Advances in Animal and Veterinary Sciences
Research Article
Effectiveness of Origanum syriacum on Schistosoma mansoni Infected Mice: Parasitological, Histopathological and Scanning Electron Microscopical Studies
Hend M. Tag1,2*, Eman M.Abouelhassan3, Mona M. Henedak4, Ehssan A. Hassan2,5
1Department of Biology, College of Science and Arts at Kulis, University of Jeddah, Jeddah, Saudi Arabia; 2Department of Zoology, Faculty of Sciences, Suez Canal University, Ismailia, Egypt; 3Department of Veterinary Parasitology, Suez Canal University, Ismailia, Egypt; 4Zoology Department, Faculty of Science, El Arish University, Egypt; 5Department of Biology, College of Science and Humanities, Prince Sattam Bin Abdulaziz University, Al -Kharj11942, Saudi Arabia.
Abstract | Schistosomiasis remains one of the most prevalent parasitic infections worldwide. Millions of people suffer severe illness related to schistosomiasis. The present studies aimed to evaluate the anti-parasitic effects of the crude, aqueous, and hexane fractions of Origanum syriacum against Schistosoma mansoni infected mice, the anti-schistosomal activity of the crude extracts of O. syriacum, their aqueous and hexane fractions were assessed by some parasitological and histopathological studies as worm load, liver egg load, intestine egg load, oogram pattern and also the SEM studies of the ultrastructure of schistosome tegument. Sixty male albino mice were used in the studies; twenty mice were observed for the acute toxicity of the Origanum syriacum extract and its fractions, for the evaluation of the anti-parasitic effects, twenty mice were infected with S. mansoni cercariae via subcutaneous route by a rate of by 80±10 cercariae per mouse and randomly divided into 4 groups (5 mice in each cage). The other uninfected 20 mice were randomly divided into 4 groups, after 6 weeks post-infection all experiment trials were conducted for 14 consecutive days. The results revealed that the groups treated with crude extracts of Origanum syriacum, and their aqueous fractions might be considered a promising anti-inflammatory and anti-schistosomal agent as they have schistosomicidal, ovicidal, histopathological effects and they reduced the total worm burden as compared with the untreated infected group.
Keywords | Origanum syriacum, Schistosoma mansoni, Parasitological, Histopathological, Scanning
Received | September 24, 2020; Accepted | September 30, 2020; Published | January 01, 2021
*Correspondence | Hend M Tag, Department of Zoology, Faculty of Sciences, Suez Canal University, Ismailia 41522, Egypt; Email: [email protected]
Citation | Tag HM, Abouelhassan EM, Henedak MM, Hassan EA (2021). Effectiveness of origanum syriacum on schistosoma mansoni infected mice: parasitological, histopathological and scanning electron microscopical studies. Adv. Anim. Vet. Sci. 9(2): 221-229.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.221.229
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Tag et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Schistosomiasis is one of the most important public health problems affecting particularly people of the Nile Delta, the most widespread type of schistosomiasis in this region is S. mansoni (Chitsulo et al., 2000). Schistosomal infection is prevalent in Upper Egypt, along the Nile river due to agricultural purposes from 70 or 80 years ago (Chitsulo et al., 2000; EL-Khoby et al., 2000; EL-Sisi et al., 2011).
In Egypt, the beginning of schistosomiasis control programs has enhanced the decline of Schistosoma infections (El-Khoby et al., 1998; El-Khoby et al., 2000; Youssef and Uga, 2014). Although the use of various controls programs, Schistosoma infections are still recorded with a relatively high prevalence of S. mansoni (Youssef and Uga, 2014). Several medications are used in the treatment of schistosomiasis as praziquantel, oxamniquine, metrifonate, antimonials, hycanthone, and niridazole (Fahmy et al., 2014; Tag et al., 2019) but developing resistances against drugs is a serious problem.
