Advances in Animal and Veterinary Sciences
Research Article
Diagnostic Studies on Gastroenteritis in Conjunction with the Anatomical and Histological Studies on the Gastrointestinal Tract of the Egyptian Tortoise (Testudo kleinmanni)
Reem R Tahon1*, Hitham Abdel-Saeed2, Mohamed A Khattab3, Zainab Sabry Othman Ahmed3, Yara S Abouelela1
1Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Cairo University, Egypt; 2Department of Medicine, Faculty of Veterinary Medicine, Cairo University, Egypt; 3Department of Cytology and Histology, Faculty of Veterinary Medicine, Cairo University, Egypt.
Abstract | Digestive problem is one of the most important complain of desert tortoises. A total of twenty four Egyptian tortoises (Testudo kleinmanni) were examined for clinical, anatomical andhistological studies. Gastroenteritis in our study was mainly occurred from parasitic infestations; large numbers of Oxyurid adult worms, small sized larvae and large number of Oxyurid eggs. In addition, protozoal infection included the presence of Nyctotherius and Plantadium species. Marked anorexia and greenish watery diarrhea were the main clinical signs. Also, significant increase in relative eosinophilic count was recorded. Marked significant decrease was recorded for serum calcium level leading to areas of discoloration at the carapace. Anatomical studies were applied to manage the Plastron celiotomy for intestinal impactions. In this study, we compared anatomically the healthy and unhealthy gastrointestinal tracts. Normal anatomical positions of the GIT were changed as, in case of severe gastroenteritis and accumulation of gases, the cecum was displaced to the left side, and the ascending colon reached the right side of the coelomic cavity. Also, the stomach was displaced more laterally to the left side in the diseased GIT. There was no external demarcation between jejunum and ileum. Densely backed mucous glandular tissue was found in the esophageal mucosa. No evidence for sub mucosal Brunner’s glands of duodenum were observed. More abundant gut associated lymphoid tissue was recorded in cecal region samples. Microscopic examination of intestinal/duodenal samples revealed many intra epithelial parasitic stages all over intestinal villi accompanied with underlying lamina propria or intra epithelial eosinophilic infiltrations.
Keywords | Gastroenteritis, Parasitic infestation, Gastrointestinal tract, Tortoise, Anatomy, Histology.
Received | January 08, 2021; Accepted | January 22, 2021; Published | February 20, 2021
*Correspondence | Reem R Tahon, Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: [email protected]
Citation | Reem RT, Abdel-Saeed H, Khattab MA, Ahmed ZSO, Abouelela YS (2021). Diagnostic studies on gastroenteritis in conjunction with the anatomical and histological studies on the gastrointestinal tract of the egyptian tortoise (testudo kleinmanni). Adv. Anim. Vet. Sci. 9(4): 500-507.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.500.507
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Reem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Many affections linked to digestive tract of tortoises included protozoa and helminthes. Both affections are of the major causes for tortoise admission to veterinary clinics as these affections cause weight loss, diarrhea, general malaise, lethargy, apathy with severe dehydration and death may be an end result. Parasites possess a harmful effect on their host through utilization of major resources of the tortoises for their development and multiplication. Also, parasites may cause a deleterious effect on the hematobiochemical status of the tortoise. Nematodes are one of the most common parasites found in tortoises beside cestodes, trematodes and protozoal parasites.
The critically endangered Egyptian tortoise (T. kleinmanni) is the smallest tortoise in the northern hemisphere (McArthur et al., 2008) (Tortoises are vertebrates with similarities with mammals but also differences as lower metabolic rates, renal portal circulation and ecdysis (Divers, 2015). Taxonomy Class: Reptilia Order: Chelonia/Testudines Family: Testudinidae Genus: Testudo, Testudo kleinmanni (Pollock and Kanis, 2015). Legler (1993) described that a sheet of connective tissue partially divides the lungs and all ventral viscera occur. It is named the horizontal pleuroperitoneal membrane (horizontal septum), or pseudo diaphragm (McArthur et al., 2004).
Materials and methods
For the clinical examinations fourteen tortoises of different age and sexes were examined in different pet clinics. Hematological, serum biochemical and fecal examinations were applied on the blood and fecal samples respectively. Parasites were classified on the basis of key described by (Gango, 2006). Statistical analysis was applied using student T-test. P-values less or equal 0.05 considered significant.
