GM–CSF), and transforming growth factor (TGF) β (Ducusin et al., 2003 and (Ohtsuka et al., 2006). Ozone gas optimizes the regeneration of tissue by creating the epithelialization and granulation. Djuicer et al., (2012) studied the effect of two preparations of ozone gas on the reproductive performance of cattle suffering from RFM. The gas preparations were applied into body of uterus and the parameters of reproductive efficiency like calving to first insemination, interval from calving to pregnancy, relative pregnancy rate and number of services per conception was measured. The cows which were treated ozone spray have the same or improved reproductive performance parameters when compared to control group. The ethno veterinary practices have significant importance to veterinary treatment (Lin et al., 2003) due to low cost and clear effectiveness (Mwale et al., 2005). It is the common perception of the farmer that RFM is due to weakness and difficult birth of animal. Therefore, mixture of oil and milk is administered as source of energy. The milk of camel is preferred due to high mineral contents. The common salt is also rubbed on the back of animal for the expulsion of fetal membranes with this idea that it stimulates the uterus for contraction (Dilshad et al., 2008).
CONCLUSION
The RFM is an important reproductive disorder in dairy animals. It causes the great economic losses via reduction in milk production and reproductive efficiency of dairy cattle. The exact etiology of RFM is not completely understood due to multiple causes. The incidence of RFM can be reduced by improving the management and adopting the new techniques of treatments.
CONFLICT OF INTEREST
Authors have no conflict of interest to declare.
REFERENCES
Akar Y, Yeldiz H (2005). Concentrations of some minerals in cows with retained placenta and abortion. Turk. J. Vet. Anim. Sci. 29: 1157 – 1162.
Au YP, Montgomery KF, Clowes AW (1992). Heparin inhibits collagenase gene expression mediated by phorbol ester–responsiveelement in primate arterial smooth muscle cells. Circ. Res 70: 1062 – 1069.
http://dx.doi.org/10.1161/01.RES.70.5.1062
PMid:1314715
Bjorkman NH, Sollen P (1960). Morphology of bovine placenta at normal delivery. Acta. Vet. Scand 1: 157 – 177.
Bocci V, Borrelli E, Travagli V, Zanardi I (2009). The ozone paradox: ozone is a strong oxidant as well as a medical drug. Med. Res. 29: 646 – 682.
http://dx.doi.org/10.1002/med.20150
PMid:19260079
Bolinder A, Seguin B, Kindahl H, Bouley D, Otterby D (1988). Retained fetal membranesin cows: Manual removal versus non–removal and its effect on reproductive performance. Theriogenology 30: 45 – 56.
http://dx.doi.org/10.1016/0093-691X(88)90262-2
Bourne N, Laven R, Wathes DC, Martinez, T, McGowan, M (2007). A meta–analysis ofthe effects of vitamin E supplementation on the incidence of retained foetal membranes in dairy cows. Theriogenology.67: 494 – 501.
http://dx.doi.org/10.1016/j.theriogenology.2006.08.015
PMid:17007917
Bruun J, Ersb AK, Alban L (2002). Risk factors for metritis in Danish dairy cows. Prev. Vet. Med.54: 179 – 190.
http://dx.doi.org/10.1016/S0167-5877(02)00026-0
Correa MT, Erb HN, Scarlett J (1993). Path analysis for seven postpartum disorders of Holstein cows. J. Dairy. Sci.76: 1305 – 1312.
http://dx.doi.org/10.3168/jds.S0022-0302(93)77461-5
Curtis CR, Erb HN, Sniffen CJ, Smith RD, Powers PA, Smith MC (1983). Association of parturient hypocalcemia with eight periparturient disorders in Holsteincows. J. Am. Vet. Med. Assoc.183: 559 – 561.
PMid:6618988
Davies CJ, Hill JR, Edwards JL, Schrick FN, Fisher PJ, Eldridge JA (2004). Major histocompatibility antigen expression on the bovine placenta: Its relationship to abnormal pregnancies and retained placenta. Anim. Reprod. Sci. 82 – 83: 267 – 280.