Phytomedicines play a vital role in the human health care system (Prakash et al., 2012). Among the medicinal plants and aromatic, Origanum sp. is an important genus belonging to the family Lamiaceae (Chishti et al., 2013). In folk medicine, Origanum species are used as, flavoring agents and powerful disinfectants (Chishti et al., 2013). Dried Origanum species are used as antitussives, analgesics, expectorants, sedatives, antirheumatics, antiparasitics, and gastrointestinal complaints in folk medicine (El-Beyrouthy et al., 2008; Loizzo et al., 2009). The present study aimed to evaluate the effects of Origanum syriacum, and their fractions against Schistosoma mansoni infected mice.
MATERIAL AND METHODS
Plants Extraction
Plant materials were collected from Arish, North Sinai, Egypt, in March 2015 and were identified and authenticated by Botany Department, Faculty of Science, Suez Canal University based on taxonomic characters and by direct comparison with the herbarium specimens available at the herbarium of Botany department. The plant extracts were prepared as described by (Azwanida, 2015). Fractionation of ethanolic plant extracts was prepared as described by (Tag et al., 2020).
Experimental Design (Figure 1)
Sixty male albino mice were used in the studies, the studies divided in to:
1- Experiment 1: Acute Toxicity Study of Origanum syriacum extract and its fractions in mice.
2- Experiment 2: Evaluation of the anti-parasitic effects of the crude, aqueous, and hexane fractions of Origanum syriacum against Schistosoma mansoni infected mice.
Experiment 1: Acute Toxicity Study of Origanum syriacum extract and its fractions in mice
Twenty male albino mice of average weight (20-22g) were acclimatized for a week in cleaned cages and randomly divided into 4 groups of 5 animals each for each extract. Groups 1, 2, and 3 were intraperitoneally injected with crude, aqueous, and hexane extracts either for Origanum syriacum extracts (10, 100 and 1000 mg/kg body weight) following the method of (Lorke, 1983). The last group was the control group which was administered saline intraperitoneally. Immediately after dosing, the mice were continuously observed for at least 4 h, and occasionally up to 6 h. They were then observed for up to 14 days (frequency of 12 h/day) for signs of toxicity and mortality (Tag et al., 2019).
Experiment 2: Evaluation of the anti-parasitic effects of the crude, aqueous and hexane fractions of Origanum syriacum against Schistosoma mansoni infected mice
Forty male albino mice were used in this study; they were purchased from the animal unit from the Schistosome Biological Supply Centre (SBSC), Theodor Bilharz Research Institute (TBRI) (Giza, Egypt) where they had been bred under conventional conditions for research purposes. Mice were selected and individually marked. The animals were matched, as closely as possible with an average weight (20-22g) to reduce the variability of their responses according to guidelines of the Organization for Economic Co-operation and Development (OECD) (OECD, 1998; 2000), they were fed on a standard commercial pelleted diet (El-Kahira Company for oils and soap) in an air-conditioned animal house at 20-22°C, humidity 65±5% and light/dark cycles (12/12hrs). The animal experiments were conducted in the animal house at the Department of Zoology Faculty of Science, Suez Canal University. Each of the used cages has a card with the data of the experiment (Tag et al., 2019).
S. mansoni cercariae were obtained from laboratory-bred infected Biomphalaria alexandrina snails in SBSP at TBRI, Giza, Egypt. Following the methodologies previously described by (Wasilewski et al., 1996). Twenty mice were infected with S. mansoni cercariae via a subcutaneous route by a rate of 80±10 cercariae per mouse (He et al., 2005) and randomly divided into 4 groups (5 mice in each cage). The other uninfected 20 mice were randomly divided into 4 groups (Table 1) (Tag et al., 2019).
