Advances in Animal and Veterinary Sciences
Research Article
Egyptian Propolis 13: Influence of Propolis and Alginate Propolis NPs on Egyptian-Nubian Goats Serum Immunoglobulins and Cytokines Level
Ahmed G. Hegazi1, Asmaa S. El-Houssiny2, Walid M.A. Sadek3, Faiz M. Al-Guthami4, Ahmed F.M. Al-Gethami4, Ahmed M.A. Sadik3, Tarek Korany Farag5*
1Zoonotic Diseases Department, National Research Centre, Dokki-Giza, Egypt; 2Microwave Physics and Dielectric Department, National Research Centre, Dokki-Giza, Egypt; 3Sheep and Goat Research Department, Agriculture Research Center, Dokki-Giza, Egypt; 4Al Guthami Foundation, Saudi Arabia; 5Department of Parasitology and Animal Diseases, National Research Centre, Giza, Egypt.
Abstract | This investigation aimed to improve and enhance the immune system status of newborn Egyptian-Nubian goats using Alginate nanoparticles (ALg NPs) as a new drug carrier for the oral delivery of propolis. Propolis was selected as a natural additive of colostrum due to its amazing functional properties. In addition, through its implementation into ALg NPs, its handling properties have been improved and potentiated. Alginate-propolis NPs were prepared by the controlled gellification method. Morphological analysis of the ALg-propolis NPs was examined using a Transmission Electron Microscope (TEM). The flavonoids content of the propolis was analyzed by HPLC. Thirty twins Egyptian Nubian goats (Zaraibi) kids were randomly allotted into three groups; 10 in each group. The rearing systems during the suckling period were extended to 13 weeks as follows: C: Free suckling (FS), where the born kids were kept with their dams until being 13 weeks old (control). T1: (FS) + 0.6 ml propolis (twice/week). T2: (FS) + 0.06 ml Alg-propolis NPs (twice/week). The kids were weighed biweekly and the daily body weight gains were recorded. The serum levels of immunoglobulins; IgA and IgG, serum total protein as well as the serum cytokine levels; IFN-γ, TNFα, IL1β, and IL6 after treatment with propolis and ALg-Propolis NPs were determined at different treatment time. The HPLC analysis revealed 15 flavonoid compounds that are characteristic of propolis. The TEM result showed that the ALg-Propolis NPs are discrete and have spherical shapes with small particle sizes in the nanometer scale (10 nm). Also, the results revealed that both propolis and ALg-Propolis NPs caused a significant increase (P<0.05) of the serum IgG and IgA immunoglobulin levels and a decrease of the serum cytokine levels (IFN-γ, TNFα, IL1β, and IL6) of newborn Egyptian-Nubian goats. However, the ALg-Propolis NPs have a more potent effect on the IgG and IgA immunoglobulin levels and cytokine levels than pure propolis. The results indicated that the nano-encapsulation of propolis within ALg NPs reflected on the health status of the kids, increased the titer of the immunoglobulins; IgG and IgA and reduced the pro-inflammatory cytokines. It could conclude that the feasibility of developing a successful propolis oral delivery nano-system on an industrial scale using the ALg NPs to improve the immune status of the Egyptian-Nubian newborn kids.