The anatomical study was applied on five Egyptian tortoises (Testudo kleinmanni). Those tortoises were immediately administered dead at the hospital of faculty of veterinary medicine The shell was opened then the coelomic cavity was opened by careful reflection of either the carapace or the plastron, so that the digestive tract was viewed and dissected. All the fresh specimens were photographed by a camera 20.1 megapixels.
Statement of Animal Rights: This study has the approval number of Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Medicine, Cairo University for using life animal in this research Vet CU01102020222.
For microscopic investigations, five tortoises were used. The turtles were anesthetized with xylazine 1mg/Kg/IM and ketamine 20mg/Kg/IM in forelimb muscles, and then they euthanized by means of an injection of sodium thiopental at 2.5% in the lethal doses of 60mg/Kg/IVin jugular vein. Tissue samples were flushed and fixed in 10% neutral buffered formalin for 72 hrs. Samples were trimmed, processed in serial grades of ethanol, cleared in xylene, and then samples were infiltrated and embedded into Paraplast wax tissue embedding media. 4μn thick tissue sections were cut by rotatory microtome and mounted on glass slides from different anatomical regions samples. Tissue sections were stained by Hematoxylin and Eosin as a general morphological examination staining method (Bancroft and Gamble, 2008). All data and micrographs were obtained by Full HD microscopic camera operated by Leica application module (Leica Microsystems GmbH, Wetzlar, Germany). All standard procedures for samples fixation and staining were according to (Culling, 2013).
Results
Clinical examination
Regarding clinical examination, diseased desert tortoises revealed dullness and depression with marked degree of dehydration showed by dry skin and increased skin tenting time in the skin of neck and limbs at the time of admission to clinic. By careful examination to the status of carapace and plastron, there were areas of discoloration at the carapace (fig. 1/A) beside pores and cracks that were scattered on both carapace and plastron (fig. 1/B). Marked anorexia was recorded as a main complain. During examination, diseased tortoises showed greenish watery diarrhea (fig. 1/C) that contained several motile fine worms which detected by naked eyes. Diarrhea samples were examined microscopically to scrutinize and confirm the gross appearance. Massive infestation with large numbers of Oxyurid adult worms (fig. 1/E) was detected. Adult female worms (fig. 1/F) were easily detected as they carrying eggs while males (fig. 1/G) show no eggs. Small sized larvae (fig. 1/H) were also detected beside large number of Oxyurid eggs (fig. 1/I). Protozoal infection was also detected during fecal microscopic examination and included the presence of Nyctotherius (fig. 1/L) and Plantadium (fig. 1/K) species.
Regarding hematological examination (Table 1) of diseased tortoises, statistical analysis showed significant decrease (P≤0.001) in PCV and relative lymphocytic count. This decrease was also significant (P≤0.01) for both hemoglobin concentration and RBCs count. Significant (P≤0.001) increase was detected in both WBCs count and relative eosinophilic count. All the results were compared with apparent healthy group.
Table 1: Hematological parameters in diseased tortoises compared with healthy group:
| Parameter | Control group | Diseased group | |
|
Hematological parameters |
PCV (%) | 24.8 ± 1.31 |
17.7 ± 1.20 a |
| Hb (g/L) | 90 ± 6.6 |
62 ± 5.2b |
|
|
RBCs count (×106 /µl) |
0.84 ± 0.06 |
0.51 ± 0.05 b |
|
|
TLC (×103/ µl) |
4.52 ± 0.35 |
6.34 ± 0.13 a |
|
| Heterophils (%) | 45.2 ± 4.3 | 57.7 ± 5.00 | |
| Lymphocytes (%) | 29.4 ± 2.53 |
15 ± 2.2 a |
|
| Monocytes (%) | 20.4 ± 1.25 | 15.7 ± 2.61 | |
| Eosinophils (%) | 4.8 ± 0.76 |
11.5 ± 1.41 a |
|
| Basophils (%) | 0 ± 0.00 |
0 ± 0.00 |
|
(a: P≤0.001), (b: P≤0.01) and (c: P≤0.05)
In terms of serum biochemical examination (Table 2) among diseased tortoises, statistical analysis showed significant (P≤0.01) decrease in total protein, albumin and glucose levels. Marked significant decrease (P≤0.001) was recorded for serum calcium level while both potassium and phosphorus levels showed mild significant (P≤0.05) decrease. All the results were compared with apparent healthy group.