Sampling and examination
Parasitological examination: The experimental design was prepared as in (Table 1), Mice were decapitated and underwent Porto-mesenteric perfusion for recovery of the adult schistosome worms for the Parasitological examination, which includes:
Table 1: The experimental groups design
| Group 1 | negative control group |
| Group 2 |
Uninfected and treated with ethanolic extract of O.syriacum, (200 mg/kg,), intraperitoneal (i.p.) administration daily. |
| Group 3 |
Uninfected and treated with aqueous extract of O.syriacum, (200 mg/kg,), intraperitoneal (i.p.) administration daily. |
| Group 4 |
Uninfected and treated with hexane extract of O.syriacum, (40 mg/kg,), intraperitoneal (i.p.) administration daily. |
| Group 5 | Infected and untreated |
| Group 6 |
Infected and treated with ethanolic extract of O.syriacum, (200 mg/kg,), intraperitoneal (i.p.) administration daily. |
| Group 7 |
Infected and treated with aqueous extract of O.syriacum, (200 mg/kg,), intraperitoneal (i.p.) administration daily. |
| Group 8 |
Infected and treated with hexane extract of O.syriacum, (40 mg/kg,), intraperitoneal (i.p.) administration daily. |
Table 2: Worm burden in Schistosoma mansoni infected mice treated with O.syriacum, and their fractions after 8 weeks post-infection
| Groups | Mean worm burden | Total worm burden | Worm % reduction | |||
| Worm % reduction | Paired | Worm % reduction | Single | |||
| Infected non-treated | 4.00±1.5 | 1.00±0.53 | 5.00±0.10 | |||
| Infected +OG crude extract | 79.25 |
0.83*±0.83 |
160 |
2.60*±0.55 |
3.30*±0.60 |
34 |
| Infected +OG aqueous extract | 75 |
1.00*±0.88 |
230 |
3.30*±1.33 |
4.30*±0.30 |
8 |
| Infected +OG hexane extract | 66.75 |
1.33*±0.66 |
100 |
2.00*±1.00 |
3.30*±0.30 |
14 |
Values are means ± SE., One Way ANOVA followed by Values are means ± SE. n=6, One Way ANOVA followed by Duncan multiple comparison tests *p<0.05 compared with Schistosoma mansoni infected mice.
a- Estimation of worm burden
All mice were sacrificed at the end of the 8th week after infection by decapitation. The sacrificed mouse was placed in the supine position, a longitudinal midline incision was made in the skin of the abdomen and the abdominal skin was removed, the peritoneal cavity was opened through another longitudinal midline incision. An incision was made in the portal vein near its entry to the liver, then a fine needle (20 gauge) connected to a perfusion pump containing citrated saline was introduced in the descending aorta. Worms emerging from the portal vein incision were sucked into a 20 µ sieve fitted to the proximal end of a rubber tube connected to a suction pump. The worms were retained in the sieve. Perfusion continued until the fluid coming from the animal was free of blood and the liver became pale. The liver of each mouse was removed, washed by a saline solution to remove blood, dried by blotting between filter papers, weighted and 0.5 mg of it was used to prepare liver homogenate and 1mg for egg load counting, and then the rest of the liver was stored in an aluminum foil paper to be preserved in the deep freezer for future requirements. The loops of the intestine were lifted and washed down to dislodge any adherent worms. The sieve was inverted into a Petri-dish and the worms washed off with normal saline and counted (Tag et al., 2019).
b- Calculation of the percentage of mature and dead ova in tissues (oogram study).
After perfusion, the small intestine for each sacrificed mouse was wholly separated and transferred to a Petri-dish, three fragments of the small intestine were cut longitudinally (1 cm in length), rinsed in saline, slightly dried on filter paper and then placed between 2 slides and compressed carefully, The preparations were examined under the low power of the light microscope. (Moraes et al., 2014).
c- Estimation of the egg load by counting the number of eggs in infected tissues (intestine and liver)
The whole small intestine was removed, and then 3 fragments were taken for oogram study, The rest of the intestine was slit opened and washed with saline to remove any faecal matter present in the lumen, a representative portion of the liver was also taken after perfusion.
One gram of each liver and each fragment of intestine was weighed and then plotted between two filter papers and then placed each in a test tube containing 5 ml 5% KOH solution. The tubes were incubated at 37ºC for 24 hours until the tissues were hydrolyzed. The digest was well shaken on a magnetic mixer for at least one minute, 3 samples (0.1 ml each) were pipetted by the Eppendorf micro-pipette, placed on a slide, and examined under the low power of the microscope, one sample at a time (Tag et al., 2019).
Scanning electron microscopy (SEM) examination:
Scanning electron microscope examination by (Inspect S; FEI, Holland) at Electron Microscopy Unite of Theodor Bilharz Research Institute (TBRI). It was performed according to (Kevin and Winfried, 2006) to determine the damaging effects on the surface of the treated worms in comparison to the untreated one. The samples were centrifuged then the pellets were immediately processed (Tag et al., 2019).
