Advances in Animal and Veterinary Sciences
Research Article
Multi-Drug Resistant Pasteurella multocida and Mannheimia haemolytica Strains Isolated from Different Hosts Affected by Pneumonic Pasteurellosis in Egypt
Amany Dieb Bahr1, Fayez Awad-allah Salib1, Yousef Adel Soliman2, Mahmoud Mohamed Amin1*
1Department of Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; 2Department of Biotechnology, Central laboratory for Evaluation of Veterinary Biologics, Veterinary Serum and Vaccine Research Institute, Cairo, Egypt.
Abstract | Pasteurella multocida (P. multocida) and Mannheimia haemolytica (M. haemolytica) are widespread pathogens, resulting in economically significant animal diseases. This study aimed to investigate some of the epidemiological aspects of P. multocida and M. haemolytica infections in Egypt, proper diagnosis of P. multocida and M. haemolytica infections, describe the distribution of capsular types of P. multocida isolates and measure the prevalence of multi-drug resistance in bacterial isolates recovered from cattle, buffaloes, sheep, and goats (ruminants) suffering from respiratory manifestations. A total of 155 deep nasal swabs were collected from 20 cattle, 37 buffaloes, 80 sheep, and 18 goats. Detection of 24 P. multocida and 12 M. haemolytica isolates from tested samples was carried out by bacteriological isolation, then identified by biochemical tests, and confirmed by polymerase chain reaction (PCR). The highest rate of infection with P. multocida and M. haemolytica has been found in young males (0-6 months age group). P. multocida capsular group A was found in the majority of the P. multocida strains (87.5%), while group D bacteria were identified in only three samples. Capsular groups B, E, and F have not been detected. The antimicrobial susceptibility pattern of P. multocida and M. haemolytica isolates indicated a high prevalence of multi-resistance to the majority of antimicrobials used as high resistance was detected against ampicillin, amoxicillin, penicillin-G, tetracycline, streptomycin, cefotaxime and chloramphenicol, however, 100% sensitivity was demonstrated by M. haemolytica isolates to gentamicin. Therefore, continuous monitoring of antimicrobial resistance is important to prevent the dissemination of resistant bacteria..
Keywords | Antimicrobial resistance, Capsular groups, M. haemolytica, P. multocida, Ruminants
Received | October 17, 2020; Accepted | December 03, 2020; Published | January 15, 2021
*Correspondence | Mahmoud Mohamed Amin, Department of Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; Email: [email protected]
Citation | Bahr AD, Salib FA, Soliman YA, Amin MA (2021). Multi-drug resistant Pasteurella multocida and Mannheimia haemolytica strains isolated from different hosts affected by pneumonic pasteurellosis in Egypt. Adv. Anim. Vet. Sci. 9(3): 356-364.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.356.364
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Bahr et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pneumonic pasteurellosis is among the most economically important infectious diseases of ruminants with a high prevalence all over the world. P. multocida and M. haemolytica are the causative agents of the disease. They are commensals in the upper respiratory tract of clinically healthy ruminants, they can gain access to the lungs and are able to induce disease in animals with impaired pulmonary defense mechanisms. Physical or physiological stress induced by unfavourable climatic and ecological conditions, including severely bad weather, improper management, co-mingling, overcrowding, shipping or prior infection with mycoplasma, respiratory viruses or certain other pathogens, may therefore contribute to pneumonic pasteurellosis (Tadesse et al., 2017).
The acute febrile course of pneumonic pasteurellosis is characterized by death of infected animals within few days after the onset of clinical signs if they are not properly diagnosed and treated, while animals that withstand the acute attack can get chronically infected (Tadesse et al., 2017; Jesse et al., 2019).
The animal breed, sex, age, and season can be effective in the occurrence of pasteurellosis (Karimkhani et al., 2011).
In 1881, Louis Pasteur showed that P. multocida was the cause of fowl cholera. Since then, it has been known that several other economically significant diseases in different animal species are caused by this Gram-negative bacteria (Harper et al., 2006). P. multocida is the cause of pneumonic pasteurellosis in cattle, water buffaloes, sheep, and goats (E-Kobon et al., 2017; Homayoon et al., 2018) and haemorrhagic septicemia in cattle and buffaloes (Quinn et al., 2002). Five P. multocida capsular serogroups (A, B, D, E and F) and 16 somatic serotypes (1 to 16) have been identified by serotyping techniques. P. multocida-PCR and capsular PCR are effective methods for early identification as well as serogrouping of P. multocida isolates, particularly in epidemiological studies compared to traditional serogrouping methods, which is time-consuming and involves the development and maintenance of a hyperimmune sera battery (Townsend et al., 2001; Kumar et al., 2009).