Table 2: Serum biochemical parameters in diseased tortoises compared with healthy tortoises
| Parameter | Control group | Diseased group | |
|
Biochemical parameters |
Total proteins (g/L) | 48 ± 3.1 |
34 ± 2.3 b |
| Albumin (g/L) | 24 ± 1.7 |
15 ± 1.3 b |
|
| Globulin (g/L) | 23 ± 2.6 | 18 ± 1.3 | |
| A/G ratio | 1.11 ± 0.11 | 0.84 ± 0.05 | |
| ALT (µkat/L) | 0.05 ± 0.01 | 0.05 ± 0.01 | |
| Glucose (mmol/L) | 3.9 ± 0.33 |
2.2 ± 0.32 b |
|
| Sodium (mmol/L) | 120.3 ± 4.2 | 112.1 ± 5.03 | |
| Potassium (mmol/L) | 3.6 ± 0.26 |
3 ± 0.13 c |
|
| Calcium (mmol/L) | 2.6 ± 0.15 |
1.37 ± 0.20 a |
|
| Phosphorus (mmol/L) | 1.7 ± 0.17 |
1.07 ± 0.17 c |
|
(a: P≤0.001), (b: P≤0.01) and (c: P≤0.05)
Anatomical study
Concerning the anatomical study, the esophagus (fig. 2B, C&E/7) was short, dorsal to the trachea at midline then moved toward the left side to reach the cardia of stomach. Also, it has small longitudinal folds without papillae (fig. 4J/1). Gastro esophageal sphincter (fig. 2C/8) was wide and conical in shape with small longitudinal folds internally without papillae (fig. 4J/2). The diseased esophageal and Gastro esophageal sphincter mucosa has decreased number of folds in addition to thinning of the mucosa (fig4N/1 &2).
The stomach (fig.2C/9) was J-shaped which began by a left cardiac region, a wide fundic region dorsoventral to the left and middle lobes of liver in case of healthy GIT or displaced more laterally to the left side in the diseased GIT (fig.3F/9). The pyloric region passed to the right side and ends by a wide pyloric sphincter (fig.2 B&C/10) dorsal to the right lobe of liver. The healthy stomach mucosa has large corrugated longitudinal folds (fig.4J/3), while the affected one has mostly sloughed mucosa (fig.4N/3).
The small intestine was a slightly convoluted tube composed of duodenum (fig.2B &C/11), jejunum (fig.2E/12) and ileum (fig.2E/13) forming a nearly similar in diameter tube except at the duodenal flexures was narrower. Duodenum started from the pyloric region at the right side then descended caudally at the right dorsal to the cecum (fig.2C/14). Duodenum has large longitudinal folds inter nally, while the jejunum (fig.4K/6) has dense thin longitudinal folds with small papillae, also the same was found in ileum (fig.4L/7) with fewer folds. There was no external demarcation between jejunum and ileum (fig.2E/12&13).