Histopathological examination
Control and treated groups were anesthetized and dissected immediately. The tissues of the liver were fixed and prepared for histopathological examination according to (Drury and Wallington, 1980), measuring the size of hepatic granulomas in S. mansoni-infected mice utilizing the method previously described (Jacobs et al., 1997).
The classification of evolutionary stages of granulomas was performed according to previous studies (Costa-Silva et al., 2002; Lins et al., 2008), and for the morphometric analysis, Image J software was used to measure granuloma diameter using spatial calibration. The process of spatial calibration involves calibrating a single image against known values, then applying that calibration to the uncalibrated image, both images are at the same magnification. For each treatment granuloma size was measured and the mean values from 5 mice for each group were used for statistical analysis.
Statistical analysis
The collected data were tabulated and analyzed using IBM’s personal computer using SPSS 16 microstate software package. ANOVA (analysis of variance) was used as the test of significance. P-value was considered significant if it was <0.05 (Field, 2000). Duncan test was used as the multiple comparison tests after obtaining significant results by i.e. post-hoc comparison to determine which pair was significantly different (Abdi and Sidak, 2007).
Ethics:
This study was conducted following legal ethical guidelines of the Medical Ethical Committee of the National Research Center, Dokki, Egypt (approval no. 09210).
RESULTS
Experiment 1: Acute Toxicity Study of Origanum syriacum extract and its fractions in mice
The acute toxicity study was conducted in mice weighting 20-22g. The whole plant ethanolic extract, their aqueous and hexane fractions were given by intraperitoneal injection. Initially, the animals were administrated with a single dose of 10,100 mg/kg of body weight, and the dose was increased to 1000 mg. All animals were observed for clinical signs and pre-terminal deaths for the first 4 hours after dose administration on the first day of treatment and once daily on days 2 to 14 for changes in skin and fur, eyes, mucous membrane, musculature, and respiratory, symptoms. There was no abnormality observation in all groups, all extracts had no significant toxic effect in mice and the plant materials were found to be nontoxic.
Experiment 2: Evaluation of the anti-parasitic effects of the crude, aqueous, and hexane fractions of Origanum syriacum against Schistosoma mansoni infected mice.
Parasitological examinations
Worm burden:
The present result showed significant differences between the total worm load of mice treated with O. syriacum or their fractions as compared to the untreated animals (Table 2). The treatment with crude, aqueous and the hexane fractions of O. syriacum significantly decreased (P < 0.05) the total worm load as compared to the untreated ones. In addition to there was a reduction in the number of paired worms in all the infected treated mice compared to the infected untreated one and the number of single worms showed a significant increase in the treated groups compared to the untreated one (Table 2).
Oogram study
The viable mature ova of the treated groups with crude, aqueous, and the hexane fractions of O. syriacum showed significant reductions (335.2±36.08, 318.2±26.16 and 374.0±25.80, respectively) (P< 0.05) as compared to the untreated group (681.60±56.85) (Table 3). Also, there were significant increases (3.6±0.67 and 7.0±0.00 respectively) (P< 0.05) in numbers of dead ova found in groups treated with the crude extracts and the aqueous fraction of the plants as compared to the untreated group (1.80±0.48) (Table 3).
Tissue egg load in intestine and liver
The effect of crude O. syriacum and their fractions on the worm ability for oviposition was assessed by counting S. mansoni eggs /g retained in both intestine and liver tissues (Table 3). The result revealed significant reductions were recorded in the mean number of eggs/g intestine in groups treated with the aqueous, crude extracts and hexane fractions of the plants (3.4±1.40, 4.46±1.40& 4.45±1.52 respectively) when compared to the untreated one (24.05±6.10) (P < 0.05). Regarding the liver egg load, significant reductions were also recorded in the mean number of eggs/g liver in infected mice treated with the crude and hexane frac tions of O. syriacum (2.20±0.53,1.40±0.46 respectively) as compared with the untreated infected group (9.50±3.60) (P< 0.05). While there was a highly significant decrease in the mean number of eggs/g liver in infected mice treated with an aqueous fraction of O. syriacum (1.34±0.60) as compared with untreated infected mice (P<0.05).