Keywords | Propolis, Alginate NPs, Egyptian-Nubian goat, Immunoglobulins, Cytokines
Received | October 05, 2020; Accepted | November 03, 2020; Published | January 01, 2021
*Correspondence | Tarek Korany Farag, Department of Parasitology and Animal Diseases, National Research Centre, Giza, Egypt; Email: [email protected]
Citation | Hegazi AG, El-Houssiny AS, Sadek WMA, Al-Guthami FM, Al-Gethami AFM, Sadik AMA, Farag TK (2021). Egyptian propolis 13: Influence of propolis and alginate-propolis NPs on Egyptian nubian goats serum immunoglobulins and cytokines level. Adv. Anim. Vet. Sci. 9(2): 280-288.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.280.288
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hegazi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The immune systems of newborn ruminants are developed during the first two months of their life (Scheerlinck, 2016). But, an increased risk of infection is occurred due to the immune system is not fully functional at birth (Ulfman et al., 2018). Final animal performance was affected by the management of newly born kids, in particular, in the early period of life (Htoo et al., 2015) because the offspring do not have a fully developed immune system (Polizel et al., 2016). Colostrum and milk contain many elements with nutritional importance such as growth factors, cytokines, enzymes, bioactive peptides, and immunoglobulins (Kuralkar and Kuralkar, 2010). The mother transfers passive protection to their kids, mainly in the form of antibodies (Rashid et al., 2012). The colostrum and milk make protection to the newly born kids against infection-related mortality which has been recognized in many cases leading to death (van Neerven, 2014). However, in the first hours of life, small ruminants are more susceptible to disease and mortality as an inadequate amount of colostrum was taken (Hernández-Castellano et al., 2014). Therefore, there is a crucial need to augment this period of immune immaturity and to reduce the risk of infection (Ulfman et al., 2018).
The phenomenon of microorganism’s resistance to antibiotics is leading to the use of natural products as an alternative for chemotherapeutic agents (Ishihara et al., 2001). Since ancient times, propolis has been used in medicine (Hegazi, 2012) due to its biological and pharmaceutical effects such as immunomodulatory (Takagi et al., 2005), antimicrobial (Hegazi et al., 2014), and antiparasitic (Hegazi et al., 2018). It contains biologically active compounds such as phenol compounds, flavonoids (primuletin, chrysine, tecochrysine, akacetine, galangine, morin, robinetin), terpenes, and steroidal alkaloids (Sahinler and Kaftanoglu, 2005). Propolis induces inhibitory effects on the lymphoproliferation, which is associated with its anti-inflammatory property (Sforcin, 2007). It also affects the antibody production as well as different cells of the immune system which are involved in the innate and adaptive immune response (Freitas et al., 2011).
Nanoparticles could exhibit better efficacy in the fields of medical science and biology (Hamouda, 2012). Their nanometer-size could promote effective permeation through cell membranes and stability in the bloodstream. Alginate is a natural polymer found in all species of brown algae (Afrouzan et al., 2012). ALg NPs are well known for their controlled drug release properties and used for the encapsulation of many active pharmaceutical agents (El-Houssiny et al., 2016, 2017).
Several clinical studies have been developed for testing the ability of the nanoparticles such as the highly negatively charged PLGA NPs to reduce the severe inflammation in some diseases as myocardial infarction and acute encephalitis syndrome (Getts, 2014; Getts et al., 2015). Studies have highlighted the mechanistic insight into how the nanoparticles interact with the mononuclear phagocyte system and impact its function during the homeostasis and inflammation (Smith, 2013; Song et al., 2014). Also, several studies have shown the potential of nanoparticle-based therapies for controlling severe inflammation and peripheral immune tolerance in autoimmune disease (Serra and Santamaria, 2015).
However, there is not yet enough common use of nanotechnology in the market (Farjadian et al., 2019) as well as animal production. Therefore, the purpose of this study was to improve the potency of the propolis by encapsulating it within ALg NPs as a natural additive of colostrum. Moreover, it was aimed to enhance the immune system of the Egyptian-Nubian newly born kids which reflected on their health status.
MATERIALS AND METHODS
Materials
Propolis sample was collected from the apiary farm near El-Mansoura City, Dakahlia Governorate, Egypt. The resinous materials were kept in a dark bag in the refrigerator until being extracted with ethyl alcohol. Sodium Alginate was supplied by ROTH, Germany. Calcium chloride was supplied by Qualikems, India. The materials used are with analytical grade.
