Advances in Animal and Veterinary Sciences
Research Article
Histological Characterization of Some Feline Mammary Gland Tumors with Whole Slide Images Scan as a Trial of Remote Diagnosis
Mawada M. Ali, Bardes B. Hassan, Asmaa K. Al-Mokaddem, Iman B. Shaheed*
Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt, Giza 12211.
Abstract | Twelve feline mammary gland tissue samples were collected over a period of one year (Jan 2018-Jan 2019) from pets’ clinics in Cairo, Egypt. Aiming to identify the histologic patterns of feline mammary tumors and generation of whole slides scans for remote consultation. Each collected sample was accompanied by a complete animal data and case history. Samples were routinely processed and stained with hematoxylin and eosin (H&E) for histological examination, Masson’s trichrome (MTC) stain was used in some cases and immunohistochemical (IHC) staining using anti- CK8/18 and vimentin markers was performed as well. The tissue slides were scanned using a digital camera to obtain whole slide images (WSI) that were sent to three expert pathologists for teleconsulting. The histological criteria of each tumor type were carefully described under light microscope. Generally, all the examined cases showed marked histological heterogenicity. Ductal papillary or tubule-papillary carcinoma were the most common types of the examined cases. Also, few cases of cribriform and solid carcinomas were diagnosed. All carcinomas (11 cases) were CK8/18 positive. One case exhibited proliferation of pleomorphic myoepithelial cells that were positive for vimentin by IHC staining. Three rare cases of glycogen rich, mucinous and lipid rich carcinoma were recorded. Intense stromal reaction was observed within the examined cases. The trial of remote consulting of scanned slides, despite the presence of some described difficulties, revealed that the degree of accuracy in identifying the histological criteria ranged from 98 to 100% suggesting the positive impact of using whole slide images as a cheap alternative method for digital slide scanning.
Keywords | Feline, mammary gland tumors, histopathology, Whole slide image (WSI).
Received | October 12, 2020; Accepted | October 20, 2020; Published | December 10, 2020
*Correspondence | Iman B Shaheed, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt, Giza 12211; Email: [email protected]
Citation | Ali MM, Hassan BB, Al-Mokaddem AK, Shaheed IB (2021). Histological characterization of some feline mammary gland tumors with whole slide images scan as a trial of remote diagnosis. Adv. Anim. Vet. Sci. 9(1): 117-123.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.117.123
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shaheed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Feline mammary tumors (FMT) are aggressive in nature and commonly observed in veterinary practice (Mills et al., 2015). They are considered as the main cause of death in elder female cats (Shafiee et al., 2013). FMT occupy the 3rd rank of all tumors that affect queens (Shafiee et al., 2013; Morris, 2013; Campos et al., 2016; Silva et al., 2017; Chocteau et al., 2019; Chen et al., 2020).
About 80-90% of FMT are malignant as being aggressive carcinomas (Simeonov and Simeonova, 2009; Shafiee et al., 2013; V Zappulli et al., 2013; Valentina et al., 2015; Mills et al., 2015; Chocteau et al., 2019) Simple malignant neoplasms predominate in FMT as they are more homogenous than mammary tumors of both canines and humans (Zappulli et al., 2013) (Chen et al., 2020). The most common patterns of adenocarcinomas are tubular, papillary, solid, cribriform, and in situ carcinomas (Campos et al., 2016), although solid carcinoma is infrequent in comparison to tubular and papillary adenocarcinomas (Senna et al., 2019).
Feline mucinous mammary gland carcinoma is a rare tumor that characterized by excessive mucin production either in pure or non-pure form when associated with another histotypes of carcinoma (Sarli et al., 2006). Also, feline mammary gland lipid-rich carcinoma was firstly reported by (Kamstock et al., 2005) as a rare type of FMT.
Benign types of FMT are infrequent (Zappulli et al., 2013). The most common lesion among them is fibro adenomatous hyperplasia (Viste et al., 2002), characterized by rapid growth of both epithelium and stroma (Mayayo et al., 2018) (Senna et al., 2019).
Digital microscopy (DM) or digital pathology is a term given to the process of digitalization of glass slides into a high-resolution whole slide images (WSI) usually using slide scanners (Bertram and Klopfleisch, 2017).