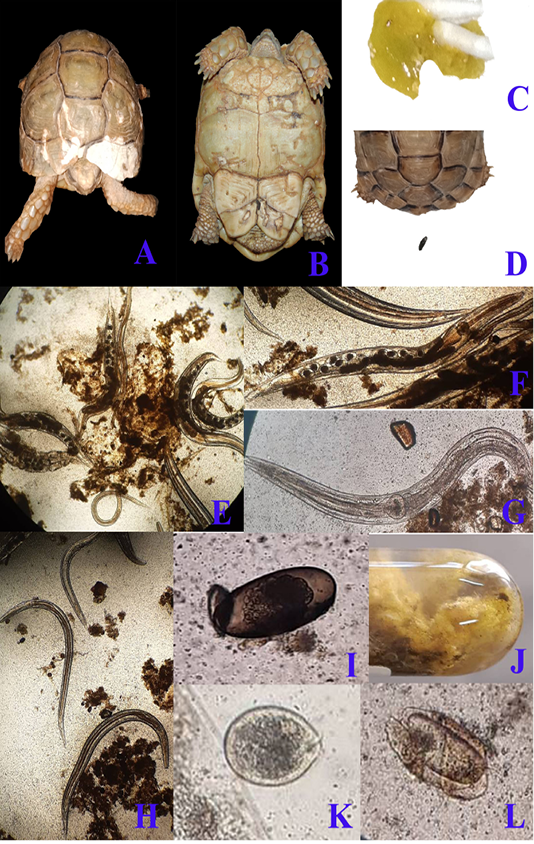
Figure 1: A & B; Clinical presentation of gastroenteritis in Egyptian tortoise, photographs showing multiple areas of white discoloration, pores and cracks in carapace and plastron respectively which indicates severe minerals deficiency due to diarrhea. C: photograph showing diarrhea with the presence of numerous motile worms. D: photograph showing the normal configuration of stool. Fecal samples analysis of diarrheic tortoises; E: Severe infestation with large numbers of adult males, females and larvae of Oxyurid species (4X). F: Adult female Oxyurid species contains large number of eggs (10X). G: Adult male Oxyurid species (10X). H: Larvae of Oxyurid species (4X). I: Oxyurid species egg showing central embryo (40X). J: Gross appearance of diarrheic stool with adult pin worms (Oxyurid species). K: Plantadium species trophozoite (40X). L: Nyctotheris species trophozoite (40X).
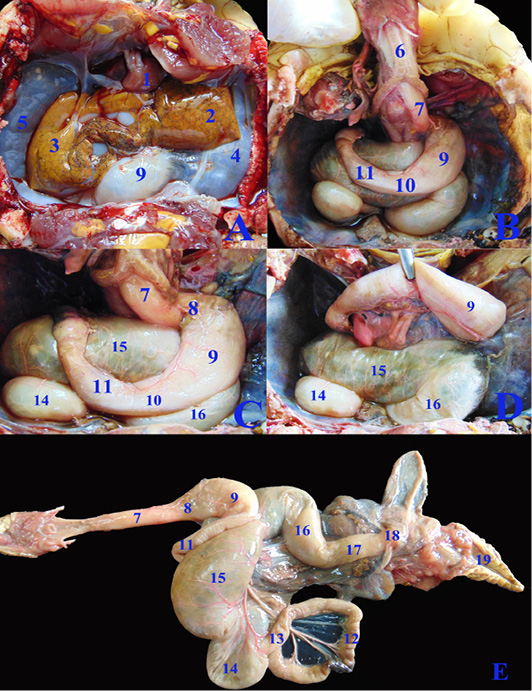
Figure 2: A, B, C, D; The coelomic cavity of Healthy female Desert Egyptian tortoise after removing the plastron (fresh specimen). E: Photograph showing separated healthy GIT. 1. Heart 2. Left lobe of liver 3. Right lobe of liver 4. Left lung 5. Right lung 6. Trachea 7. Esophagus 8. Gastro esophageal sphincter 9. Stomach 10. Pyloric sphincter 11. Duodenum 12. Jejunal loops. 13. Ileum 14. Cecum 15. Ascending colon 16. Transverse colon 17. Descending colon 18. Cloaca.
The cecum (fig.2C, D & E/14) was a large sac like dilation at the right caudal part of coelomic cavity, just caudal to the duodenum and the ascending colon. The largest part of colon, the ascending colon (fig.2C, D & E/15) at the mid dle region of coelomic cavity, dorsal to the stomach and duodenum then the transverse colon (fig.2C, D & E/16) which passed from the left side to the middle region to be converted into the straight, narrower and short part, the descending colon (fig.2E/17) To reach finally the cloaca (fig.2E/18). Internally the large intestine (fig.4L&M) had several sacculations with smooth mucosa and followed by narrow parts of small misshapen folds in the mucosa. The cloaca was the common for both digestive and urogenital organs. It had internally medium sized longitudinal folds (fig.4M/12).
In case of severe gastroenteritis and accumulation of gases in the large intestine, the cecum (fig.3F/14) displaced to the left side covering most of the dorsal surface of the stomach (fig.3F/9) and the duodenum (fig.3G/11), also the ascending colon (fig.3F&G/15) extended from the middle coelomic cavity to reach also the right side of coelomic cavity.
