Advances in Animal and Veterinary Sciences
Research Article
Marjoram Oil and Propolis Modulate Hepatic Injury Induced by Diclofenac Sodium in Albino Rats via Reducing Oxidative Stress, Inflammation and BAX Gene Expression
Olfat Shehata1, Randa M. Hassan2, Doaa Sh. Mohamed3, Abeer M. Radi4*
1 Department of Clinical Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62515, Egypt; 2Department of Cytology and Histology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62515, Egypt; 3Department of Biochemistry, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62515, Egypt; 4Department of Pharmacology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62515, Egypt.
Abstract | Diclofenac sodium (DS) is a non-steroidal anti-inflammatory drug used to relief pain and inflammation, it ranks in the top reasons of drug-induced liver injury. This study investigated the modulatory effect of marjoram oil (MO) and propolis on DS induced liver injury in rats. Rats were administrated MO (0.5 mL/kg b.wt.) or propolis (600 mg/kg b.wt) orally for 12 days where I.p injection of Diclofenac sodium (50 mg/kg b.wt ) was started on 11th and 12th days of experiment. Administration of DS induced liver injury as manifested by abnormalities of hepatic profile (AST, ALT, total protein and albumin) together with increased level of Malondialdehyde (MDA) in liver homogenate and serum level of inflammatory cytokines (IL-1β and TNF-α). DS induced liver injury was confirmed by histological and immunohistochemical alterations, diminished reduced glutathione (GSH) and catalase (CAT) and up regulated BAX gene. Both MO and propolis improved liver injury, histological alterations, lipid peroxidation and enhanced antioxidant status in diclofenac-induced rats. In addition, MO and propolis reduced serum liver pro-inflammatory cytokines, and down regulated the expression of BAX gene. In conclusion, marjoram oil and propolis effectively improved the oxidative stress, reduced inflammation and damage induced by Diclofenac sodium in rats.
Keywords | Diclofenac sodium, Marjoram oil, Propolis, Oxidative stress, Inflammatory cytokine, Gene expression
Received | August 30, 2020; Accepted | October 07, 2020; Published | January 01, 2021
*Correspondence | Abeer M Radi, Department of Pharmacology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62515, Egypt; Email: [email protected]; [email protected]
Citation | Shehata O, Hassan RM, Mohamed DS, Radi AM (2021). Marjoram oil and propolis modulate hepatic injury induced by diclofenac sodium in albino rats via reducing oxidative stress, inflammation and bax gene expression Adv. Anim. Vet. Sci. 9(2): 162-174.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.162.174
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Radi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Diclofenac sodium is one of the most commonly used non-steroidal anti-inflammatory drugs (NSAIDs). It is frequently used to treat pain in musculoskeletal injuries, osteoarthritis and rheumatoid (Francio et al., 2017) and used generally in human and veterinary practice (Ramesh et al., 2002). Drug-induced liver injury considered as a major problem and has become a leading cause of acute liver failure and transplantation.
The mechanism of DS hepatotoxicity including CYP450 enzyme that oxidizes DS to form reactive metabolites (5‐OH and 4‐OH DcNa) which oxidizes the benzoquinone imine. Reduced glutathione (GSH) plays an important role in detoxification, conjugation and removing of these metabolites outside the cells. Thus, an elevated dose of DS may lead to GSH depletion which reflected liver injury (Lauer et al., 2009) and inducing hepatocytes apoptosis by the generation of oxidative stress and mitochondrial dysfunction (Gómez-Lechón, et al., 2003). Therefore, many therapeutic agents are used to reverse or arrest cytotoxic action activated by diclofenac.
Marjoram oil is a product of Origanum majorana L. (O. majorana) which is a member of the mint family Lamiaceae, commonly known as sweet marjoram. In folk medicine, marjoram is considered as carminative, antiseptic, antispasmodic, antidiabetic, diaphoretic, stimulant and diuretic. It is used for the treatment of coughs, asthma, rheumatism, indigestion, headaches, and cancer (Rau et al., 2006). MO is shown to have antimicrobial activity against both Gram-positive and Gram-negative bacteria (Barbosa et al., 2009) which may be attributed to its thymol, carvacrol and ursolic acid content (El-Ashmawy et al., 2005). In addition, MO possesses antioxidant activity (Hossain et al., 2012) due to phenolic terpenoids, tannins, flavonoids, phenolic glycosides and sitosterol content.
Propolis is one of the most important natural antioxidants. It is a resinous natural product collected by honeybees from various plant sources. Propolis has attracted attention in the latest years as a potential substance used in medicine. It has revealed several biological properties, such as anti-inflammatory, immunomodulatory, antiallergic properties, anticarcinogenic and antioxidant, chemopreventive, neuroprotective, antimicrobial, antiparasitic, antifungal and hepatoprotective (Bhadauria et al., 2007).
It has been reported that a daily intake of propolis dampens lipid peroxidation and rises superoxide dismutase (SOD) activity in healthy humans (Jasprica et al., 2007). Moreover, propolis has been shown to prohibit macrophage apoptosis through the effect on glutathione (Claus et al., 2000) and it is more potent in counteracting free radical-induced damage owing to its content of flavonoids and esters of phenolic acids (Bhadauria and Nirala, 2009). Propolis was showed a protective effect against epirubicin-induced hepatotoxicity (Chaa et al., 2019) and ameliorates cerebral injury in focal cerebral ischemia/reperfusion (I/R) rat model via upregulation of TGF-β1 (Abdel-Rahman et al., 2020).
Therefor the current study was undertaken to assess the effects of exposure to DS including the changes in some biochemical, immunological, molecular parameters and histological alterations and the possible protective effect of marjoram oil and propolis on hepatic injury induced by diclofenac sodium in male albino rats.