Many human pathology diagnostic laboratories and a number of veterinary pathology diagnostic laboratories have integrated DM for routine diagnostic work on-site and off-site for telepathologic diagnosis and secondary consultation (Bertram et al., 2018).
In a validation study comparing digital and light microscopy as diagnostic tools performed by Bertram et al. (2018), digital microscopy was found noninferior to differentiate epithelial and mesenchymal tumors.
The current work aimed to highlight the frequently encountered histological patterns of feline mammary tumors (FMT) with a trial for secondary consultation using digital scanning camera as a feasible and affordable replacement of digital slide scanners.
Material and method
Samples Collection
A total of 12 specimens from a diagnosed feline mammary gland tumor were collected from pets’ clinics in Cairo, Egypt. Over the period from (Jan 2019 to Jan 2020), animal data (including the animal breed and age) were recorded.
Histopathological and Immunohistochemical Examination
The tissue specimens were fixed in 10% neutral buffered formalin, washed in distilled water dehydrated in ascending grades of alcohol and cleared in xylene. After embedding in paraffin, tissues were cut into sections of 4 µm thickness and were stained with hematoxylin and eosin (H&E) (Bancroft, 2013) for routine histopathological examination. Images were captured using an Olympus BX43 microscope equipped with an Olympus DP27 digital camera with CellSens dimensions software (Olympus, Tokyo, Japan). Masons trichrome stain (MTC) and some tissue immune markers were used. Histological criteria were carefully described for each sample including, the carcinoma type, degree of necrosis, stromal scirrhous reaction, peripheral lymphocytic infiltration, desmoplasia and local invasion, vascular invasion and clearance of the surgical margins (Sammarco et al., 2020).
For immuneohistochemistry, slides were cut into 4-5 µm sections on poly-l-lysine coated slides, rehydrated. Heat induced epitope retrieval was performed in microwave for 15 minutes, followed by blocking for protein and endogenous peroxidases. Tissue sections were incubated with primary antibodies (CK8/18 (NCL-L-5D3, 1 in 30 dilution, Novocastra, Newcastle Upon Tyne, UK) and vimentin (V9, 1 in 150 dilution, Dakocytomation) overnight at 4oC. After washing with PBS, HRP labelled secondary antibody was applied for 2 hours at room temperature, followed by a detection step using DAB substrate kit (Thermo Fisher Scientific, USA). Mayer’s hematoxylin was used as counterstain (Sammarco et al., 2020).
Digital Slide Scanning and Remote Diagnosis
Stained glass slides were scanned using a digital scanning camera (Basler, Germany) fitted to light microscope CX 30 (Olympus, Tokyo, Japan) at 200 X. The generated digital images (high quality images with jpg extension files) were uploaded and sent as downloadable links to 3 pathologists with different experience levels for remote diagnosis and teleconsulting. Scans were opened using photo viewer software on laptop. Each pathologist was given the animal data and were asked to provide a histological description for each sample and to spot the pros and cons of such trial.
Results
Animals data for each feline mammary gland tissue sample and histological criteria were summarized in Table (1).
Histopathological and immunohistochemical characterization
From the collected 12 cases, 11 cases were diagnosed as malignant carcinomas and only one case was benign fibroepithelial hyperplasia. Tubulo-papillary carcinoma represented the most frequently observed histological pattern of all examined cases, in which the epithelial neoplastic cells showed the criteria of malignancy, including cellular and nuclear pleomorphism, hyperchromasia and frequent atypical mitosis. These proliferating cells projected toward the ductal lumina forming inward projections supported with fibrous connective tissue stroma (Fig. A.1). Micropapillary invasive carcinoma (Fig. A.2) was also noticed, in which a population of neoplastic cells were forming intraductal aggregates or small papillae without supporting fibrovascular stroma.