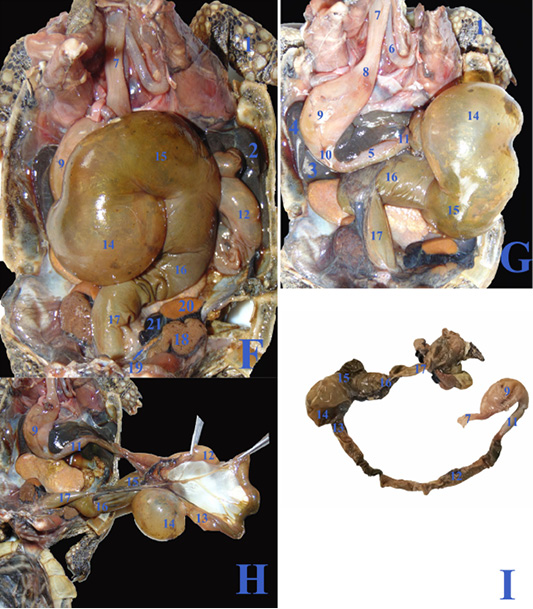
Figure 3: F, G, H; The coelomic cavity of diseased male Desert Egyptian tortoise after removing the carapace (the tortoise was dissected after death freshly). I: Photograph showing separated diseased GIT. 1. Right forelimb 2. Right lobe of liver 3. Left lobe of liver 4. Spleen 5.Pancreas 6. Right bronchus 7. Esophagus 8. Gastro esophageal sphincter 9. Stomach 10. Pyloric sphincter 11. Duodenum 12. Jejunal loops. 13. Ileum 14. Cecum 15. Ascending colon 16. Transverse colon 17. Descending colon 18. Right kidney 19. Right adrenal gland 20. Right testes 21. Right epididymis.
Histopathology
Microscopic examination of the healthy esophageal wall tissue sections showed that it was lined by stratified squamous non keratinized epithelium (10-15 cells thick) all over the esophageal regions (fig.5 A,B arrows) except near the cardiac junction, it was transformed into stratified layers of columnar epithelial cells alternated with goblet cells (fig.5 C arrow). Densely backed mucous glandular tissue was observed in the underlying lamina propria (fig.5 A, B & C star) with delicate loose connective tissue in the sub mucosal layer. Tunica muscularis was arranged into inner circular and outer longitudinal smooth muscle fibers (fig.5A dashed arrow) and surrounded by serosa.
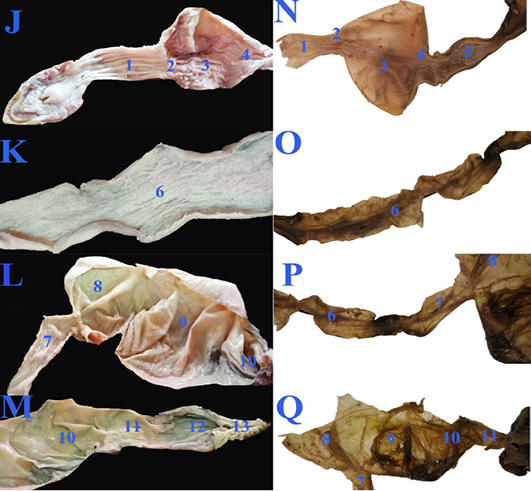
Figure 4: The macroscopic internal structure of the healthy GIT (J, K, L &M) and the diseased GIT (N, O, P &Q) of Desert Egyptian tortoise. 1. Esophageal mucosa 2. Gastro esophageal sphincter mucosa 3. Gastric mucosa 4. Pyloric sphincter mucosa 5. Duodenal mucosa 6. Jejunal mucosa 7. Ileum mucosa 8. Cecal mucosa 9. Ascending colon mucosa 10.transverse colon mucosa 11. Descending colon mucosa 12. Cloaca 13. Vent.