Scanning electron microscopy (SEM) examination:
Treating infected mice with crude O.syriacum extract led to mild to severe alterations in the tegument of adult worms,
Table 3: Effect of crude O.syriacum , and their fractions on oogram and tissue egg load(8 weeks post-infection).
| Groups | Oogram | Egg load | ||||
| % of change | Dead | % of change | Mature | Intestine | Liver | |
| Infected non-treated | 1.80±0.48 | 681.60±56.85 | 24.05 ±6.10 | 9.50 ± 3.60 | ||
| infected + OG crude extract | -128 | 3.60±0.67* | +53.3 | 335.20±36.08* | 4.46 ± 1.40* | 2.20±0.53* |
| Infected + OG aqueous extract | -28 | 7.00±0.00* | +45.1 | 318.20±26.17* | 3.41 ± 1.40* | 1.34±0.66* |
| Infected + OG hexane extract | -188 | 1.40±0.50 | +35 | 374.0±25.80* | 4.45 ± 1.52* | 2. 40±1.11* |
Values are means ± SE., One Way ANOVA followed by Duncan multiple comparison tests. Duncan multiple comparison tests *p<0.05 compared with Schistosoma mansoni infected mice
the male worms showed variable alterations in their tegument that increased with increasing the administrated dose. Tubercles appeared ruptured, collapsed, disrupted, or reduced in size with obviously diminished spines and sensory papillae. Besides, grooves between tubercles showed diminished spines (figure 2.A). While treating with an aqueous fraction of O.syriacum extracted to severe alterations in the tegument of adult worms. Showed variable alterations, accompanied by focal lesions on the tegument, disruption, tegumental peeling on its dorsal body surface, wrinkling that increased with increasing the administrated dose in addition to the formation of vesicles. Tubercles appeared ruptured, collapsed, disrupted, or reduced in size with obviously diminished spines and sensory papillae. Also, grooves between tubercles showed diminished spines (figure 2.B, C, D &E), treating with hexane fraction of O.syriacum extract leads to mild to severe alterations in the tegument of adult worms. The tubercles appeared ruptured, collapsed, disrupted, or reduced in size with obviously diminished spines and numerous membrane-bound vesicles (figure 2.F).
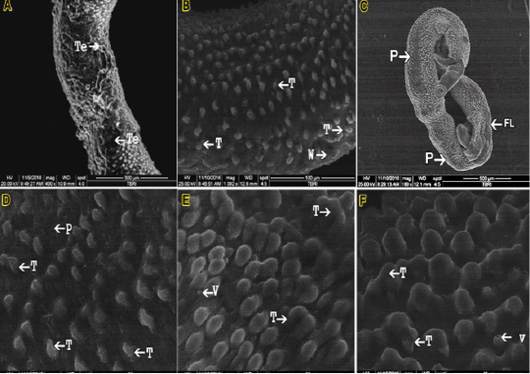
Figure 2: SEM micrograph (A) male S. mansoni after exposure to the crude Origanum syriacum; (B, C, D &E) male S. mansoni after exposure to the aqueous fraction of O.syriacum extracts; (F): male S. mansoni after exposure to the hexane fraction of O.syriacum extract Tubercles (T); Wrinkling (W); Peeling (P) Vesicles (V); Focal lesions (FL).
Histopathological studies
After 8 weeks post-infection, the liver of infected mice treated with crude O.syriacum extract showed less hydropic degeneration as compared with the untreated infected mice (figure 3.B). Kupffer cells were lesser than those of the untreated infected group. Pyknotic necrosis (an irreversibly damaged cell characterized by condensation and increased basophilia of its nucleus) is associated with granulomas lesions (figure 3.A). Moderate periportal inflammatory infiltration was observed. Also, dark brown pigments of hemozoin were observed (figure 3A).Yellowish pigments of bilirubin (figure 2.B).Exudative productive granuloma stages were observed (figure 3.A). Pre-granulomatous stages were common and less fibrotic granulomas were observed (Figure 3.B).