Some cases were bearing the criteria of non-invasive ductal carcinoma in situ (DCIS) in which the neoplastic cells
Table 1: Pathological features and animal data for mammary gland samples
| Sample number | Breed | Age (years) | Detected Histological Patterns | Necrosis | stromal scirrhous reaction | Lymphocytic infiltration | Desmoplasia and local invasion | Vascular invasion | Clear surgical margin |
| 1 | Egyptian Mau | 14 | Tubule-papillary carcinoma |
˂10% |
Present | Present | Undetected | Yes | No |
| 2 | Persian | 13 | Solid carcinoma, Comedocarcinoma, Myoepithelioma | ˃50% | Present | Present | Yes | Yes | No |
| 3 | Mixed | 15 |
Tubulo-papillary carcinoma, Solid carcinoma Mucinous carcinoma Lipid rich carcinoma |
20% | Present | Present | Yes | Undetected | No |
| 4 | Egyptian Mau | 8 |
Tubulo-papillary carcinoma, Comedocarcinoma. |
˂10% | Present | Present | Undetected | Undetected | No |
| 5 | Persian | 9 | Fibroepithelial hyperplasia (Benign) | - | - | - | - | - | - |
| 6 | Mixed | 17 | Comedocarcinoma, tubulo-papillary carcinoma | ˃50% | Present | Present | Undetected | Undetected | No |
| 7 | Mixed | 14 | Solid, glycogen-rich, cystic papillary carcinoma | ˂10% | Minimal | Present | Undetected | Undetected | No |
| 8 | Persian | 10 | Comedocarcinoma, solid carcinoma | ˃50% | Present | Present | Yes | Undetected | No |
| 9 | Mixed | 11 | Cribriform, tubulo-papillary carcinoma, Solid carcinoma | ˃50% | Present | Present | Undetected | Undetected | No |
| 10 | Mixed | 16 | Tubulopapillary carcinoma, Solid carcinoma | ˂10% | Present | Present | Yes | Yes | No |
| 11 | Siamese | 13 | Tubulopapillary carcinoma, Solid carcinoma | ˂10% | Present | Present | Undetected | Undetected | No |
| 12 | Persian | 13 | Micropapillary carcinoma, comedocarcinoma | ˂10% | Present | Present | Yes | Yes |
No |
were proliferating in the ductal lumen with no tendency for invasion or basement membrane destruction (Fig. A.3 and A.4). Cystic-papillary carcinoma (Fig. A.5) was also detected, in which the tubular lumen was greatly ectatic with presence of multiple papillary projections with some eosinophilic debris in some instances.
Tubular carcinoma (Fig. A.6 and A.7) as a simple type of tumor was quite common as well, the neoplastic cells were arranged in tubular or gland-like structure of one or two cells thickness. Some of the examined cases showed cribriform pattern (Fig. A.8 and A.9) that characterized by the sieve-like appearance of neoplastic cells as they arranged with central narrow aperture.
Comedocarcinoma (Fig. A.10 and A.11) was described as an aggregate of neoplastic cells with central area of necrosis. The necrotic foci were consisted of homogenous eosinophilic material, cell debris, degenerating neutrophils and macrophages.
Solid carcinoma (Fig. A.12) was recorded; sheets of proliferating neoplastic cells were seen without tendency for tubular or luminal formation. Solid carcinoma pattern was
usually accompanied by another pattern of the previously described carcinomas and was associated with marked local and vascular invasion.
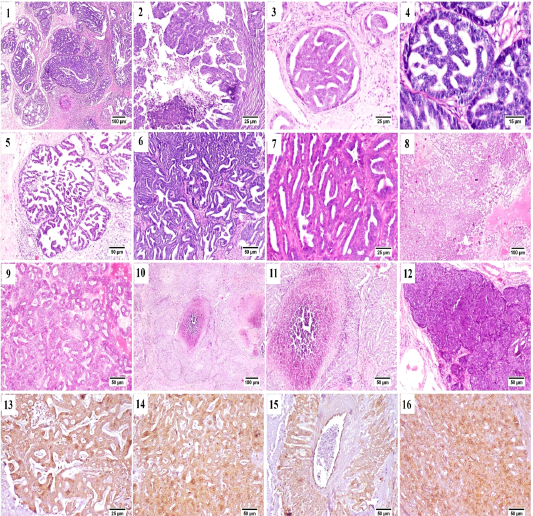
Figure (A): Mammary gland of cat , H&E stained, showing (1) papillary carcinoma, (2) Micropapillary carcinoma, (3) Ductal carcinoma in situ, (4) higher magnification, frequent mitosis of intraductal neoplastic cells, (5) cystic papillary carcinoma, (6) Tubular carcinoma, (7) higher magnification, tubular carcinoma, (8) cribriform carcinoma, (9) higher magnification, cribriform carcinoma, (10) comedocarcinoma, (11) higher magnification, comedocarcinoma with central necrotic area, (12) Solid carcinoma, (13) – (16) Positive Immunostaining of CK 8/18 in the different histologic patterns of carcinoma.