Propolis extraction
Fifty grams of propolis samples were cut into small pieces and extracted at room temperature with 500 ml of 70% ethanol (twice after 72 hours). The alcoholic extract was evaporated under vacuum at 50oC until dryness (Hegazi et al., 2019). The percentage of the extracted matter is 6.3 g/dry weight.
Preparation of the ALg NPs and propolis-ALg NPs samples
Alginate nanoparticles (ALg NPs) were prepared by the controlled gellification method based on the ionotropic gelation of polyanion with CaCl2 (Rajaonarivony et al., 1993). Alginate solution with concentration (0.1% w/v) was obtained by dissolving the polymer in distilled water at room temperature. Then, 5 ml of CaCl2 solution (36 mM) was added dropwise under constant stirring to 95 ml of alginate solution to induce gellification. The nanoparticles obtained were stirred for three hours at room temperature. For the propolis-loaded ALg NPs sample, 2 ml of propolis with concentration (5mg/ml ethanol) was mixed with the alginate solution for twenty-four hours before CaCl2 addition. This mixture was further stirred for three hours at room temperature. Finally, the nanoparticles were freeze-dried then stored (El-Houssiny et al., 2016, 2017).
Experimental
HPLC analysis of propolis
The dry extract was dissolved in methanol and filtered through a 0.45 μm filter before HPLC analysis (Bruschi et al., 2003). The Propolis sample was analyzed by HPLC (Agilent 1100 series liquid chromatography with a UV detector and an auto-sampler, United States). The column used was a Lichrochart RP-18 (Merck, Darmstadt, Germany; 12.5 x 0.4 cm, 5 μm particle size). The elution was either with water: formic acid (19:1 v: v; solvent A) or acetonitrile (solvent B) and the flow rate was 1 ml/min. Flavonoids were determined and identified by chromatographic comparisons with authentic markers (Tomàs-Barberàn et al., 1993). The flavonoids were detected with a UV detector at 340 and 290 nm. Flavonoid concentration in the propolis sample was calculated according to (Ogan and Katz, 1981).
Morphological analysis of the Propolis-ALg NPs (TEM)
The morphological characteristics of the Propolis-loaded ALg NPs were examined by transmission electron microscope (TEM) (JEM- HR- 2100, Japan). One drop of the particle suspension was spread onto a carbon-coated copper grid and stained using phosphotungstic acid. The sample was left for drying at room temperature. Then, the sample was placed for TEM imaging at 120 kV accelerating voltage (El-Houssiny et al., 2016).
Experimental treatments
Ethical approval
All experimental procedures were performed following the institutional guidelines of the National Research Centre’s Animal Research Committee under protocol number 19140.
Animals
This study was conducted at the Animal Production Research Station; Agriculture Research Center, El-Serw, and National Research Center, Egypt. Thirty twins Egyptian Nubian (Zaraibi) kids were randomly allotted to one of the three treatments (ten in each group) which extended to 13 weeks as follow:
(C): Free suckling (FS), where the born kids were kept with their dams till being 13 weeks old (control).
T1: (FS) + 0.6 ml propolis (twice/week).
T2: (FS) + 0.06 ml Alginate-propolis NPs (twice/week).
The groups which allowed to natural suckling were reared with their dams for 24h/d from born to weaning with giving propolis or ALg-propolis NPs twice/week (Ahmed et al., 2003; Htoo et al., 2015). The goat kids were kept in separate cages to facilitate the proper management. Starter and berseem hay were available posts the first 4th weeks of suckling. The kids were weighed biweekly of life and the daily body weight gains in that period were determined.
Measurement of the serum immunoglobulin
Blood samples were collected from the jugular vein once before feeding (3 animals in each group) at different suckling period. Then, the blood samples were collected into an evacuated tube containing heparin. After that, the serum portion was separated by centrifugation at 3000 × g for 5 min at 4°C and then kept in a deep freezer at -70ºC until the immunological analysis (Dini et al., 2015).