M. haemolytica formerly P. haemolytica biotype A comprises serotypes 1, 2, 5-9, 12-14, 16, and 17 (Angen et al., 1999). Serotyping methods are not specific enough for M. haemolytica reliable identification (Jaramillo-Arango et al., 2007). M. haemolytica was known to be the most significant pathogen causing severe pneumonia in bovine respiratory disease (Confer and Ayalew, 2018) and ovine pneumonic pasteurellosis (Tabatabaei and Abdollahi, 2018).
The most effective method for managing Pasteurella and Mannheimia infections is using antimicrobial agents. However, imprudent antimicrobial usage dramatically increases the risk of selecting resistant bacteria, enabling the dissemination of resistance genes located on plasmids and transposons, thereby reducing the effectiveness of presently approved antimicrobials for the treatment of food-producing animals (Kehrenberg et al., 2001).
The purpose of this study was to explore certain aspects of P. multocida and M. haemolytica epidemiology, diagnosis of P. multocida and M. haemolytica infections using bacterial isolation and identification, apply molecular diagnosis of P. multocida and M. haemolytica, describes the distribution of capsular types of P. multocida isolates and measures the prevalence of multi-drug resistance in bacterial isolates recovered from cattle, buffaloes, sheep, and goats suffering from respiratory manifestations.
MATERIAL AND METHODS
Ethical approval
Animal ethical approval was obtained from the Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Cairo University.
Animals and samples
A total of 155 deep nasal swabs were collected from 20 cattle, 37 buffaloes, 80 sheep, and 18 goats of different ages and sexes from different localities in the Giza Governorate during the period from October 2018 to December 2019. The animals were suffering from respiratory manifestations.
Clinical examination
Animals showed clinical signs of coughing and copious nasal discharge in association with rectal temperatures above 39.5°C, congested mucous membranes, and respiratory discomfort were examined.
Epidemiological studies
Prevalence of P. multocida and M. haemolytica isolation in the examined animals was reported by species, ages, sexes, and season of the year.
Isolation and identification of P. multocida and M. haemolytica
Sterile cotton swabs were used to collect the deep nasal swab samples from the nostrils of affected animals. After sampling, the tips of each nasal swab were placed into individual tubes containing Amies transport medium (Oxoid, UK) and immediately transported on ice to the laboratory. Upon arrival at the laboratory, the swabs were removed from the transport media and placed individually into brain heart infusion (BHI) broth (Oxoid, UK) and incubated aerobically at 37°C for 6-8 hours.
A loopful from the broth was streaked onto blood agar media supplemented with 7% defibrinated sheep blood and MacConkey agar media (Oxoid, UK) and incubated aerobically for 24 hours at 37°C. The suspected isolates of P. multocida and M. haemolytica were biochemically tested and microscopically examined after staining with Gram’s stains (Quinn et al., 2002).
Molecular identification of P. multocida and M. haemolytica
Whole cells obtained from single colonies grown on 7% sheep blood agar were used for DNA extraction as the template in the amplification reactions. DNA was extracted from bacterial cultures with the QIAamp DNA Mini Kit (Qiagen GmbH, Germany).
For identification of P. multocida (KMT1 gene) and M. haemolytica (Rpt2 gene), a uniplex PCR assay was applied for each species separately in a 25 μl PCR amplification mixture containing 12.5 μl of 2X Taq PCR Master Mix (Qiagen, Germany), 1 μl of each primer (Metabion company, Germany), 5 μl of DNA template and 5.5 μl of PCR grade water (Jena Bioscience, Germany).
The amplification condition was applied according to (Townsend et al., 2001) for the identification of P. multocida and according to (Deressa et al., 2010) for the identification of M. haemolytica.
Molecular capsular serogrouping of P. multocida strains
All genomic DNA of the identified P. multocida strains were subsequently PCR tested for different capsular serogroups A, B, D, E and F based on capsule biosynthesis genes (hyaD-hyaC, bcbD, dcbF, ecbJ, and fcbD, respectively) using multiplex PCR according to (Townsend et al., 2001). The PCR reaction was performed in a final volume of 50 μl PCR amplification mixture using 25 μl of 2X Taq PCR Master Mix, 1 μl of each primer, 10 μl of DNA template, and 5 μl of PCR grade water.