Materials and methods
Chemicals
Diclofenac sodium (Voltaren®) was obtained from Novartis Pharma AG, Basel, Switzerland Company in the form of ampoules; each ampoule contains (75mg/3ml). Propolis (Biopropolis®) was purchased from Sigma pharmaceutical industries Company in the form of capsules; each capsule contains 400 mg of pure propolis. Marjoram oil was obtained from Royal herbs, ltd company, Egypt.
The chemical composition of marjoram oil and propolis was analyzed using Gas Chromatograpy (GC)-Mass Spectrometer (Agilent Technologies 7890A GC system equipped with a 5975 Inert MS with triple Axis Detector, Germany). Malondialdehyde (MDA), reduced glutathione (GSH) and Catalase (CAT) commercial kits were purchased from Biodiagnostic Company, Cairo, Egypt. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein and albumin kits were delivered from Diamond Company. Determination of IL-1β and TNF-α serum levels were supplied by ELISA kits (Ray Biotech, USA).
Animals and Treatments
Thirty-two adult male albino rats (160–200g) were obtained from El-Nahda University, Beni-Suef, Egypt. Rats were provided with a balanced commercial diet and water ad libitum and kept at 25±2ºC room temperature, 45±5% humidity, with a 12:12h light: dark cycle throughout the period of experiment (12 days).
All experimental procedures were conducted in agreement with the guide for the care and use of laboratory animals and in accordance with the Research Ethical Committee of Faculty of Veterinary Medicine, Beni-Suef University, Egypt.
After one week of acclimatization, rats were randomly divided into four experimental groups of 8 rats each:
Group C: Rats served as control and were given normal saline orally 1ml/rat daily.
Group D: Rats were injected diclofenac sodium intraperitoneally once daily on11th and 12th day of the experiment at a dose of 50 mg/kg b.wt. (Simon and Evan, 2018)
Group D+M: Rats were received MO orally for 12 days at a dose of 0.5 ml (500mg)/kg/day, Nashwa and Faten (2014), at the 11th and 12th day of the experiment rats were injected by DS (50 mg/kg) after 1hour of MO administration.
Group D+P: Rats were administered propolis orally for 12 days at a dose of 600 mg/kg/day (Oluwatosin et al., 2015), at the 11th and 12th day of the experiment rats were injected by DS (50 mg/kg) after 1hour of propolis administration.
Sampling and Biochemical analysis
After 24 hours from the last dose of treatments, blood samples were collected from all rats via retro-orbital plexus under light ether anaesthesia. Clotted blood samples were centrifuged at 3000 rpm for 15 minutes for serum separation and the obtained sera were kept at – 20 ºC until use.
The rats were sacrificed by cervical dislocation. The liver was excised, then washed by physiological saline. The liver samples were divided into three parts. The first part of liver (0.5g) was suspended in 5 ml ice-cold phosphate- buffered saline (pH: 6.8) for homogenization (Teflon Homogenizer, India). The liver tissue homogenate was centrifuged at 3000 rpm for 10 minutes at 4˚C using a high-speed cooling centrifuge. The supernatants were kept on –20˚C until the time of determination of oxidative/antioxidant parameters. The second part of the liver tissues was preserved at –80˚C for molecular investigation. The Third part of the liver tissues was prepared for histopathological examination.
Liver function Biomarkers
Serum ALT and AST activities were measured according to Steven (1996). Serum total protein and albumin levels were estimated according to Peters (1968), Dumas and Biggs (1972), respectively. However globulin was measured by subtracting albumin from total protein.
Oxidative/antioxidant Parameters
Oxidant-antioxidant status was evaluated by measuring the levels of MDA, GSH and CAT activity in liver tissue homogenate according to Wills (1987), Moron et al., (1979) and Aebi (1984), respectively.
Estimation of Inflammatory Cytokine Levels
Serum levels of IL-1β and TNF-α were measured by ELISA according to the manufacturer’s guidelines.
Detection of BAX gene expression using real time-polymerase chain reaction (RT-PCR):
Total RNA Extraction
Extraction of total RNA from liver tissue homogenate was prepared by using SV Total RNA Isolation System (Promega, Madison, WI, USA). Concentrations and purity of the RNA were measured using a UV spectrophotometer “Hitachi spectrophotometer, Model U -2000, Hitachi Ltd. Tokyo, Japan”.
Complementary DNA (cDNA) Synthesis
One microgram of total RNA was reversely transcribed by oligonucleotide using (SuperScript III First-Strand Synthesis System) a reverse transcription kit as termed in the industrialization’s protocol (K1621, Fermentas, Waltham, MA, USA).
Quantitative Real-time PCR:
Real-time PCR amplification was done by using an Applied Biosystems through software version 3.1 (StepOne™, USA). The PCR assay with the primer sets (BAX and b-actin) were optimized at the annealing temperature (Mohamed et al, 2018). The primer sequence was shown in table (3). PCR amplification reactions were carried out at 50°C for 2 min, 95°C for 10 min, and 40 cycles of denaturation for 15 sec and annealing/extension at 60°C for 10 min. ABI Prism 7500-sequence detection system software was used for analysis of the data attained by real-time assays. The data were calculated by means of the v1.7 Sequence detection software through PE Biosystems (Foster City, CA). Relative expressions of BAX gene was calculated using the comparative Ct method. All values were normalized to the beta-actin gene and reported as fold change. All these steps were performed according to the method described by Livak and Schmittgen (2001).