Dilshad S, Rehmana N, Iqbal Z, Muhammad G, Iqbal A, Ahmed N (2008). An inventory of the Ethnoveterinary practices for Reproductive Disorders in cattle and buffaloes, Sargodha district of Pakistan. J. Ethnopharmacol. 117: 393 – 402.
http://dx.doi.org/10.1016/j.jep.2008.02.011
PMid:18384987
Drillich M, Klever N, Heuwieser W (2007). Comparison of twomanagement strategies for retained fetal membranes on small dairy farms in Germany. J. Dairy. Sci. 90: 4275 – 4281.
http://dx.doi.org/10.3168/jds.2007-0131
PMid:17699046
Drillich M, Reichert U, Mahlstedt M, Heuwieser W (2006). Strategies to improvethe therapy of retained fetal membranes in dairy cows. J. Dairy. Sci. 89: 627 – 635.
http://dx.doi.org/10.3168/jds.S0022-0302(06)72126-9
Drillich M, Mahlstedt M, Reichert U, Tenhagen B A, Heuwieser W (2006). Strategies to improve the therapy of retained fetal membranes in Dairy Cows. J. Dairy Sci. 89: 627 – 635.
http://dx.doi.org/10.3168/jds.S0022-0302(06)72126-9
Ducusin JT, Nishimura M, Sarashina T, Uzuka Y, Tanabe S, Otani M (2003). Phagocytosis of bovine blood and milk polymorphonuclear leukocytes after ozone gas administration in vitro. J. Vet. Med. Sci. 65: 535 – 539
http://dx.doi.org/10.1292/jvms.65.535
PMid:12736440
Eiler H, Fecteau KA (2007). Retained placenta. In: Youngquist RS, Threlfall WR, eds. Current Therapy in Large Animal Theriogenology, 2nd ed. St Louis, MO:WB Saunders: 345 – 354
http://dx.doi.org/10.1016/B978-072169323-1.50048-9
Eiler H, Hopkins FM (1992). Bovine retained placenta: Effects of collagenase and hyaluronidase on detachment of placenta. Biol. Reprod. 46: 580 –585.
http://dx.doi.org/10.1095/biolreprod46.4.580
PMid:1315581
Eiler H, Hopkins FM (1993). Successful treatment of retained placenta with umbilical cord injections of collagenase in cows. J. Am. Vet. Med. Assoc. 203: 436 – 443
PMid:8226224
Erb RE, Hinze PM, Gildow EM, Morrison RA (1958). Retained fetal membranes, the effect on prolificacy of dairy cattle. J. Am. Vet. Med. Assoc. 133: 489 – 495.
PMid:13598670
Fecteau KA, Eiler H (2001). Placenta detachment: Unexpected high concentrations of 5–hydroxytryptamine (serotonin) in fetal bloodand its mitogenic effect on placental cells in the bovine. Placenta. 22: 103 –110.
http://dx.doi.org/10.1053/plac.2000.0596
PMid:11162359
Flint APF, Rickets AP, Craig VA (1979). The control of placental steroid synthesis at parturition in domestic animals. Anim. Reprod. Sci. 2: 239 – 251.
http://dx.doi.org/10.1016/0378-4320(79)90050-2
Fuchs AR, Rust W, Fields MJ (1999). Accumulation of cyclooxygenase – 2 gene transcripts in uterine tissues of pregnant andparturient cows: Stimulation by oxytocin. Biol Reprod. 60: 341 – 348.
http://dx.doi.org/10.1095/biolreprod60.2.341
PMid:9916000
Gross T.S, Manspeaker JE, Williams WF, Russek E (1985). In vitro proteolytic activity of the late pregnant and peripartum bovine placenta. J. Anim. Sci.61 (Suppl 1): 391 – 392.
Gross TS, Williams WF, Moreland TW (1986). Prevention of the retained fetal membrane syndrome (retained placenta) during inducedcalving in dairy cattle. Theriogenology. 26: 365 – 370.
http://dx.doi.org/10.1016/0093-691X(86)90156-1
Grunert E (1986). Etiology and pathogenesis of retained placenta. In: Morrow DA, ed. Current Therapy in Theriogenology 2. Philadelphia, PA: WB Saunders: 237 – 241
Guerin P, Thiebault JJ, Delignette–Muller ML, Ménézo Y (2004). Effect of injecting collagenase into the uterine artery during a caesareansection on the placental separation of cows induced to calve with dexamethasone. Vet. Rec. 154: 326 – 328.
http://dx.doi.org/10.1136/vr.154.11.326
PMid:15068040
Gunnink JW (1984). Prepartum leukocytic activity and retained placenta. Vet. Quart. 6: 52 – 54.
http://dx.doi.org/10.1080/01652176.1984.9693910
http://dx.doi.org/10.1080/01652176.1984.9693911
http://dx.doi.org/10.1080/01652176.1984.9693909
Gupta S, Gupta H, Soni J (2005). Effect of vitamin E and selenium supplementation on concentration of plasma cortisol and erythrocytelipid peroxidases and the incidence of retained fetal membranes in crossbred dairy cattle. Theriogenology. 64: 1273 – 1286.
http://dx.doi.org/10.1016/j.theriogenology.2005.03.008
PMid:16139604
Han, Y K, Kim IH (2005). Risk factors for retained placenta and the effect of retained lacenta on the occurrence of postpartum diseases and subsequent reproductive performance in dairy cows. J. Vet. Sci. 6: 53 –59.