The serum immunoglobulins; IgA and IgG levels of newborn goats after treatment using propolis and ALg-Propolis NPs were determined at different treatment time (7, 14, and 28 days) using an enzyme-linked immunosorbent assays ELISA kit (Calokit-Cabra, Zeu-Immunotec S.L., Zaragoza, Spain) by following the manufacturer’s instruction. Complexes including antigen and antibody couples were tracked. Data were expressed as mg/ml of sample for IgG and μg/ml for IgA. Triple determinations were performed on each plate (Sunderland et al., 2003). Titer was defined as the reciprocal of the highest dilution that produced OD readings more than 0.1 OD unit above background. The absorbance was measured at 405 nm. The total protein was measured using colorimetric techniques as the Lowry method (Lowry et al., 1951).
Measurement of the serum cytokines level
Blood samples were obtained from the goats on the 4th and 12th weeks. The serum samples were diluted to 1/10 in phosphate buffer solution. Then, the cytokines level (IFN-γ, TNFα, IL1β, and IL6) were measured (Hegazi et al., 2017) using a sandwich enzyme-linked immunosorbent (ELISA) assay (Votre Fournisseur AbCys S.A., Paris, France) by following the manufacturer’s instructions. The experiment was carried out three times using three animals per group. The cytokine concentrations were assayed spectrophotometrically by reading the absorbance at wave length 450 nm. The standard curve was constructed by using the cytokine standards. Then, the cytokine concentrations of the unknown samples were calculated from the standard curve and expressed as picograms per milliliter (Chiswick et al., 2012).
Statistical analysis
The results obtained in the present study were represented as means ± standard error and analyzed using analysis of variance (ANOVA). Samples were compared using the Student’s t-test (two-tailed) for unpaired samples with equal variance and calculated using Excel (Microsoft, Seattle, WA). The significance of the difference between mean values at P<0.05 was calculated using the Duncan Multiple Range Test as SAS Institute (2003).
RESULTS AND DISCUSSION
HPLC analysis of propolis
The flavonoid profile of propolis was carried out by the HPLC-based analysis method on a C-18 reverse phase column, using a UV detector. The HPLC analysis was identified 15 flavonoids which are characteristic of propolis (Table 1). The total flavonoids concentration was 24.325 mg/g propolis. The results obtained indicated that the propolis sample in this investigation was typical poplar propolis (Hegazi et al., 2000). The chemical composition of the investigated sample was similar to those previously observed by (Hegazi and El-Hady, 2001; Hegazi et al., 2007). It was clear that the variety of phenolic acids in propolis depends on the vegetation predominating (Hegazi and El-Hady, 2002; El-Hady et al., 2007).
Table 1: Flavonoids assessed by HPLC of Egyptian propolis (conc. mg/g propolis).
| No | Name | Concentration (mg/g propolis) |
| 1 | Galangin | 4.596 |
| 2 | Pinostrobin | 2.896 |
| 3 | Chrysin | 1.981 |
| 4 | Pinobankasin-3-acetate | 1.944 |
| 5 | Luteolin | 1.911 |
| 6 | Pinocembrin | 1.906 |
| 7 | Formonontin | 1.835 |
| 8 | Pinobankasin | 1.823 |
| 9 | Apigenin | 1.641 |
| 10 | Biochanin A | 1.462 |
| 11 | Quercetin-3-methylether | 1.431 |
| 12 | Dimethylallylcaffeate | 1.312 |
| 13 | Phenylethylcaffeate | 0.917 |
| 14 | Quercetin-3,3'-dimethylether | 0.823 |
| 15 | Quercetin-7-methylether | 0.452 |
| Total flavonoids concentration mg/g propolis | 24.325 | |
Up till now, more than 300 chemical compounds have been identified in propolis (Havsteen, 2002; Khalil, 2006; Hegazi et al., 2007). Flavonoids comprise the major part of biologically active substances in propolis (Betances-Salcedo et al., 2017). Propolis has been found to contain aromatic acids and carbonic acids with the benzoic ring in the aliphatic chain (for example, phenolic derivates of cinnamic and coumaric acids), which were characterized by a very potent antimicrobial activity (Hegazi et al., 2000; Miguel and Antunes, 2011). Esters of phenolic acids such as ferulates and caffeates have shown antiviral, antibacterial, and anti-inflammatory activity (Okutan et al., 2005). Also, Caffeic acid phenethyl esters having particularly potent antioxidant activity, scavenging oxygen free radicals, and inhibiting xanthine oxidase (Ramanauskienė et al., 2009).