Histopathological Assessment
Histological and histochemical studies: liver samples from all groups were cut into small parts, washed by saline, rapidly fixed in neutral buffered formalin 10% and Bouin’s fluid for 48 hrs. , dehydrated in ascending grades of ethyl alcohol (70–100%), cleared in xylol and embedded in paraplast blocks. Subsequently, sectioned at 4-5 mm thickness by rotatory microtome and mounted on slides. Sections were stained by the general stain; Haematoxylin and eosin (H&E), Crossmon’s trichrome stain for demonstration of collagen fibers, Periodic acid- Schiff technique (PAS) for the mucopolysaccharides demonstration and Bromophenol blue stain for cytoplasmic total protein detection. All fixatives and stains were performed as outlined by Suvarna et al. (2018).
Immunohistochemical Study
Immunohistochemical detection of Desmin carried out by using monoclonal mouse Anti-Desmin anti-human antibody clone (D33, M0760) 1:100 that presented from Dako, Glostrup, Denmark and application of PAP technique. This was carried out as the standard procedure stated by Knittel et al. (1999) on tissue sections that previously fixed in neutral buffered formalin. All sections were examined and captured by LEICA (DFC290 HD system digital camera, Heerbrugg, Switzerland) connected to the light microscope using 10×objective lens.
Micropathological Analysis
Image analysis was performed on immunostained liver tissue sections for measurements of the percentage area of sinusoidal cells revealed positive Desmin expression in 3 fields (400X) in each liver tissue section (18 fields in each group) using Image-J program 1.52a freeware (National Institutes of Health, USA).
Statistical Analysis
All data resulted from image analysis were used. The statistical significance was evaluated by SPSS version 22 software (Chicago, Illinois, USA). The differences between the groups were assessed by one-way analysis of variance (ANOVA). The p values < 0.05 were considered as statistically significant.
Table 1: The chemical compositions of Marjoram oil sample by using gas chromatography-mass spectrometry.
| No. | Name of the compound | (Retention time) RT | Area % |
| 1 | Bicyclo [3.1.0] hex-2-ene, 4-methyl- 1-(1-methylethyl) | 8.368 | 1.20 |
| 2 | (1R)-2,6,6-Trimethylbicyclo[3.1.1] hept-2-ene | 8.597 | 1.20 |
| 3 | Cyclohexene, 4-methylene-1-(1-methylethyl) | 10.125 | 8.39 |
| 4 | beta.-Myrcene | 10.794 | 1.36 |
| 5 | alpha.-Phellandrene | 11.235 | 0.65 |
| 6 | 4-Carene | 11.750 | 6.26 |
| 7 | o-Cymene | 12.082 | 7.10 |
| 8 | Cyclopentene, 3-isopropenyl-5,5-dimethyl | 12.213 | 3.48 |
| 9 | Eucalyptol | 12.293 | 0.49 |
| 10 | gamma.-Terpinene | 13.421 | 9.00 |
| 11 | p-Menth-8-en-1-ol | 13.758 | 3.22 |
| 12 | Spathulenol | 30.873 | 0.39 |
| 13 | 1,6-Octadien-3-ol, 3,7-dimethyl | 15.080 | 10.62 |
| 14 | Terpinen-4-ol | 18.124 | 20.62 |
| 15 | Thymol | 18.296 | 0.19 |
| 16 | alpha.-Terpineol | 18.502 | 4.59 |
| 17 | Estragole | 18.662 | 3.13 |
| 18 | cis-Decalin, 2-syn-methyl- | 18.851 | 0.18 |
| 19 | Citronellol | 19.692 | 0.13 |
| 20 | D-Carvone | 20.201 | 0.19 |
| 21 | Linalyl acetate | 20.653 | 1.94 |
| 22 | Bornyl acetate | 21.637 | 0.19 |
| 23 | Sorbic Acid | 22.908 | 0.23 |
| 24 | Eugenol | 24.058 | 0.11 |
| 25 | Methyleugenol | 25.586 | 0.19 |
| 26 | Caryophyllene | 26.078 | 3.25 |
| 27 | Humulene | 27.085 | 0.20 |
| 28 | gamma.-Elemene | 28.435 | 1.05 |
| 29 | Caryophyllene oxide | 31.021 | 0.33 |
| 30 | Camphene | 32.589 |
0.14 |
Table 2: The chemical compositions of propolis sample by using gas chromatography-mass spectrometry.
| No. | Name of the compound | (Retention time) RT | Area % |
| 1 | Benzene, 1-(1,5-dimethyl-4-hexenyl) -4-methyl | 21.361 | 2.67 |
| 2 | Sulfurous acid, 2-propyl tridecyl ester | 36.038 | 0.95 |
| 3 | 1-Octadecene | 36.250 | 2.27 |
| 4 | 10-Methylnonadecane | 37.743 | 3.05 |
| 5 | Hentriacontane | 38.785 | 1.39 |
| 6 | Hexacosane | 39.386 | 4.66 |
| 7 | 2-Propen-1-one,1-(2,6-dihydroxy-4-methoxyphenyl)-3-phenyl | 39.546 | 20.36 |
| 8 | Tetracosane | 40.959 | 8.59 |
| 9 | Bis(2-ethylhexyl) phthalate | 41.766 | 0.66 |
| 10 | Nonadecane | 42.481 | 9.54 |
| 11 | 4H-1-Benzopyran-4-one, 5-hydroxy-7 -methoxy-2-phenyl | 42.550 | 9.59 |
| 12 | Hexadecane | 43.940 | 14.83 |
| 13 | Octacosane | 45.354 | 8.84 |
| 14 | Squalene | 45.800 | 2.18 |
| 15 | Heneicosane | 46.72 | 9.57 |
Results
Marjoram Oil and Propolis GC–MS Analysis
In the present study, the major compounds of the MO and propolis samples have been identified by GC–MS analysis (Table 1, 2). The major components of MO were Terpinen-4-ol (20.62%), 1,6-Octadien-3-ol, 3,7-dimethyl (10.62%), gamma.-Terpinene (9%), Cyclohexene, 4-methylene-1-(1-methyl ethyl) (8.39), o-Cymene (7.1), 4-Carene (6.26). The main constituents of propolis were 2-Propen-1-one (anti-inflammatory and antioxidant),1-(2,6-dihydroxy-4-methoxyphenyl)-3-phenyl (flavo) (20.36), Hexadecane (14.83), 4H-1-Benzopyran-4-one, (flavo) 5-hydroxy-7 -methoxy-2-phenyl (flavor) (9.59), Heneicosane (9.57), Nonadecane (9.54), Octacosane (8.84), Tetracosane (8.59).