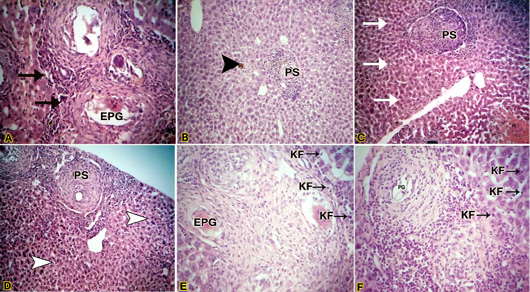
Figure 3: (A) Light micrograph of liver of infected mouse with S. mansoni treated with crude Origanum syriacum extract H&E. Magnification (400x); (B): Light micrograph of liver of infected mouse with S. mansoni treated with crude O.syriacum extract Magnification (100x); (C & D) Light micrograph of liver of infected mouse with S. mansoni treated with aqueous fraction of O.syriacum extract H&E. Magnification (100x); (E&F): Light micrograph of liver of infected mouse with S. mansoni treated with hexane fraction of O.syriacum extract H&E. Magnification (200x). Productive granuloma (PG); Exudative-productive granuloma (EPG); Kupffer cells (KF).
Regarding the liver of infected mice treated with an aqueous fraction of O.syriacum extract showed normal hepatocytes away from granulomas (figure 3.C). At granulomatous areas, hydropic degeneration and pyctonic nuclei of some hepatocytes were recorded (figure 2.D). Pre-granulomatous stages were common (figure 3.C) as well as productive granuloma (figure 3.D), besides, Kupffer cells were lesser than those of the untreated infected group. Dark brown pigmentation was minor or not observed totally in some mice.
Concerning, the liver of infected mice treated with hexane fraction of O.syriacum extract showed relatively normal hepatocytes and dilated sinusoids (figure 3.F). The Kupffer cells were lesser than untreated infected mice (figures 3.E & F). Also, some pyknotic hepatocytes were observed near granulomas. However, necrosis in this group was minor when compared with untreated infected liver. Productive granuloma (Figure 3F) and exudative productive granuloma were observed.
Measuring the size of hepatic granulomas:
The diameter of granulomas showed highly significant differences (P < 0.05) between the untreated infected group (645.01±67.86) and groups treated with the crude and aqueous fractions while The diameter changed in granulomas of groups treated with the hexane fraction of O. syriacum extract was not significantly different (P > 0.05) from the untreated infected group (Table 4).
Table 4: Differences in hepatic granulomas mean diameter (µm ± SE) in the liver of Schistosoma mansoni infected mice treated with crude O.syriacum, and their fractions after 8 weeks post-infection.
| Groups | Granuloma size (µm ± SE ) |
| Infected non-treated | 645.01 ± 67.86 |
| Infected +OG crude extract | 301.00 ±39.55* |
| Infected+ OG aqueous extract | 264.33 ±52.717* |
| Infected +OG hexane extract | 502.33 ±50.23 |
Values are means ± SE., One Way ANOVA followed by Duncan multiple comparison tests *p<0.05 compared with Schistosoma mansoni infected mice.
DISCUSSION
Schistosomiasis remains one of the most prevalent parasitic infections in the world and is endemic in more than 75 countries (World Health Organization, 2010). Medical plant extracts have been used since ancient times and they considered the first anthelmintic products in traditional medicine (Ndjonka et al., 2013). Previous attempts have been done to combat schistosomiasis using traditional medicinal plants (Soliman and El-Shenawy, 2003; Tag et al., 2019).