Confirmatory uniplex PCR
Based on the findings of the multiplex PCR, the existence of certain capsular serogroups in the isolates under investigation was confirmed by uniplex PCR according to (Townsend et al., 2001). All amplifications were performed with the (Biometra, Germany) thermal cycler. Target genes, oligonucleotide primer sequences, the expected product size in different PCR assays with references are illustrated in Table 1.
Gel Analysis of the PCR Products: Aliquots of 7μL of the amplified PCR products were separated by electrophoresis on 1.5% agarose gel stained with ethidium bromide in 1x TAE EDTA [pH 8.0] buffer at room temperature. Finally, results were visualized under UV light and documented with a GelDoc 1000 fluorescent imaging system (Bio-Rad).
Antimicrobial resistance testing
Antimicrobial resistance test was conducted on P. multocida and M. haemolytica isolates using the Kirby Bauer disk diffusion method (Bauer et al., 1966). The isolates were tested to 12 different antimicrobials across 7 antimicrobial classes: (I) beta lactams (ampicillin, amoxicillin, penicillin-G); (II) tetracyclines (tetracycline); (III) fluoroquinolones (ciprofloxacin, enrofloxacin); (IV) aminoglycosides (gentamicin, spectinomycin, streptomycin); (V) cephalosporins (cefotaxime); (VI) folate pathway antagonists (trimethoprim-sulfamethoxazole), (VII) phenicols (chloramphenicol). The interpretations were carried out according to (NCCLS, 2002; CLSI, 2006, 2008).
RESULTS
Epidemiological studies
Isolation findings for P. multocida and M. haemolytica in the examined cattle, buffaloes, sheep, and goats regarding sex, season, and age are shown in Tables 2 and 3.
Isolation and identification of P. multocida and M. haemolytica
On blood agar, P. multocida appeared as moderate size, round, greyish mucoid colonies, non-haemolytic, and has a characteristic sweetish odour while M. haemolytica is beta-haemolytic and has no odour. On MacConkey agar, M. haemolytica grew as pinpoint red colonies while P. multocida did not grow on MacConkey agar.
Table 1: Primer sequences for detection of P. multocida and M. haemolytica genes.
| Bacterial species | Gene | Name | PCR primer sequence (5’-3’) | Amplicon size (bp) | References |
| P. multocida | KMT1 |
KMT1T7 KMT1SP6 |
ATCCGCTATTTACCCAGTGG GCTGTAAACGAACTCGCCAC |
460 | |
| hyaD-hyaC |
CAPA-FWD CAPA-REV |
TGCCAAAATCGCAGTCAG TTGCCATCATTGTCAGTG |
1044 | ||
| bcbD |
CAPB-FWD CAPB-REV |
CATTTATCCAAGCTCCACC GCCCGAGAGTTTCAATCC |
760 | ||
| dcbF |
CAPD-FWD CAPD-REV |
TTACAAAAGAAAGACTAGGAGCCC CATCTACCCACTCAACCATATCAG |
657 | ||
| ecbJ |
CAPE-FWD CAPE-REV |
TCCGCAGAAAATTATTGACTC GCTTGCTGCTTGATTTTGTC |
511 | ||
| fcbD |
CAPF-FWD CAPF-REV |
AATCGGAGAACGCAGAAATCAG TTCCGCCGTCAATTACTCTG |
851 | ||
| M. haemolytica | Rpt2 |
Rpt2-FWD Rpt2-REV |
GTTTGTAAGATATCCCATTT CGTTTTCCACTTGCGTGA |
1022 |
Table 2: Number and percentage of positive P. multocida samples in the examined animals regarding sex, season and age.