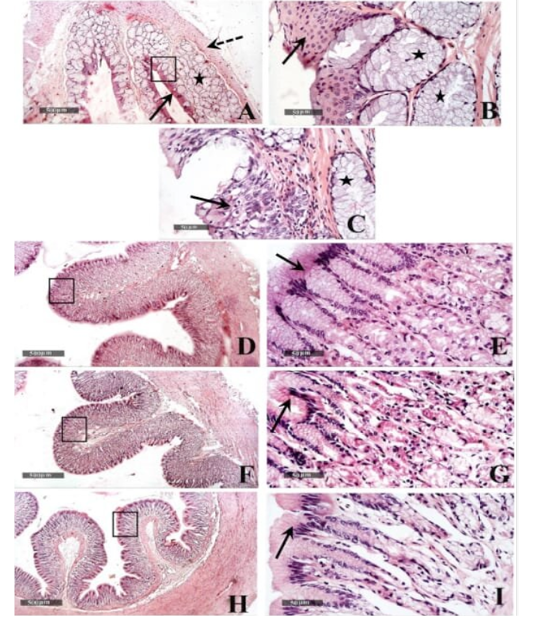
Figure 5: Histological features of esophageal samples (A, B & C), cardiac region and glands (D & E), fundic region and glands (F& G); pyloric region and glands (H & I). H&E stain – 40X & 400 X magnifications.
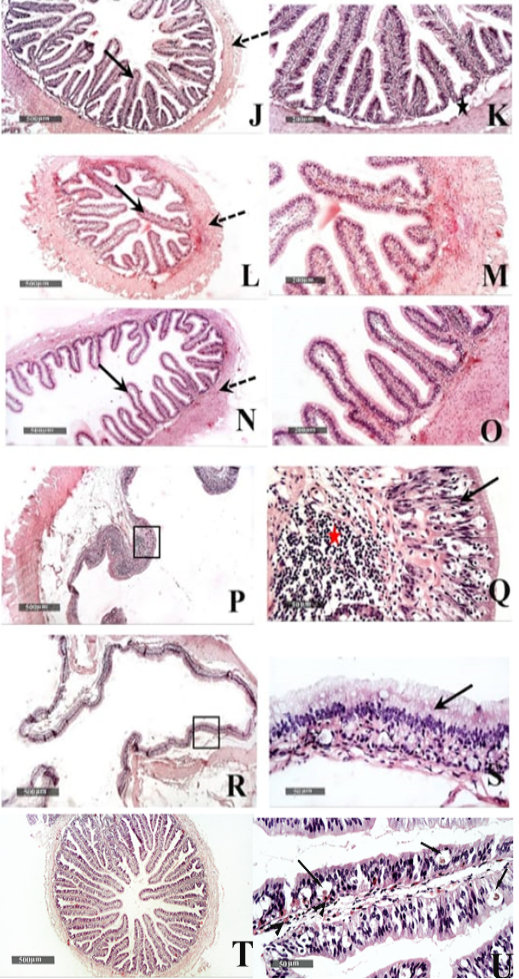
Figure 6: Histological features of Duodenum (J & K), Jejunum (L & M), and Ileum (N &O) - 40 X & 200 X magnifications. Cecum (P & Q) and colon (R & S) H&E stain – 40X & 400 X magnifications. T&U: showing Microscopic examination of intestinal/duodenal samples revealed many intra epithelial parasitic stages all over intestinal villi (arrow) accompanied with underlying lamina propria or intra epithelial eosinophilic infiltrates in (arrowhead).
Gastric tissue samples examination from the healthy tortoises demonstrated normal morphological features of dif ferent cardiac (fig.5 D & E), fundic (fig.5 F & G) and pyloric regions (fig.5 H & I), with simple columnar secretory epithelial lining (fig.5E, G & I arrows). Cardiac glands showed shallow coiled mucous tubular glands (fig.5 E star) unlike more deep straight pyloric glands (fig.5 I star). Evident mucous neck cells were shown lining tubular fundic glands; however, basophilic chief cells and eosinophilic parietal cells couldn’t be distinguished by routine light microscopic examination in all samples (fig.5 G). Outer muscular layers were arranged into inner circular and outer longitudinal smooth muscle fibers with surrounding thin serosal membrane.
Small intestinal samples from different anatomical regions including duodenal (fig.6 J &K), jejunal (fig.6 L & M) and ileum (fig.6 N & O) regions of the healthy tortoises demonstrated normal organization of intestinal villi (fig.6 J, L&N arrows) with simple columnar absorptive epithelium lining and lacking of evident crypts of Lieberkühn. Fibrous connective tissue of propria and submucosa (fig.6 K, M& O stars) as well as thick outer muscular coat (fig.6 J, L & N dashed arrow) were demonstrated. No evidence for sub mucosal Brunner’s glands of duodenum was observed in serial tissue sections of all processed samples (fig.6 K star).