Morphological analysis of the Propolis-ALg NPs (TEM)
The particle size and surface morphology of the ALg-Propolis NPs were examined by a transmission electron microscopy, Figure 1. It shows that the NPs have a small particle size in the nanometer range with an average particle size of 24 ± 17 nm (Gaumet et al., 2008). Moreover, the TEM view showed that the NPs are spherical in shape and discrete with a smooth surface. Therefore, the TEM results suggested that the ALg-propolis NPs can permeate through the cell membranes more effectively and are stable in the bloodstream due to its small particle size. Thus, this may be reflected in the health status of newborn kids and may enhance their performance (El-Houssiny et al., 2016, 2017).
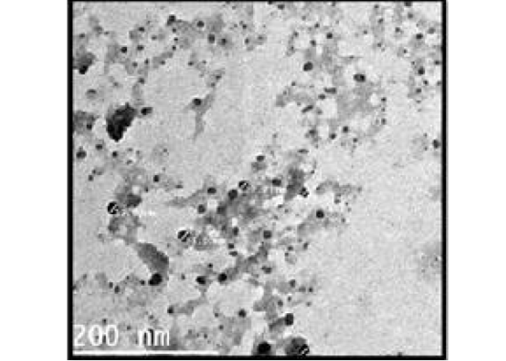
Figure 1: TEM micrograph of ALg-propolis NPs.
Bodyweight
The body weights of the goat kids of different suckling groups were measured biweekly as shown in Figure 2a, b, c. In Figure 2a, the mean live daily body gain differences were significant (P<0.05) with the T2 group versus the other groups. The highest value of the daily body weight was noted with T2 and T1 (146.13 gm and 133.63 gm respectively) than the control group (92.85 gm). Figure 2b showed a significant increase in the mean live total body gain in the group (T2) treated with ALg-propolis NPs (12.28 kg) as compared with T1 (11.23 kg) and control group (7.8 kg). Also, it was clear from Figure 2c, the mean live body weight changes in the different treatments of newly born goat kids were significantly increased in the (T1) and (T2) groups as compared with the control group in the birth and the weaning weight. It is interesting to find that the group that treated with the ALg-propolis NPs (T2) has a higher average body gain (14.18 kg) than the group (T1) treated with propolis alone (13.15 kg) compared with the control group (9.53 kg). The lack of daily gains in the control group may be explained by the fact that, in several kids, moderate diarrhea symptoms were noted during the first two months of life. As a result, these kids consumed smaller amounts of starter feed. Similar results were obtained by (Freitas et al., 2011) who found that the preventive application of 10 % ethanol extract of propolis (EEP) improved the health status of the calves in the neonatal period. After an application of propolis in a dose of 4 ml/day, higher daily gains were noted when compared to the control calves.
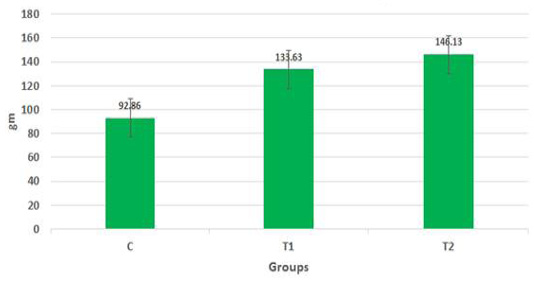
Figure 2a: Mean live daily body gain in the different treatments of Egyptian-Nubian goat kids (gm) (x̅ ± Sx̅).