| Species |
Sex |
Season |
Age |
Total |
||||||
| Male | Female | Winter | Summer | 0-6 m | 7-12 m | 13-24 m | >24 m | No. | Percentage | |
| Cattle | 6/11 | 1/9 | 4/14 | 3/6 | 5/5 | 0/3 | 1/8 | 1/4 | 7/20 | 35% |
| Buffaloes | 6/23 | 0/14 | 5/5 | 1/32 | 6/12 | - | 0/15 | 0/10 | 6/37 | 16.22% |
| Sheep | 6/54 | 5/26 | 11/75 | 0/5 | - | - | 11/79 | 0/1 | 11/80 | 13.75% |
| Goats | 0/11 | 0/7 | 0/15 | 0/3 | - | - | 0/18 | - | 0/18 | - |
| Total | 18/99 | 6/56 | 20/109 | 4/46 | 11/17 | 0/3 | 12/120 | 1/15 | 24/155 | 15.48% |
| Percentage | 18.18% | 10.7% | 18.35% | 8.69% | 64.7% | - | 10% | 6.66% | 15.48% | - |
Table 3: Number and percentage of positive M. haemolytica samples in the examined animals regarding sex, season and age.
| Species |
Sex |
Season |
Age |
Total |
||||||
| Male | Female | Winter | Summer | 0-6 m | 7-12 m | 13-24m | >24 m | No. | Percentage | |
| Cattle | 4/11 | 2/9 | 5/14 | 1/6 | 0/5 | 1/3 | 5/8 | 0/4 | 6/20 | 30% |
| Buffaloes | 5/23 | 1/14 | 0/5 | 6/32 | 6/12 | - | 0/15 | 0/10 | 6/37 | 16.22% |
| Sheep | 0/54 | 0/26 | 0/75 | 0/5 | - | - | 0/79 | 0/1 | 0/80 | - |
| Goats | 0/11 | 0/7 | 0/15 | 0/3 | - | - | 0/18 | - | 0/18 | - |
| Total | 9/99 | 3/56 | 5/109 | 7/46 | 6/17 | 1/3 | 5/120 | 0/15 | 12/155 | 7.74% |
| Percentage | 9.09% | 5.36% | 4.59% | 15.22% | 35.29% | 33.33% | 4.17% | - | 7.74% | - |
Table 4: Antimicrobial resistance test result of the P. multocida and M. haemolytica isolates.
| Antimicrobials tested |
M. haemolytica |
P. multocida |
||||
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| Ampicillin | - | - | 12(100%) | - | - | 24(100%) |
| Amoxicillin | - | - | 12(100%) | - | - | 24(100%) |
| Penicillin-G | - | - | 12(100%) | - | - | 24(100%) |
| Tetracycline | 1(8.3%) | 1(8.3%) | 10(83.3%) | - | - | 24(100%) |
| Ciprofloxacin | 7(58.3%) | 2(16.7%) | 3(25%) | 7(29.2%) | 2(8.3%) | 15(62.5%) |
| Enrofloxacin | 6(50%) | 1(8.3%) | 5(41.7%) | 3(12.5%) | 3(12.5%) | 18(75%) |
| Gentamicin | 12(100%) | - | - | 8(33.3%) | 2(8.3%) | 14(58.3%) |
| Spectinomycin | 5(41.7%) | 3(25%) | 4(33.3%) | 5(20.8%) | 12(50%) | 7(29.2%) |
| Streptomycin | 1(8.3%) | 2(16.7%) | 9(75%) | 1(4.2%) | 1(4.2%) | 22(91.6%) |
| Cefotaxime | 3(25%) | 2(16.7%) | 7(58.3%) | 1(4.2%) | 2(8.3%) | 21(87.5%) |
|
Trimethoprim-Sulfamethoxazole |
10(83.3%) | - | 2(16.7%) | 6(25%) | 8(33.3%) | 10(41.7%) |
| Chloramphenicol | 3(25%) | 2(16.7%) | 7(58.3%) | 6(25%) | - | 18(75%) |
On TSI agar, both P. multocida and M. haemolytica fermented all sugars and produced yellow slant and butt without H2S or gas production. In the Indole reaction, P. multocida gave red ring at the interface while M. haemolytica gave yellow ring. Both P. multocida and M. haemolytica are catalase, oxidase-positive, and citrate negative.
Both P. multocida and M. haemolytica appeared as small, Gram-negative rods or coccobacilli in Gram’s stained slides.
The frequency of micro-organism detection in this study was 15.48 % (24/155) for P. multocida and 7.74 % (12/155) for M. haemolytica.
Molecular identification of P. multocida and M. haemolytica
PCR using the KMT1 gene confirmed the 24 culture-positive P. multocida isolates, which developed specific 460 bp molecular size bands (Figure 1). Also, the 12 culturally positive M. haemolytica isolates have been confirmed using the Rpt2 gene, which developed specific 1022 bp molecular size bands (Figure 2).