Table 3: Sequences of the primers used for amplification of mRNAs encoding BAX by using quantitative real-time PCR.
| mRNA |
Sequences (5`→3`) |
Gene Accession No |
|
BAX
|
Forward primer: GGGGACGAACTGGACAGTAACAT Reverse primer: GGAGTCTCACCCAACCACCCT |
NM_017059 |
|
β-actin |
Forward primer : ATGAGCCCCAGCCTTCTCCAT Reverse primer: CCAGCCGAGCCACATCGCTC |
NM_007393 |
Liver Function Biomarkers
From the results in a Table (4), diclofenac sodium administration significantly increases the serum ALT and AST activities and significantly decreases serum total protein and albumin levels when compared to the control group. Administration of MO or propolis ameliorated these effects where they significantly decrease the liver enzyme activities and also significantly increase the level of total protein and albumin when compared to DS administrated group. Globulins results showed significant decrease in all experimental groups when compared to normal control group. However, there were significant increase in serum globulins of Propolis treated group when compared to both diclofenac treated group and marjoram oil treated group.
Oxidative/antioxidant Parameters
The hepatic MDA level of DS administrated rats was significantly higher than that of control group while this high level was significantly decreased by MO or propolis treatment when compared to DS group. The treatment with MO or propolis significantly improved the changes of GSH levels and CAT activities induced by DS. The ameliorative effect of propolis on CAT activities was more obvious than that of MO as it was non-significant as compared to C group as illustrated in (Table 5).
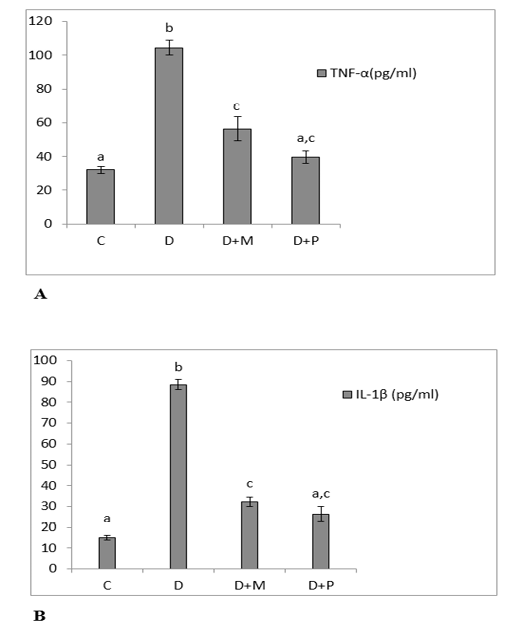
Figure 1: (A) Serum levels of TNF-α. (B): Serum levels of IL-1β in the various studied rat groups. Values are represented as mean ± standard error of mean.
The different superscript letters mean a significant difference at (P> 0.05) between different groups. Group C: control, Group D: diclofenac sodium, Group (D+M): diclofenac sodium & MO, Group (D+P): diclofenac sodium & propolis.
Inflammatory cytokines (TNF-α and IL-1β) and relative expression of BAX gene
The serum levels of TNF-α and IL-1β were increased significantly in diclofenac treated rats in comparison with control group and these increases were significantly lowered as compared to MO and propolis treated groups. The serum levels of both TNF-α and IL-1β of propolis group
Table 4: Changing in Liver function biomarkers of different experimental groups.
| Group C | Group D | Group (D+M) | Group (D+P) | |
| ALT (IU/L) |
44.67 ± 2.60 a |
80.00 ± 2.89 b |
57.22 ± 1.39 c |
55.33 ± 3.04 c |
| AST (IU/L) |
90.05 ± 2.09 a |
116.25 ± 1.59 b |
103.04 ± 1.41 c |
105.08 ± 0.57 c |
| Total protein (g/dl) |
7.16 ± 0.15 a |
4.53 ± 0.28 b |
5.25 ± 0.35 c |
5.57 ± 0.39 c |
| Albumin (g/dl) |
3.70 ± 0.27 a |
2.28 ± 0.83b |
3.02 ± 0.11c |
3.19 ± 0.58 c |
| Globulins (g/dl) |
3.45± 0.01 a |
2.25 ± 0.01 b |
2.23 ± 0.02 b |
2.37 ± 0.01 c |
Values are represented as mean ± standard error of mean. The different superscript letters mean a significant difference at
(P > 0.05) between different groups in the same row. Group C: control, Group D: diclofenac sodium, Group (D+M): diclofenac sodium & MO, Group (D+P): diclofenac sodium & propolis.
Table 5: Changes in MDA, GSH levels and CAT activity in the liver of different groups.
| Group C | Group D | Group (D+M) | Group (D+P) | |
| MDA (nmol/g tissue) |
7.97 ± 0.51 a |
61.03 ± 2.01 b |
20.13± 1.13 c |
21.47 ± 1.35 c |
| GSH (mmol/mg tissue) |
60.03 ± 2.15 a |
21.20 ± 0.49 b |
45.03 ± 1.04 c |
47.87 ± 2.17 c |
| CAT (U/g tissue) |
130.97 ± 4.16 a |
62.43 ± 2.54 b |
103.17 ± 4.74 c |
117.30 ± 3.89 a |
Values are represented as mean ± standard error of mean. The different superscript letters mean a significant difference at (P > 0.05) between different groups in the same row. Group C: control, Group D: diclofenac sodium, Group (D+M): diclofenac sodium & MO, Group (D+P): diclofenac sodium & propolis.
became non-significant as compared to control group (Figure 1. A, B). Both MO and propolis treatment significantly decreased the high BAX expression level induced by diclofenac and became non-significant as compared to control group (Figure 2).