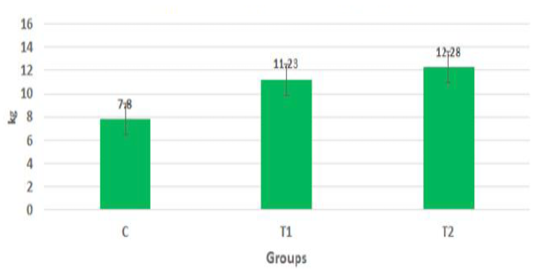
Figure 2b: Mean live total body gain in the different treatments of born Egyptian-Nubian goat kids (kg) (x̅ ± Sx̅).
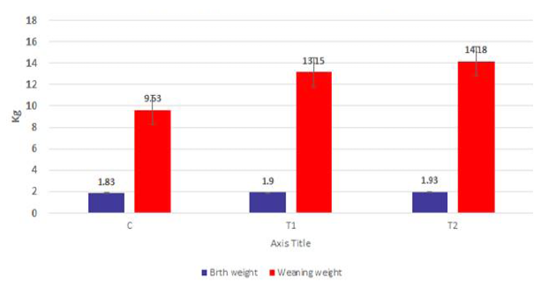
Figure 2c: Mean live body weight changes in different treatments of born Egyptian-Nubian goat kids (kg) (x̅ ± Sx̅).
Measurement of total serum protein, IgA, and IgG
The data in Figure 3a, b, c show the effect of propolis (0.6 ml) and ALg-propolis NPs (0.06 ml) on the total protein and serum immunoglobulin IgA and IgG levels of newborn Egyptian-Nubian goats. It was observed that the propolis and ALg-propolis NPs increased significantly (P<0.05) the serum total protein (7.2 and 8.2 g/dl respectively) as compared to the control group (7.1 g/dl). Also, it was noted that the propolis and ALg-propolis NPs increased significantly (P<0.05) the globulin levels (4.47 and 5.47 g/dl respectively) in newborn Egyptian-Nubian goats as compared with the control group (4.37 dl/g). While the albumin levels were not affected in all groups. Similar findings were observed by Takagi et al. (2005) and Morsy et al. (2013) who found that the Brazilian red propolis increased (P<0.01) the total protein and globulin.
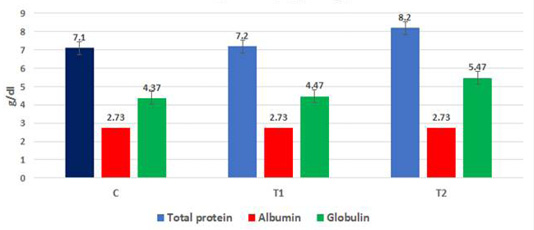
Figure 3a: Mean total protein, albumin and globulins in born Egyptian-Nubian goat kids (kg) (x̅ ± Sx̅)
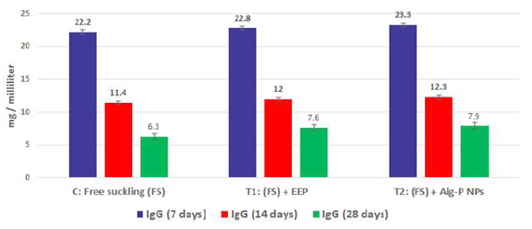
Figure 3b: Immunoglobulin G measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.
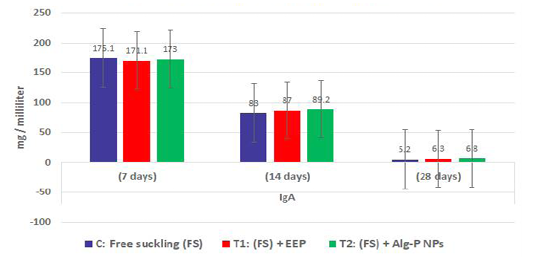
Figure 3c: Immunoglobulin A measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.