PMid:15785124
Holt LC, Whittier WD, Gwazdauskas FC (1989). Early postpartum reproductive profiles in Holstein cows with retainedplacenta and uterine discharges. J. Dairy. Sci. 72: 533 – 539.
http://dx.doi.org/10.3168/jds.S0022-0302(89)79137-2
Izhar M, Pasmanik M, Marcus S (1992). Dexamethasone inhibition of cyclooxygenase expression in bovine term placenta.Prostaglandins. 43: 239 – 254.
http://dx.doi.org/10.1016/0090-6980(92)90092-8
Janszen BPM, Bevers MM, Ravenshorst MM, van der Weijden GC, Dieleman SJ, Taverne MAM (1993). The relation between prostaglandin–induced luteolysis and temporaryinhibition of myometrial activity in late pregnant cows with progestagen containing ear implants. J. Reprod. Fert. 97: 457 – 461.
http://dx.doi.org/10.1530/jrf.0.0970457
PMid:8501715
Joosten I, Van Eldik P, Elving L, Vander Mey GJW (1987). Factors related to the etiology of retained placenta in dairy cattle. Anim. Reprod. Sci. 14: 251 – 262.
http://dx.doi.org/10.1016/0378-4320(87)90015-7
Julien WE, Conrad HR (1976). Selenium and vitamin E and incidence of retained placenta in parturient dairy cows. J. Dairy. Sci. 59: 1954 – 1959.
http://dx.doi.org/10.3168/jds.S0022-0302(76)84467-0
http://dx.doi.org/10.3168/jds.S0022-0302(76)84468-2
Kimura K, Goff JP, Kehrli ME, Reinhardt TA (2002). Decreased neutrophil function as a cause of retained placenta in dairy cattle.J. Dairy. Sci. 85: 544 – 550.
http://dx.doi.org/10.3168/jds.S0022-0302(02)74107-6
Laven RA, Peters AR (1996). Bovine retained placenta: Aetiology, pathogenesis, and economic loss. Vet. Rec. 139: 465 – 471.
http://dx.doi.org/10.1136/vr.139.19.465
PMid:8938967
Lee LA, Ferguson JD, Galligan DT (1989). Effect of disease on days open assessed by survival analysis. J. Dairy. Sci. 22: 1020 – 1026.
http://dx.doi.org/10.3168/jds.S0022-0302(89)79197-9
Lin J, Kaphle K, Yang N, Lu H, Yamada, Rogers P (2003). Sustainable veterinary medicine for the new era. Review. Scienti?c andTechnical Of?ceInternational Epizootics. 22: 949 – 96.
Lye SJ (1996). Initiation of parturition. Anim Reprod Sci. 42: 495 – 501.
http://dx.doi.org/10.1016/0378-4320(96)01529-1
Maj JG, Kankofer M (1997). Activity of 72–kDa and 92–kDa matrix metalloproteinases in placental tissues of cows with and withoutretained fetal membranes. Placenta. 18: 683 – 687.
http://dx.doi.org/10.1016/S0143-4004(97)90010-2
Majeed AF, Taha MB, Azawi OI (1991). Hormonal treatment of retained placenta in local breed of cattle. Iraqi J. Vet. Sci. 4 (1): 61 – 72.
Markiewicz H, Kuma K, Malinowski E (2001). Predisposing factors for puerperal metritis in cows. Bull. Vet. Inst. Pulawy. 45: 281 –288.
McDougall S (2001). Effects of periparturient diseases and conditions on the reproductive performance of New Zealand dairy cows. NZ. Vet. J. 49: 60 – 68.
http://dx.doi.org/10.1080/00480169.2001.36204
http://dx.doi.org/10.1080/00480169.2001.36223
Melendez P, Donovan A, Risco C, Jesse P (2004). Plasma mineral and energy metabolite concentrations in dairy cows fed an anionic prepartum diet that did or did not have retained fetal membranes after parturition. Am. J. Vet. Res. 65: 1071 – 1076.
http://dx.doi.org/10.2460/ajvr.2004.65.1071
PMid:15334840
Melendez P, Risco CA, Donovan GA, Littell R, Goff JP (2003). Effect of calcium– energy supplements on calving–related disorders, fertility and milk yield during the transition period in cows fed anionic salts. Theriogenology. 60: 843 – 854.
http://dx.doi.org/10.1016/S0093-691X(03)00103-1
Muller LD, Owens MJ (1974). Factors associated with the incidence of retained placentas. J Dairy Sci. 57: 725 –728.
http://dx.doi.org/10.3168/jds.S0022-0302(74)84956-8
Mwale, M, Bhebhe E, Chimonyo M, Halimani T (2005). Use of herbal plants in poultry health management in the Mushagashe small–scale commercial farming area in Zimbabwe. Intl. J. Appl. Res. Veterinary. Medicine. 3: 163 – 170.