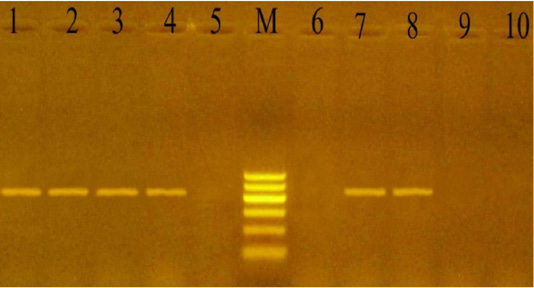
Figure 1: PCR for detection of Kmt1 gene in P. multocida isolates at amplicon size 460 bp. Lane M: Molecular weight marker, 100-600 bp (100bp plus DNA ladder, Vivantis, Malaysia). Lanes 1-4 and 7-8: positive samples. Lanes 5, 6 and 9-10: negative samples.
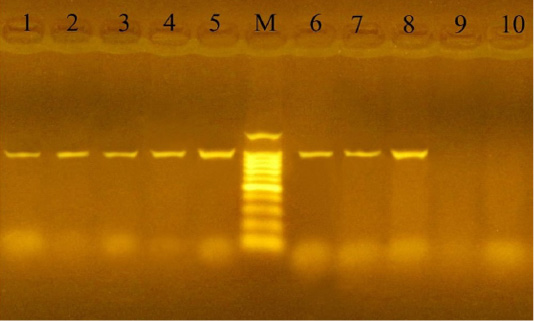
Figure 2: PCR for detection of Rpt2 gene in M. haemolytica isolates at amplicon size 1022 bp. Lane M: Molecular weight marker, 100-1500 bp (100bp plus DNA ladder, Vivantis, Malaysia). Lanes 1-8: positive samples. Lanes 9-10: negative samples.
Molecular capsular serogrouping of P. multocida strains
Capsular serogrouping of the 24 confirmed P. multocida strains revealed that 21 out of the 24 strains belonged to serogroup A (11 from sheep, 7 from cattle, and 3 from buffaloes) with an approximate molecular size of 1044 bp (Figure 3). The isolation percentages of P. multocida serogroup A were, therefore, estimated to be 87.5%. The other 3 P. multocida isolates (isolated from buffaloes) belonged to serogroup D with an approximate molecular size of 657 bp (Figure 4). The isolation percentages of P. multocida serogroup D were, therefore, estimated to be 12.5%. Serogroups B, E, and F have not been identified in the isolated P. multocida.
Antimicrobial resistance testing
P. multocida (n= 24) and M. haemolytica (n= 12) isolates were subjected to a panel of 12 antimicrobials. The antimicrobial resistance pattern of the isolates indicated a high prevalence of multi-resistance to the majority of antimicrobials commonly used to control or treat respiratory diseases as illustrated in Table 4.
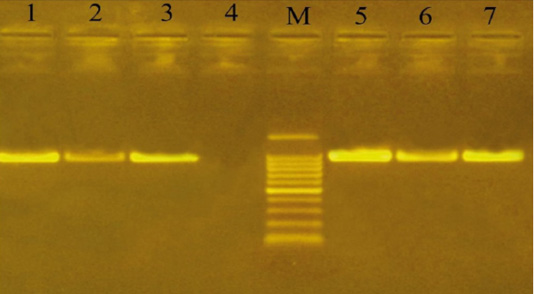
Figure 3: Uniplex PCR for detection of cap-A (hyaD-hyaC gene) in P. multocida isolates at amplicon size 1044 bp. Lane M: Molecular weight marker, 100-1500 bp. Lanes 1-3 and 5-7, positive samples. Lane 4: negative sample.
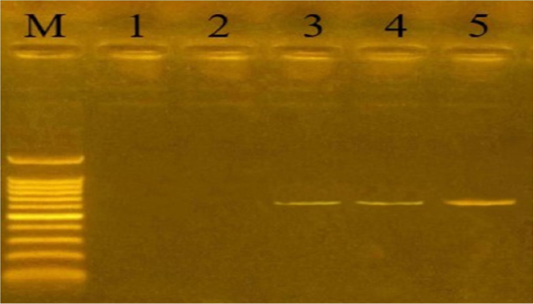
Figure 4: Uniplex PCR for detection of cap-D (dcbF gene) in P. multocida isolates at amplicon size 657 bp. Lane M: Molecular weight marker, 100-1500 bp. Lanes 1-2: negative samples. Lanes 3-5: positive samples.