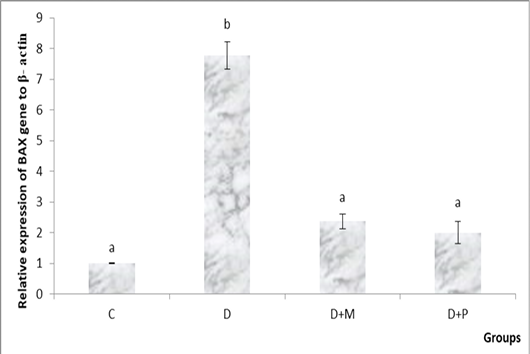
Figure 2: Relative expression levels of BAX gene in the various studied rat groups. The different superscript letters mean a significant difference at (P> 0.05) between different groups. Group C: control, Group D: diclofenac sodium, Group (D+M): diclofenac sodium & MO, Group (D+P): diclofenac sodium & propolis.
Histopathological studies:
a. General histological results: in H&E stained sections; the liver of control group showed normal hepatic architecture; ill distinct classic lobules. Each lobule contained a normal central vein lined with intact tunica intima and portal areas at its periphery. The hepatic cords, radiated from the central vein and separated by thin walled irregular blood sinusoids lined with endothelial and Von Kupffer cells. The hepatocytes appeared polyhedral in shape with acidophilic cytoplasm containing central, vesicular rounded nuclei (Fig. 3a-b), some of them were binucleated and others appeared as apoptotic cells with condensed nuclei. While, in the treated group with DS, the central vein, portal vein and lymphatic vessels appeared congested, containing desquamated cells and inflammatory cells infiltration. A large number of the hepatic plates appeared disarranged with many degenerated cells and lymphocytic infiltration in the portal areas and around the central vein. Swollen hepatocytes with vacuolar degeneration and different signs of nuclear degenerative changes besides an increased number of apoptotic cells could be observed. The blood sinusoids appeared dilated and congested with numerous Von Kupffer cells (Fig. 3c-d). On the other hand, administration of MO make improvement and occurred overcoming to the pathological pictures which appeared in D group, there were a normal number of the apoptotic cells, lymphocytic infiltration and central vein with intact lining, no dilation or congestion in the hepatic sinusoids with normal lining and all hepatocytes to a great extent resembling the normal (Fig. 3e). However, in treated D+P group, occurred partial overcoming to the pathological changes appeared in D group when compared to D+M group. It revealed minimal pathological changes as dilation and congestion in some blood sinusoids with inflammatory cell infiltration (Fig. 3f).
Sections of rat liver stained with Crossmon’s trichrome stain showed normal distribution of collagen fibers appeared with green color in the interlobular connective tissue septa and in the perivascular tissue of the control group (Fig. 4.a). While, in the treated group D, there was dilated and congested lymphatic vessel that surrounded by a mild proliferation of fibrous tissue when compared with normal (Fig. 4b). On the other hand, the treated groups D+M and D+P (Fig. 4 c-d respectively) showed interstitial collagen fibers with normal distribution when compared to treated D group.
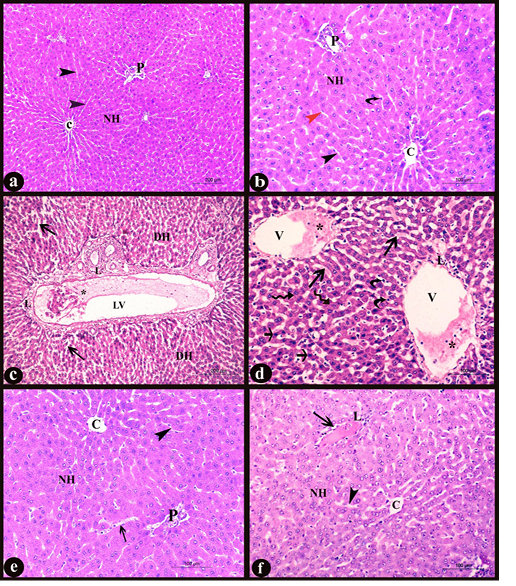
Figure 3: Sections in liver of albino rat showing the histopathological changes a-b) Control liver, c-d) Rat liver of D group, e) Rat liver of D+M group and f) Rat liver of D+P group. Notice, normal central vein with intact lining (C), normal portal area (P), normal polyhedral hepatocytes with rounded vesicular nuclei forming hepatic plates (NH), normal hepatic sinusoids (black arrowheads), endothelial cells (red arrowhead), Von kupffer cells (curved arrows), degenerated hepatic cells (DH), dilated and congested lymphatic vessel (LV), desquamated cells and inflammatory cells infiltration (*), diffuse lymphocytic infiltration (L), dilated and congested hepatic sinusoids (long arrows), dilated and congested central vein (V), swollen hepatocytes with vacuolar degeneration (irregular arrows) and numerous apoptotic cells (short arrows). H&E stain X100, X200, X100, X 200, X200, and X200 respectively)
b. Histochemical results: according to PAS stain, the general mucopolysaccharides appeared as a strong positive reaction of magenta red coloration in the cytoplasm of hepatocytes in the control group (Fig. 5a). While, in D group, great depletion of cytoplasmic mucopolysaccharides occurred which manifested by a weak reaction of faint magenta color when compared with C group (Fig.