All previously described carcinoma patterns were CK8/18+ positive by immunostaining (Fig. A.13-A.16).
Some of the examined cases were accompanied by desmoplastic stromal reaction, in which the proliferating fibrous tissue stroma were separating an islands of tumor cells (Fig. B.1). Vascular invasion was observed in many cases that was represented by the presence of intravascular aggregates of neoplastic cells (Fig. B.2).
A case of proliferating pleomorphic myoepithelial cells was detected (Fig. B.3) these cells were strong vimentin positive by immunostaining (Fig. B.4).
In the current study three types of rare carcinomas were spotted, the first one was glycogen-rich or clear cell carcinoma (Fig. B.5) in which the neoplastic cells were arranged predominately in solid form with their characteristic clear vacuolated cytoplasm. The second case was that of mucinous carcinoma (Fig. B.6); which was characterized by the presence of blue stained mucus within the tumor cells. The last case was lipid-rich carcinoma (Fig. B.7) characterized by microvacuolated cytoplasm of neoplastic cells with presence of variably sized, intracytoplasmic, distinct vacuoles pushing the nucleus to the periphery of the cells.
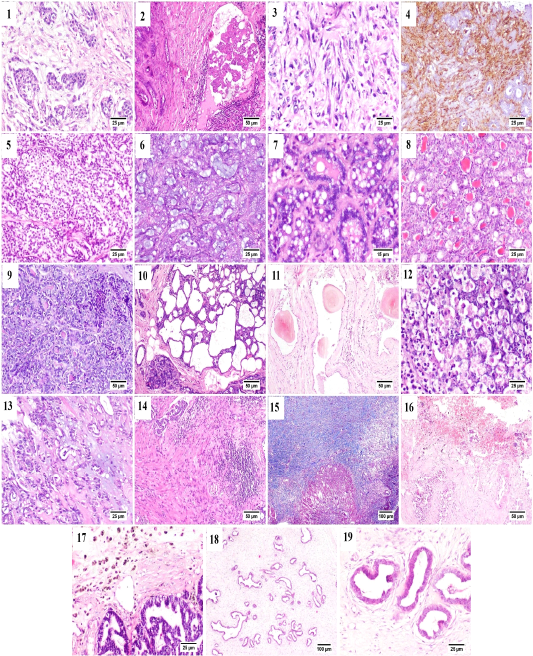
Figure (B): Mammary gland of cat, showing (1) Desmoplasia, (2) vascular invasion by anaplastic cells, (3) Pleomorphic population of myoepithelial cells, (4) Vimentin positive cells (immunostaining), (5) Glycogen-rich carcinoma, (6) mucinous carcinoma, (7) lipid-rich carcinoma, (8) lobular hyperplasia, (9) lobular hyperplasia with atypia, (10) cystically dilated ductules, (11) Duct ectasia with corpora amylacea, (12) Apoptosis with karyorrhexis, (13) Myxomatous change, (14) Stromal fibroplasia with lymphocytic infiltration, (15) proliferating fibrous tissue stained blue with MTC stain, (16) Extensive hemorrhage, (17) hemosiderin-laden macrophages, (18) fibroepithelial hyperplasia and (19) higher magnification, proliferating ductal epithelial tissue with surrounding myoepithelial cells and fibrous stroma.