For M. haemolytica, 100% of the isolates were resistant to ampicillin, amoxicillin, and penicillin-G. Resistance to tetracycline (83.3%) and streptomycin (75%), followed by cefotaxime and chloramphenicol (58.3%) while they were 100% sensitive to gentamycin followed by 83.3%, 58.3% and 50% sensitivity to trimethoprim-sulfamethoxazole, ciprofloxacin, and enrofloxacin respectively.
Similarly, P. multocida isolates showed 100% resistance to ampicillin, amoxicillin, penicillin-G, and tetracycline. Resistance to streptomycin (91.6%), cefotaxime (87.5%), and (75%) of the isolates were resistant to both enrofloxacin and chloramphenicol.
DISCUSSION
Pneumonic pasteurellosis is a very complex multifactorial disease with a widespread prevalence in ruminants. It is often caused by a combination of stress, immunity, and the causative bacteria (P. multocida and M. haemolytica) that commensally occur in the upper respiratory system of susceptible animals (Tadesse et al., 2017).
This study used 155 deep nasal swabs for isolation, identification, and detection of antimicrobial resistance of P. multocida and M. haemolytica which were obtained from cattle, buffaloes, sheep, and goats in Egypt; P. multocida was isolated from 24 samples and M. haemolytica was isolated from 12 samples.
The findings of this study revealed that P. multocida was more prevalent in samples (15.48 %) compared to M. haemolytica (7.74 %); (El-Seedy et al., 2019) has reported similar results in Egypt, probably the lack of effective vaccines, contribute to the increased prevalence of P. multocida (Guo et al., 2020).
P. multocida is an important micro-organism related to pneumonia in cattle. This study has isolated P. multocida from 35% of the samples collected from cattle in accordance with (França Dias de Oliveira et al., 2016) who found that P. multocida (15.50 %) was the most prevalent bacterial species in healthy and unhealthy cattle suffering from respiratory diseases and not isolated M. haemolytica. Also (Nefedchenko et al., 2016) analysis revealed 50% of P. multocida and 11.2% of M. haemolytica in all examined samples of biological material from the infected animals.
In our study, M. haemolytica has not been isolated from sheep which is inconsistent with (Legesse et al., 2018) who demonstrated that M. haemolytica has been correlated with one-third of pneumonic cases suggesting its major role in sheep pneumonia in central Ethiopia, which may be due to annual vaccination using a monovalent vaccine (inactivated P. multocida biotype A) there.
No isolation of either P. multocida or M. haemolytica was carried out from goats in contrast to (Rawat et al., 2019) who identified (7) M. haemolytica and (5) P. multocida isolates out of 10 clinical cases of goat pneumonia.
Young animals especially (0-6 months age group) were found to be at risk for developing pneumonic pasteurellosis. This may be due to the fact that the animal’s immune status is capable of predisposing to bacterial infection and many other predisposing etiological agents as mentioned by (Abera et al., 2014).
Colonization by P. multocida was high in the winter, this is because of environmental stresses such as cold weather and overcrowding as recorded by (Karimkhani et al., 2011) in contrary to M. haemolytica which is higher in the summer.
Males were found to be more vulnerable to respiratory tract infections than females as mentioned by (Karimkhani et al., 2011). This may be because males are used for meat production, which renders them susceptible to the stress of transportation, the stress of repeated unloading, loading, handling, lack of food and water, exposure to inclement weather during transportation, and co-mingling with other animals from multiple sources that may be diseased or latent carrier of respiratory pathogens and shed these pathogens which can cause respiratory diseases in animals (Taylor et al., 2010).
The used PCR protocol identified the P. multocida and M. haemolytica isolates accurately, hence it should be used as an easy, available, and highly repeatable serological typing analogue in veterinary laboratories that enables the identification and genotyping of P. multocida and M. haemolytica strains at every step of bacteriological surveys and, thereby reduce the time needed to approach a diagnosis, making it possible to improve epizootic measures (Nefedchenko et al., 2016).
P. multocida serogroup A was present in the majority of samples (87.5%); in only three cases, group D bacteria were identified. Identification of the P. multocida bacteria of capsule group A in a larger number of samples can indicate that it played a more significant role in developing various diseases in domestic animals than the P. multocida bacteria of capsule group D did as mentioned by (Nefedchenko et al., 2016; Cucco et al., 2017). Bacterial distribution of P. multocida capsule groups B, E, and F among affected animals has not been determined.