The present study demonstrated that the administration of crude O. syriacum extracts and their aqueous fractions to infected mice revealed less pathological features than untreated ones. Granuloma size is the most variable frequently used to evaluate the immunopathogenesis of schistosome infections (Shaha et al., 2017). The size of the granuloma is controlled by many immune factors related to the persistence of the egg in the lesion and the ability of the host cells to destroy antigens (Zuim et al., 2012). In the present study, the size of hepatic granulomas in infected mice treated with all extracts showed a significant decrease except the group treated with the hexane fraction of O. syriacum did not show a significant decrease as compared with untreated infected mice. The variation in the activity of different extracts might be due to the different nature and amount of active components released with the solvent used in the extraction processes (Mansour et al., 2002; Tag et al., 2019). The studies showed that there was a significant decrease in the paired worms’ burden and an increase the single worms’ burden in all treated groups. This indicates that O. syriacum crude extracts and their fractions negatively affected the coupling of the worms (Tag et al., 2020). Besides, the treatments of the infected animals with crude and aqueous extracts of O. syriacum were effective at reducing the total worm burden with untreated infected mice, indicating its useful antischistosomal action. Some authors have demonstrated that the death of worms upon treatment with antischistosomal drugs resulted from metabolic disorders, mechanical destruction, or muscular contraction of treated worms (Doenhoff et al., 2002; El Ridi and Tallima, 2013). The efficacy of O. syriacum and their aqueous fractions as an antischistosomal agent was not studied before but some authors studied their efficacy as anti-inflammatory and antioxidant agents and found that low concentration of crude O. syriacum had anti-inflammatory and antioxidant properties due to its content of flavonoids, triterpenoids, glycoside, and steroids (Soliman et al., 2007; Tag et al., 2019).
Concerning, the oogram pattern, they showed a significant reduction in the number of viable mature eggs at groups treated with the crude and aqueous extracts of O. syriacum as well as hexane fraction of O. syriacum. This reduction in viable egg numbers may be to the death of adult worms. Additionally, the number of dead ova increased in groups treated with the crude and aqueous extracts of O. syriacum while there wasn’t a significant difference in groups treated with the hexane fraction of O. syriacum compared with untreated infected mice. Such changes may indicate the possible lethal effects of the crude and aqueous extracts of O. syriacum on released eggs in the lumen of the intestines. Concerning effects of Origanum syriacum crude extract and their fractions on egg load in the liver and intestine tissues, the present data revealed a reduction in egg count in these tissues It is clear from the results that there was a suppression of egg-laying capacity; which may be attributed to a reduced worm burden and/or the extracts may affect the ability of the worms to copulate, thereby affecting egg production by the female adult worms (Abdel-Ghaffar, 2004; Zhang et al., 2009; Rabia et al., 2010). Moreover, other factors may also explain such a reduction in schistosomal egg count. These factors are probably diminished fecundity of the worm pairs and an increased rate of egg excretion due to the egg death (Riad et al., 2009). The anti-schistosomal activity of treated groups of O. syriacum and their fractions was also assessed by the SEM studies of the ultrastructure of schistosome tegument. The present results showed alteration in male worm tegument including tubercles collapsing, tubercles with reduced spines, tegument swelling or tearing, and presences of vesicles. These changes confirm the anti-schistosomal activities of treated groups of O. syriacum and their fractions as vesicle formation are an indicator of stress and swelling of tegument and focal lysis of worm muscles (Manneck et al., 2011; Zhang et al., 2009).
Our results are in agreement with Frezza et al. (2013) who pointed out that the treatment of experimentally infected mice with 500 mg/kg of Artemisia annua, showed significant erosion, peeling, sensory structure damage, and vesicle formation on the tegument of S. mansoni 30 days post-infection. Moreover, worm tegument tearing increases antigen exposure on the worm surface to the host immune system that results subsequently in worm death (Brindley et al., 1989; Soliman and Ibrahim, 2005). Tegumental changes recorded in this study were observed by other anti-schistosomal drugs and compounds (Munro and McLaren, 1990; Xiong et al., 2000; Xiao et al., 2002; Jiraungkoorskula et al., 2005; Soliman and Ibrahim, 2005; Tag et al., 2019).
In conclusion, Schistosomiasis is one of the important wide spread tropical parasitic diseases, the present study reflected that the groups treated with crude extracts of O. syriacum and their aqueous fractions might be considered a promising anti-inflammatory and anti-schistosomal agent especially after the findings of the safe use of them in animals in this study, they have schistosomicidal and ovicidal effects besides they reduced the histopathological effects and the size of granulomas induced by S. mansoni, Further studies are required to increase the efficacy of complete the elimination of worms and ova and to improve the anti-inflammatory and antioxidant properties of the infected host.
conflict of interest
The authors have no conflicts of interest relevant to this article.
Authors contribution
All the authors contributed to the design and the performance of the experiments, the analysis of the results and to the writing of the manuscript.
REFERENCES