Large intestine samples from cecum (fig.6 P & Q) and colon (fig.6 R &S) regions of the healthy tortoises showed lining epithelium of single layer of tall thin columnar epithelium (fig.6 Q & S arrow) alternated with abundant goblet cells. More abundant gut associated lymphoid tissue were recorded in cecal region samples (fig.6 Q red star) and surrounded with outer inner circular and outer longitudinal smooth muscles coat. The intestinal/duodenal samples of the diseased tortoises revealed many intra epithelial parasitic stages all over intestinal villi (fig. 6 U arrows) accompanied with underlying lamina propria or intra epithelial eosinophilic infiltrates (fig. 6 U arrowhead).
Discussion
The clinical presentation among diseased desert tortoises included dullness, anorexia, lethargy, dehydration and weight loss. All these deteriorations were resulted from the heavy infestation with intestinal parasites. These findings were in the same context of (Pasmans et al., 2008; Martinez-Silvestre, 2011). Also, Loukopoulos et al. (2007) added that nematode infection may result in deleterious effects on the intestinal absorptive capacity that leads to severe weight loss and death.
Numerous pores and cracks found on the carapace and plastron were attributed to severe calcium deficiency and metabolic bone disease made by parasites. Fecal examination applied at the time of admission revealed Oxyurid species eggs, adult male and female worms. These results were in agreement with (Shanker et al., 2015; Hallinger et al., 2018).
Hematological examination in diseased tortoises revealed significant decrease for PCV, hemoglobin and RBCs count which come in the same findings of (Diaz-Figueroa, 2005; Ali et al., 2018). Relative lymphocytic count showed significant decrease in proportion to increase of relative eosinophilic and heterophilic count during acute phase of the disease. Significant increase in WBCs count in the present study was explained by severe gastro-enteritis and parasitic damage of GIT mucosa.
In the term of serum biochemical examination in diseased tortoises, the present results showed significant decrease in total protein, albumin and glucose levels. These findings were similar to some extent that of Martinez-Silvestre, (2011). who recorded marked decrease to such levels but without obvious significance. The present study revealed significant decrease in serum calcium level in diseased tortoises. This result explained as Balantidium spp. may interfere with intestinal calcium absorptive capacity which may resulted in metabolic bone disease, cracked and porous carapace and plastron (Pasmans et al., 2008). Mild significant decrease was recorded for serum potassium and phosphorus levels. These findings may result from severe diarrhea and heavy worm infestation. Decreased phosphorus level comes in the line with calcium deficiency due to Balantidium spp. infection.
In the present anatomical study the esophagus was short, dorsal to the trachea at midline then moved toward the left side to reach the cardia of stomach. Also, it has small longitudinal folds without papillae, In accordance with (Wyneken, 2011). Per-contra, Barboza (1995) stated that in the herbivorous wild tortoise Xerobates agassizii, the esophagus has Cornified esophageal epithelia. In addition to Melo et al. (2019) revealed that the green turtle (Chelonia mydas) esophagus has thin and conical keratinized dermal papillae.
Gastro esophageal sphincter was wide and conical in shape with small longitudinal folds internally without papillae, similar to observations of, Magalhães et al. (2012) cited that the sea turtles Gastro esophageal sphincter was devoid of papillae internally which demarcated the stomach.
Similar to the observations of Mansoori et al. (2018) as they mentioned that the stomach was J in shape, Per-contra Barboza, (1995) stated that the stomach was L-shaped, also McArthur et al. (2004) stated that it was fusiform. In accordance with Taylor et al. (1996) stomach was situated ventrally to the left lobe of the liver. In our study the healthy stomach mucosa has large corrugated longitudinal folds, conversely, Mansoori et al. (2018) documented that the mucosal folds were absent cranially and caudally at the stomach.