The effect of propolis (0.6 ml) and ALg-propolis NPs (0.06 ml) on the serum immunoglobulin IgA and IgG levels of newborn Egyptian-Nubian goats are illustrated in Figure 3b, c. This figure showed that the propolis and ALg-propolis caused an increase in the immunoglobulin; IgA and IgG serum levels of Egyptian-Nubian newborn kids as compared to the control group. These results indicated that the propolis and ALg-propolis activate the macrophages which stimulated the interferon (IFN)-γ production in association with the secondary activation of T-lymphocytes, and increasing the IgG and IgA production as observed by (Morsy et al., 2013). Also, Freitas et al. (2011) found that the propolis administration to laying hens increased the production of IgG specific to SRBC and natural antibodies.
Measurement of serum cytokine levels
The proinflammatory cytokines or inflammatory cytokines are types of signaling molecules that are secreted from the immune cells and other certain cell types that promote inflammation. They play an important role in mediating the innate immune response (Zhang and An, 2007). The proinflammatory cytokines involved are interleukin-1β (IL-1β), interleukin-6 (IL-6), interferon-gamma (IFN-γ), and tumor necrosis factor-α (TNF-α) (Nagai et al., 2003).
Figure 4a, b, c, d show the effect of propolis (0.6 ml) and ALg-propolis NPs (0.06 ml) on the serum cytokine levels of Egyptian-Nubian newly born kids. In this study, it was observed that the administration of propolis and ALg-propolis NPs decreased significantly (P<0.05) the concentration of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) as compared to control. The reduction of the proinflammatory cytokines may be due to the administration of propolis which contains a variety of phenolic acids and Caffeic acid phenethyl ester (CAPE) (Hegazi and El-Hady, 2002; El-Hady et al., 2007).
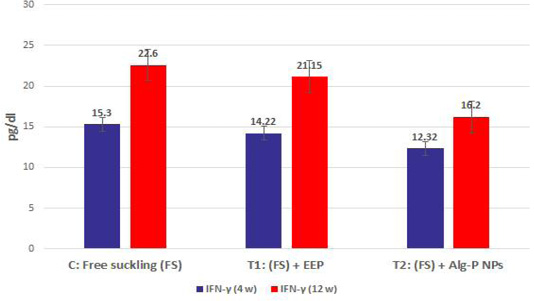
Figure 4a: IFN-α measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.
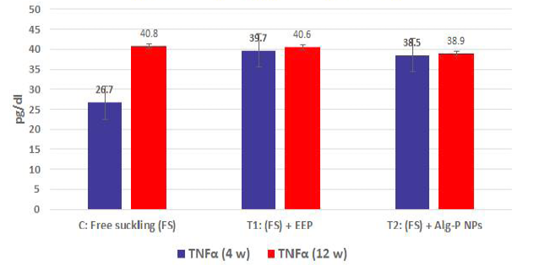
Figure 4b: TNFα measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.
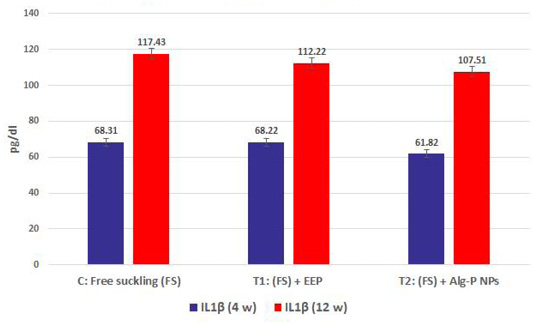
Figure 4c: IL1β measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.)