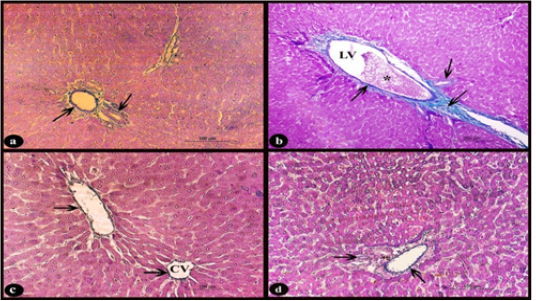
Figure 4: Sections stained with Crossmon’s trichrome stain showing; a) Rat liver of control group, normal distribution of collagen fibers of green color in the interlobular connective tissue septa and perivascular tissue of the portal area (arrows), X 200. b) Rat liver of D group, obvious slight increase of collagen fibers (arrows) around dilated lymphatic vessel (LV) with congestion (*), X 100. c-d) Rat liver of D+M and D+P groups, normal distribution of collagen fibers (arrows) nearly to that of control group around the central vein (CV) and in the perivascular tissue compared to D group, X200
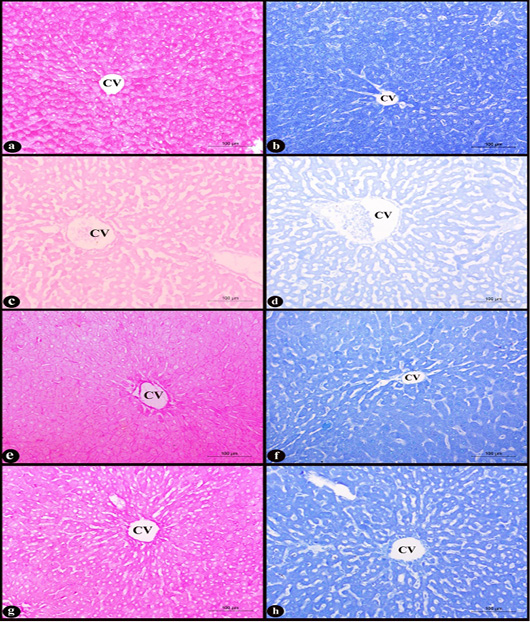
Figure 5: Sections in rat liver stained with PAS stain (left column) and Bromophenol blue stain (right column) X200 in the area of central vein (CV) showing; a-b) control group, normal hepatocytes with strong cytoplasmic positive reactions, c-d) D group, degenerated hepatocytes with weak positive reactions, e-f) D+M and g-h) D+P groups, slight depletion of muccopolysaccharides and protein indicated by moderate cytoplasmic positive colors compared with (D) group.
5c). On the contrary, in the two treated D+M and D+P groups, mild changes in the amount of cytoplasmic mucopolysaccharides were observed by moderate positive reaction when compared with D group (Fig. 5e-g respectively). Regarding to the Bromophenol blue stain, the cytoplasmic total proteins of the hepatocytes were seen as intensely dark blue coloration in the rat liver sections of control group (Fig. 5b). Marked depletion of the cytoplasmic total proteins in the treated D group indicated by faint blue color when compared with control (Fig. 5d). While, in the two treated D+M and D+P groups, improvement to the previous depletion occurred in D group can be observed and only showed a mild decrease in the amount, appeared as a moderate reaction of blue color (Fig. 5f-h respectively).
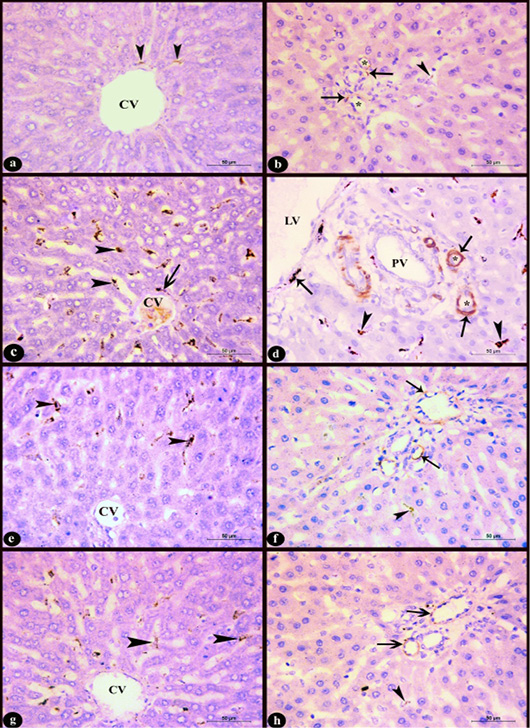
Figure 6: Sections in rat liver stained immunohistochemistry with Anti-Desmin antibody clone (D33, M0760) X400 in the area near to the central vein (CV) (left column) and the region of the portal area (right column) showing; a-b) Control group; few desmin positive cells, c-d) Group D; increased number of desmin positive cells with high intensity, e-f) D+M and g-h) D+P groups; decreased number of desmin positive cells compared to group D. Notice, positive sinusoidal cells near to the central vein (arrow heads), desmin positive cells in the perivascular tissue (arrows), lymphatic vessel (LV), portal vein (PV), hepatic arteries (*).
c. Immunohistochemical results: In control group few sinusoidal cells showed positive Desmin expression of brown color in the hepatic sinusoids near to the central vein, portal area and in the perivascular tissue (Fig. 6a-b). In D group, an obvious increase of desmin positive sinusoidal cells in the number and staining intensity compared to control group was observed. It appeared in the hepatic sinusoids near to the central vein and portal area as well as in the perivascular tissue (Fig. 6c-d). Administration of marjoram oil D+M group decreased the number of hepatic sinusoidal and perivascular desmin positive cells (Fig. 6e-f) compared to D group. Also, the group treated with propolis D+P (Fig. 6g-h) showed a decrease in Desmin expression nearly to that observed in control group.
d. Micropathological analysis: The significant increase of the desmin positive cells in the D group compared to control group and their significant decrease in D+M and D+P groups compared to D and C groups (Table 6) concluded that the P value 0.0057 (< 0.05) was considered very significant and the difference between columns mean was significantly great (Figure 7).