In the present study as well as Taylor et al. (1996) mentioned that the small intestine was convoluted. There was no external demarcation between jejunum and ileum as stated by Magalhães et al. (2012). Similar to the observations of Mansoori et al. (2018), the duodenum has two flexures. Also, Magalhães et al. (2012) documented that the small intestine internally had reticular folds. In accordance with McArthur et al. (2004) as they cited that the small intestine lies in the caudal coelomic cavity, On the other hand, Magalhães et al. (2012) cited that the small intestine had a constant diameter throughout its length and the duodenum was located medially in the coelomic cavity.
In the desert tortoise of our study, the healthy cecum was large sac like dilation; conversely, the cecum formed a small dilatation as mentioned by (Taylor et al., 1996). In accordance with Magalhães et al. (2012) that cecum and colon has saccular and narrow areas, in addition to uneven mucosal folds.
Similar to the observations of McArthur et al. (2004) the caecum situated at the right caudal region of the coelom. The rest of large intestine was the ascending, transverse and descending colons. The cloaca was the last part of the urogenital and digestive tracts (Divers, 2015). The cloacal mucosal had rectilinear folds which was in agreement with (Magalhães et al., 2012).
Histologically, similar to the observations of Barboza (1995), our study revealed that the esophagus had large number of mucus glands. Also, the esophageal region near the cardiac junction transformed into stratified layers of columnar epithelial cells alternated with goblet cells, which was in conjunction with Wyneken (2011) who cited that it contained secretory cells and with Perez‐Tomas et al. (1990) who reported that the lower third of the esophagus of was lined by a stratified columnar epithelium composed of a superficial layer of columnar mucous cells, a middle layer of polyhedral cells, and a cuboidal cells forming the basal layer.
In accordance with Stevens and Hume (2004), our results showed that the esophageal outer muscular layers were arranged into inner circular and outer longitudinal smooth muscle fibers.
In addition, the results of the current study revealed that the stomach was lined with simple columnar secretory epithelium in the cardiac, fundic and the pyloric regions, and that the fundic gland was lined with the mucous cells without clear distinguish between the acidophilic oxyntic (parietal) cells and the basophilic peptic (chief) cells. These findings were in consistence with the observation of Perez‐Tomas et al. (1990) that revealed the mucous secretory columnar cells of the stomach lining epithelium, in addition to the lining of the fundic gland with mucous neck cells and oxyntic peptic cells.
Similar to observations of McArthur et al. (2004), the small intestine mucosa had simple columnar epithelium. On the other hand, Tlachia et al. (2014) mentioned that the small intestine mucosa had pseudo-stratified columnar cells with goblet cells. In addition, our study added that there was no evidence for sub mucosal Brunner’s glands of duodenum. These findings come in line with the results of Perez-Tomas et al. (1990) exhibited the absence of Lieberkiihn crypts, Brunner glands, and Paneth cells in the small intestine. They also reported the columnar lining of the large intestine which was observed in our study alternated with abundant goblet cells.
Conclusions
In our study the examined Egyptian tortoises (Testudo kleinmanni) suffering from Gastroenteritis were diagnosed as parasitic infestations with Oxyurid worms. In addition to Protozoal infection included the presence of Nyctotherius and Plantadium species. Marked anorexia and greenish watery diarrhea were the main clinical signs. A significant increase in relative eosinophilic count was recorded. Marked significant decrease was recorded for serum calcium level leading to areas of discoloration at the carapace. Normal anatomical positions of the GIT was changed as, in case of severe gastroenteritis and accumulation of gases, the cecum was displaced to the left side and the ascending colon reached the right side of coelomic cavity. Also the stomach was displaced more laterally to the left side in the diseased GIT. There was no external demarcation between jejunum and ileum. Densely backed mucous glandular tissue was found in the esophageal mucosa. No evidence for sub mucosal Brunner’s glands of duodenum were observed. More abundant gut associated lymphoid tissue was recorded in cecal region samples. Microscopic examination of intestinal/duodenal samples revealed many intra epithelial parasitic stages all over intestinal villi accompanied with underlying lamina propria or intra epithelial eosinophilic infiltrations.
Authors Contribution
Reem Rashad Tahon, Yara Sayed Abouelela we studied the anatomical part as a whole. Hitham Abdel-Saeed: he made all the clinical, hematological, biochemical examinations as well as the statistical analysis. Mohamed Abdel-Razik Khattab, Zainab Sabry Othman Ahmed they studied the histological part as a whole.
Conflict of interest
There is no conflict.
References