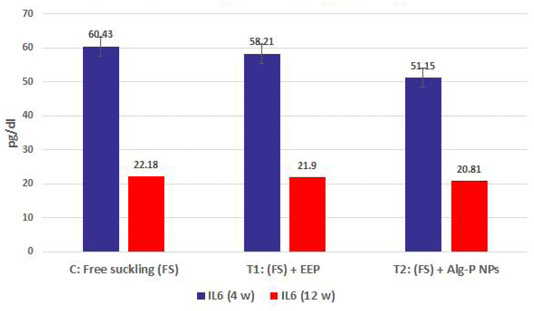
Figure 4d: IL6 measured from plasma at different ages and feeding systems in Egyptian-Nubian goat kids.
Flavonoids comprise a major part of biologically active substances in the propolis (Betances-Salcedo et al., 2017). Propolis has been found to contain esters of phenolic acids such as ferulates and caffeates which have antiviral, antibacterial, and anti-inflammatory activity (Okutan et al., 2005). Caffeic acid phenethyl ester (CAPE), a natural derivative of the honeybee propolis (Yildiz et al, 2009), is a small lipid-soluble potent flavonoid with multiple biological effects that emerged in recent research as an antioxidant and anti-inflammatory agent (Tao et al., 2014).
Our results were confirmed with the results obtained by Khan et al. (2018) who found that the Caffeic acid phenethyl ester (CAPE) decreased the inflammatory cytokines and increased the levels of the anti-inflammatory cytokine. Also, Fitzpatrick et al. (2001) found that the CAPE is an effective inhibitor of the NF-κβ and related cytokines in vitro. Also, it can induce apoptosis in the inflammatory cells. Moreover, propolis Caffeic acid phenethyl ester (CAPE), quercetin, hesperidin, and other flavonoids strongly inhibit the DNA synthesis and inflammatory cytokine production of Th1 as well as Th2 type T cells.
Also, it was interesting to find that the serum level of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) was reduced in the kids’ group that were taken the ALg-propolis NPs than the kids’ group that were taken the pure propolis. This means that the ALg-propolis NPs have a more potent effect on the immune system than pure propolis.
CONCLUSION
These results indicated that using propolis and ALg-propolis NPs as a natural additive improve the health status of newborn kids. However, the nano-encapsulation of propolis within ALg NPs gives better results than pure propolis even with its low dose as it led to a good impact on the immune system of newborn Egyptian-Nubian (zariba goats). Therefore, these results confirmed that the ALg-propolis NPs can be considered as a suitable alternative natural anti-inflammatory agent.
ACKNOWLEDGMENTS
The author’s acknowledgment of the financially supported by the project number 11020201 entitled: “Evaluation of the antimicrobial effect of propolis and Moringa on Fasciola gigantica and Clostridium oedematous (Clostridium novyi) type B” which is funded by a grant from the National Research Centre (NRC), Egypt.
Author’s Contribution
Ahmed G. Hegazi: prepared the original idea, Designer of the experiment, Coordination of the team, Preparation and extraction of the propolis conceptualized the aim, design, plan of the study, cytokines evaluation, Discussion of the results and wrote and revising the original manuscript draft. Asmaa S. El-Houssiny: Preparing the ALg nanoparticles encapsulation of propolis within ALg NPs, Characterization of the NPs, Discussion of the results, Writing the paper. Walid M.A. Sadek: Collect and grouping of Kids, Preparing the Feeding for kids, Observation of animals. Faiz M. Al-Guthami: Collecting the propolis, Help in Preparation and extraction of the propolis, help in financial of some cytokine kits. Ahmed F.M. Al-Gethami: prepared the tables and the figures, Help in publication. Ahmed M.A. Sadik: body weights of the goat kids, prepared the tables and the figures, Did the statistical analysis.Tarek Korany Farag: Collection of the serum samples, immunological analysis., Treatment of dehydration and wrote the discussion related to study in the original manuscript draft.
All authors contributed to revising the final draft of the manuscript. All authors read and approved the final manuscript
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References