Table 6: The differences in the percentage area of Desmin expression between all different groups (Mean ± SE)
| Group | Desmin expression (Mean ± SE) |
| Group C | 0.4107 ± 0.1546 |
| Group D | 1.222 ± 0.06300 |
| Group (D+M) | 0.9988 ± 0.1261 |
| Group (D+P) | 0.9488 ± 0.1998 |
Group C: control, Group D: diclofenac sodium, Group (D+M): diclofenac sodium & MO, Group (D+P): diclofenac sodium & propolis.
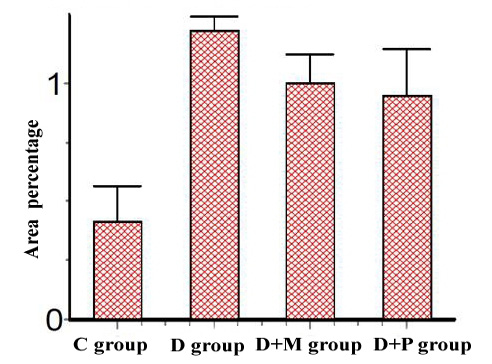
Figure 7: Area percentage of Desmin Immunoexpression in various studied rat groups, (The p value is < 0.05)
Discussion
The liver is an organ implicated with various metabolic capacities and is liable to damage of xenobiotics in view of its focal part in xenobiotics detoxification. NSAID is frequently used for the treatment of rheumatic infections in light of the fact that have antipyretic, pain relieving and palliating activities (Gupta et al., 2004). The study of pathogenesis of hepatic injury as well as the role of lipid peroxidation and inflammation, preventing or retarding the oxidative and inflammatory chain reactions process could be hopeful therapeutic strategies for remediation of liver injury (Tacke et al., 2009).
The rat liver of D treated group exhibited a significant elevation in serum ALT and AST activities, hypoalbuminemia and hypoproteinemia when compared to normal control group. These results are compatible with those reported by Yogesh et al. (2011) who stated that i.p injection of DS (50 mg/kg b.wt.) for 2 days in rats elevated the serum levels of ALT and AST and decrease the total protein and albumin. The liver injury is fundamentally due to the production of reactive metabolites resulted from the metabolism of diclofenac in the liver by CYCP3A4, CYP2C9, and UGT2B7 (Daly et al., 2007). The reactive oxygen species (ROS) interact with the hepatocytes causing lipid peroxidation and exhibiting their toxic effects leading to an increase in the liver enzymes (Linden et al., 2008). The alteration in total protein, albumin and globulins could be related to lesions in intestine, liver, reduced feed intake and absorption. Similar results were observed by Darwish and Eldakroury (2020).
Pretreatment of MO or Propolis restored the levels of liver enzymes, total protein and albumin towards normalization. Propolis treatment only improved the globulin level in D+P group. Increase of globulin concentration may be attributed to stimulation of immune system by propolis flavonoid (Havsteen, 2002).The hepatoprotective effects of propolis and MO on diclofenac induced liver injury have been linked to their anti-oxidative properties (Valente et al., 2011).
In the current study, rats in D group showed a decrease in GSH and CAT whereas MDA increased due to the excess formation of free radicals produced by DS metabolites causing peroxidation of lipids (Alabi et al., 2017). MO or Propolis pretreatment improved the status of antioxidants and decreased the lipid peroxidation induced by DS. MO contains polyphenols, terpenes, phenolic glycosides, flavonoids, tannins, phenolic derivatives, sitosterol and essential oil. Polyphenols are the most plentiful secondary metabolites of plants, which have various properties including antibacterial, anti-viral, antioxidant and anti-inflammatory activity (Bennick, 2002). The high capability of phenolic components to scavenger radicals might be explained by their ability to give a hydrogen atom from the phenolic hydroxyl groups (Abu Aita and Mohammed, 2014). Propolis contains plant phenolic compounds and flavonoids that have strong antioxidant properties. Propolis also inhibits lipid peroxidation and scavenges directly free radicals (Zhu et al., 2011). A prior study showed that oral administration of propolis for seven sequential days decreased CYP2E1 activity and increased the activity of glutathione S-transferase and sulfotransferase, resulting in a decrease in paracetamol-induced liver injury (Seo et al., 2003). In addition, it was reported that administration of caffeic acid phenethyl ester (CAPE), a unique propolis constituent, for three successive days decreased the level and the enzymatic activity of CYP2E1 protein (Lee et al., 2008).
The involvement of mitochondria in diclofenac sodium-induced apoptosis has been confirmed by several studies. Reactive oxygen species produced by DS opens the mitochondrial permeability transition pores and liberates cytochrome c and subsequent initiation of mitochondrial pathway of apoptosis causing cell death (Anarkooli et al., 2008). Members of the BCL- 2 family proteins play regulatory roles in the apoptosis. BAX is a member of the BCL-2 family proteins, which form channels in the outer mitochondrial membrane enabling translocation of cytochrome c into cytosol. Apoptosome formation was stimulated by the cytochrome c released from mitochondria resulting in caspase-9 activation. The latter activates caspase-3, which enhances apoptosis by means of cleaving cellular substrates which responsible for the morphological features and biochemical changes of apoptosis (Chipuk et al., 2004).