Niskanen R, Emanuelson U, Sundberg J, Larsson B, Alenius S (1995). Effects of infection with bovine virus diarrhoea virus on health and reproductive performance in 213 dairy herds in one country in Sweden. Prev. Vet. Med. 23: 229 – 237.
http://dx.doi.org/10.1016/0167-5877(94)00437-N
Ohtsuka A, Ogata N, Terasaki M, Koiwa S (2006). Changes in leukocyte population after ozonated autohemoadministration in cows with inflammatory diseases J. Vet. Med. Sci. 68 175 – 178
http://dx.doi.org/10.1292/jvms.68.175
PMid:16520542
Paisley LG, Mickelsen WD, Anderson PB (1986). Mechanisms and therapy for retained fetal membranes and uterine infections of cows: A review. Theriogenology. 25: 353 –38
http://dx.doi.org/10.1016/0093-691X(86)90045-2
Peter AT, Bosu WTK (1987). Peripartal endocrine changes associated with retained placenta in dairy cows. Theriogenology. 28: 383 – 394.
http://dx.doi.org/10.1016/0093-691X(87)90026-4
Peters AR, Laven RA (1996). Treatment of bovine retained placenta and its effects. Vet. Rec. 139: 539 – 541.
http://dx.doi.org/10.1136/vr.139.22.535
Rajala PJ, Grohn YT (1998). Effects of dystocia, retained placenta, and metritis on milk yield in dairy cows. J. Dairy. Sci. 81: 3172 – 3181.
http://dx.doi.org/10.3168/jds.S0022-0302(98)75883-7
Roberts SJ, Woodstock VT (1986). Veterinary Obstetrics and Genital Diseases. 373 –393.
Ronning M, Berousek ER, Kuhlman AH, Gallup WD (1953). The carotenerequirements for reproduction in Guernsey cattle. J. Dairy. Sci. 36: 52 – 56.
http://dx.doi.org/10.3168/jds.S0022-0302(53)91455-6
Stevens RD, Dinsmore RP (1997). Treatment of dairy cows at parturition with prostaglandin F2 a or oxytocin for prevention of retained fetal membranes. J. Am. Vet. Med. Assoc. 21: 1280 – 1284.
Terblanche HM, Kritzinger LJ, Van Heerden JS (1976). Induced parturition in cattle. 1. Clinical studies. J. S. Afr. Vet. Assoc. 47: 113 – 115.
PMid:940094
Travagli I, Zanardi V (2009). Bocci Topical applications of ozone and ozonated oils as antiinfective agents: an insight into the patent claims Recent Patents on Anti–infective Drug Discovery. 4: 130 – 142
http://dx.doi.org/10.2174/157489109788490271
Van Werven T, Schukken YH, Lloyd J, Brand A, Heeringa HT, Shea M (1992). The effects of duration of retained placenta onreproduction, milk production, postpartum disease and culling rate. Theriogenology. 37: 1191 – 1203
http://dx.doi.org/10.1016/0093-691X(92)90175-Q
Vandeplassche M, Bouters R (1982). The impact of gynaecologicaland obstetrical problems resulting out of pregnancy and parturition. In: Karg H, Schallenberger E, eds. Factors Influencing Fertility in the Post–Partum Cow. The Hague: Martinus Nijhoff: 30 – 44.
Wehrend AT, Reinle K, Herfen H, Bostedt H (2002). Fetotomy in cattle with special reference to postoperative complications–an evaluationof 131 cases. Dtsch. Tierarztl. Wochenschr. 109: 56 – 61.
PMid:11889843
Wischral A, Nishiyama–Naruke A, Curi R, Barnabe RC (2001). Plasma concentrations of estradiol 17b and PGF2a metabolite and placental fatty acid composition and antioxidant enzyme activity in cows with and without retained fetal membranes. Prostaglandins. Other. Lipid. Mediat. 65: 117 – 124.
http://dx.doi.org/10.1016/S0090-6980(01)00123-X