In sheep, all P. multocida isolates are of capsular type A, this is similar to the results obtained by (Kumar et al., 2009) indicating the role of this capsular type in the development of sheep bronchopneumonia.
Data analyzed in the present study indicate that multiple antimicrobial resistance against P. multocida and M. haemolytica in cattle, buffaloes, and sheep has been established (Table 4) as stated by (Klima et al., 2014) who found that M. haemolytica and P. multocida isolates exhibited a high rate of antimicrobial resistance, with 45% exhibiting resistance to three or more antimicrobials. Antimicrobials impose selection pressure on bacterial populations that can contribute to the emergence of antimicrobial resistant organisms. Bacterial pre-exposure to antimicrobials was also involved as a risk factor for antimicrobial resistance evolution during subsequent antimicrobial treatments (Gould and MacKenzie, 2002).
The in-vitro sensitivity testing of P. multocida isolates showed 100% resistance to ampicillin, amoxicillin, penicillin-G, and tetracycline, and high resistance to streptomycin, cefotaxime, enrofloxacin, and chloramphenicol. Meanwhile, the in vitro antimicrobial susceptibility testing of M. haemolytica isolates demonstrated 100% resistance to ampicillin, amoxicillin, and penicillin-G and high resistance to tetracycline, streptomycin, cefotaxime, and chloramphenicol. On the other hand, M. haemolytica isolates were 100% sensitive to gentamicin followed by 83.3%, 58.3%, and 50% sensitivity to trimethoprim-sulfamethoxazole, ciprofloxacin, and enrofloxacin respectively.
Tetracycline resistance was prevalent in our study in accordance with (El-Seedy et al., 2019; Guo et al., 2020), this may be due to increased administration of this drug for the treatment of respiratory diseases in animals. Resistance to aminoglycoside in P. multocida isolates is also documented by (Wang et al., 2017) who found that all of the isolates were resistant to at least two types of aminoglycosides.
Resistance to β-lactams among P. multocida and M. haemolytica isolates detected in this study was agreed with (El-Seedy et al., 2019) which is mostly mediated by β-lactam resistance plasmids (Livrelli et al., 1988; Schwarz et al., 1989).
Pasteurella infections are widely managed at the group level with fluoroquinolones and cephalosporins as important antibiotics in human medicine, leading to their overall use in food-producing animals (Vasseur et al., 2017) which contributes to the emergence of resistant strains against these antibiotics. Fluoroquinolone resistance can also be mediated by plasmid (Rodríguez-Martínez et al., 2011).
High resistance was detected against chloramphenicol which disagreed with (Choudhary et al., 2019) who demonstrated that 100% of P. multocida isolates were responsive to chloramphenicol. (Vassort-Bruneau et al., 1996) reported that chloramphenicol resistance is typically attributed to plasmids that encode inactivating enzymes; the chloramphenicol acetyltransferases.
All of M. haemolytica isolates were sensitive to gentamicin, likely due to decreased use of gentamicin in the treatment of animals and 83.3% of the isolates were sensitive to trimethoprim-sulfamethoxazole which is nearly similar to the results obtained by (De Jong et al., 2014).
CONCLUSION
In conclusion, the current investigation indicated that animal age and sex, along with the season of the year, play an important role in the occurrence of pneumonic pasteurellosis. The high prevalence of P. multocida serogroup A indicates its role in the pathogenesis of respiratory diseases in ruminants. This study provided unique information on resistance development in P. multocida and M. haemolytica isolates to most of the antimicrobials used for the treatment of ruminants. Continuous monitoring of antimicrobial resistance is therefore important to assist practitioners in the appropriate selection of antimicrobial agents and the prudent use of these drugs. This also includes the development of new vaccines to effectively prevent pneumonic diseases in food-producing animals and thus reduce the overall use of antimicrobial agents.
ACKNOWLEDGEMENTS
The authors thank the staff members and colleagues in the Department of Medicine and Infectious Diseases, Faculty of Veterinary Medicine, Cairo University, for their continuous help.
AUTHOR’S CONTRIBUTION
Amin MM and Salib FA designed the experiment protocol and the study. Bahr AD and Soliman YA collected and analyzed the samples. All authors were involved in data analysis, scientific discussion, and writing of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