In the current study, the severity of the apoptosis was indicated by the hepatic gene expression level of BAX as a pro-apoptotic gene. DS administration significantly increased the gene expression level of BAX as compared to control group and this comes in accordance with Jianchun et al. (2016). A previous study declared that antioxidants prevent mitochondrial dysfunction induced by studied DS (Salimi et al., 2019). Supplementation with MO showed a cytoprotective activity through a significant decrease in the level of BAX gene expression in comparison with D group. The anti-apoptotic activity of MO noticed in our study reflected its anti-apoptotic properties and explained that the cytoprotective activity of MO may be attributed to its antioxidant effect, scavenging of free radicals and suppression of oxidative DNA damage (Misharina et al., 2009). Propolis significantly down regulated BAX gene expression level as compared to D group and became non-significant as compared to control group. Izuta et al. (2008) showed that propolis protected nerve cell death via inhibition of caspase-9 and caspase-3 and so inhibition of the mitochondrial apoptotic pathway. Darendelioglua et al. (2016) stated that propolis significantly weakened the oxidized low density lipoprotein-induced apoptosis via inhibiting oxidative stress and that comes in a harmony with our proposal which explained that the cytoprotective effect of propolis against DS-induced apoptosis might be due to reduction of ROS generation as a result of its antioxidant properties.
TNF-α is a pro-inflammatory cytokine which plays a main role in the pathogenesis of numerous liver diseases. IL-1β is a very effective pro-inflammatory cytokine and it was involved in the liver injury caused by DS (Yano et al., 2012).
In the current study, the serum level of TNF-α in D group was higher than that of the control group as reported by Ramezannezhad et al. (2019). Excessive inhibition of prostaglandins may increase the levels of TNF-α Pinheiro and Calixto, 2002). IL-1β serum level of the D group was significantly greater than the control group. Increased levels of reactive species can elicit inflammasome (a macrophages cytosolic multiprotein complex) activation that mediates the processing of the cytokines secretion (such as IL-1β) and pro-inflammatory caspases, (Mehal, 2014).
Pretreatment with MO significantly decreased the serum levels of both TNF-α and IL-1β as compared to those of D group. These results are in harmony with Bina and Rahimi (2017) who reported that MO has anti-inflammatory activity as it suppressed the production of TNF-α, IL-1β, IL-6 and IL-10 and inhibited cyclooxygenase 2 (COX2) and NFkB gene expression. The anti-inflammatory activity of propolis was obvious through the significant decrease in the serum levels of both TNF-α and IL-1β as compared to those of D group and these corroborate with the findings of Miranda et al. (2019) who stated that propolis acted in the maintenance of a regulated inflammatory profile. We postulate that both MO and propolis exerted their prophylactic effects through limitation of the production and release of inflammatory mediators may be due to their antioxidant activities.
In the control group, the hepatic parenchyma of rats appeared with normal ill distinct classic lobules containing intact central vein, hepatocytes and blood sinusoids as mentioned by El-Maddawy and El-Ashmawy (2013). The administrations of diclofenac sodium lead to dilation and congestion of all hepatic vessels with desquamated cells and inflammatory cells infiltration. Klastskin and Oconn (1993) attributed the dilatation and congestion to the direct toxic effect of any toxin which leading to hepatocellular damage and increasing of von kupffer cells. The hepatic plates appeared disarranged with degenerative changes. Also, slight fibrous tissue proliferation and periportal inflammatory cell infiltration could be observed as mentioned by Taha et al. (2015). Latter authors showed that these changes became progressive with high doses and augmented that the hepatocellular damage was triggered by mitochondrial dysfunction. The structural hepatotoxic changes reported in D group could be alleviated by administration of marjoram oil as revealed by Mossa et al. (2013).
The hepatocytes normally exhibited strong positive PAS reaction indicating localization of neutral mucopolysaccharides as mentioned by Kiernan (1999). The high content of cytoplasmic total proteins in the normal hepatocytes appeared as intensely dark blue coloration with bromophenol blue as stated by Bhandari (1997). The latter authors reported that the dye binds itself to the basic proteins and the amount of dye bound is proportional to the quantity of proteins present. The marked depletion in neutral polysaccharides and total proteins in D group was attributed to the hepatocellular damage. While there was a mild decrease in their amount in the two treated D+M and D+P groups when compared with D group.
Immunohistochemical detection of Desmin expression was run out to assess if this dose of diclofenac could make an induction of acute hepatic fibrosis or not. The increase of Desmin expression in D group indicating that diclofenac could be involved in liver fibrosis after hepatocellular damage and Von Kupffer cells activation (Wu and Zern, 2000). Ballardani et al. (1988) reported strong positive Desmin expression of numerous lobular Ito cells in septae of fibrotic liver. Rockey et al. (1992) suggested that these positive cells derived from lipocytes after their activation then conversion to myofibroblasts, which produce too much extracellular matrix in hepatotoxicity. This was explained by Roberts et al. (2007) that Von Kupffer cells activate lipocytes by releasing of growth and inflammatory mediators. While the pretreatment with marjoram oil and propolis leads to a decrease Desmin expression. The significant increase of Desmin expression in D group and its reduction in the two pretreated groups was statistically very significant (P value is 0.0057 (< 0.05). It was a prospect that occurred due to the antioxidant activity obtained by these materials which protect the liver tissue from fibrogenesis progression by inhibiting lipocytes activation (Boigk et al., 1997; Darbar et al., 2010).
Conclusion
The present study revealed that pretreatment with marjoram oil or propolis potentially ameliorated Diclofenac sodium liver injury, make hepatocellular improvement and overcoming the pathological pictures.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contribution
Olfat Shehata and Abeer M. Radi: Conceived, designed and Performed the experiments.
Randa M. Hassan. Perform histopathological analysis
Doaa Sh. Mohamed. Contributed in biochemical analysis. All authors contributed in writing the paper.
The authors declare that all data were generated in-house and that no paper mill as used.
References