Generally, in all the diagnosed cases of carcinoma, the non-affected lobules also suffered from some changes including; lobular hyperplasia (Fig. B.8) was detected in some cases in which the proliferation of non-neoplastic cells resulting in increased number of acini and ductules per lobule. Lobular hyperplasia with atypia (Fig. B.9) was noticed as well, in which the proliferating cells bearing some resemblance to malignant cells as pleomorphism, anisokaryosis and frequent mitosis. Cystic dilatation of the ductules was frequently detected (Fig. B.10) as well as duct ectasia with presence of several corpora amylacea (Fig. B.11).
Within the proliferating tumor cells, massive necrosis, apoptosis and karyorrhexis (Fig. B.12) and presence of extended areas of myxomatous change (Fig. B.13) were frequently seen.
Stromal inflammatory and scirrhous reactions (Fig. B.14 and B.15) were quite common. Some carcinomas were accompanied by extensive stromal hemorrhages (Fig. B.16) with presence of increased numbers of hemosiderin laden macrophages (Fig. B.17). The benign case of fibroadenomatous hyperplasia was characterized histologically by the presence of hyperplastic ducts lined by one to three layers of proliferating cells surrounded by multiple layers of myoepithelial cells and finally embedded within a loose fibrillar stroma (Fig. B.18 and B.19).
The benign case of fibroadenomatous hyperplasia was characterized histologically by the presence of hyperplastic ducts lined by one to three layers of proliferating cells surrounded by multiple layers of myoepithelial cells and finally embedded within a loose fibrillar stroma (Fig. B.18 and B.19).
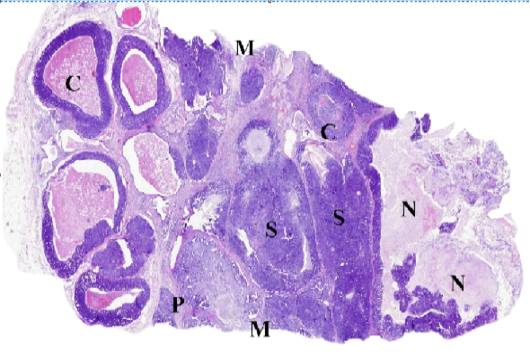
Figure (C): WSI of feline mammary gland tumor, resized, illustrating (C) Comedocarcinoma, (P) Tubulopapillary carcinoma, (S) Solid carcinoma, (N) necrosis and (M) surgical margins.
Whole slide images (WSI) examination
The accuracy of diagnosis and the histological description from the digital scans ranged from 98 to 100 % compared to stained glass slides. As illustrated in (Fig. C), WSI helped to describe in detail the different histologic pattens of the FMT and associated criteria including the stromal reaction and the clearance of surgical margins. The most addressed drawbacks included the increased files size and difficulties in viewing images using domestic photo viewer software, bad color display on LCD screens and some difficulties in identifying cellular characters
Discussion
Consistent with our findings, feline mammary tumors (FMT) were frequently malignant and of epithelial origin (Zappulli et al., 2013; Shafiee et al., 2013; Valentina et al., 2015; Mills et al., 2015; Chocteau et al., 2019). In our study among the collected 12 cases, only one benign case was recorded against eleven malignant carcinomas, that agreed with the findings of Filgueira et al. (2015) who recorded quite similar proportions.
Similar to the results of Campos et al. (2016), our histologic characterization of the collected FMT samples showed great heterogenicity in the patterns of mammary carcinoma that was contrast with the findings of Zappulli et al. (2013) and Chen et al. (2020) who confirmed the homogenic nature of FMT.
In agreement with Winston et al. (2005); Burrai et al. (2010) and Silva et al. (2017) the most commonly found histologic pattern of FMT in our study was tubulopapillary carcinoma, while Skorupski et al. (2005) and Chocteau et al. (2019) reported that the cribriform pattern was the most common pattern in their studies. Moreover, Filgueira et al. (2015) demonstrated that micropapillary was the most common type.
All recorded carcinomas were CK8/18 positive confirming their luminal epithelial origin (Meuten, 2016) as expression of this type of intermediate filaments is preserved in malignant epithelial change.
As previously described by Sammarco et al. (2020), presence of biphasic FMT not a quite common finding that could not be distinguished easily by histologic examination, in our results a case of proliferating pleomorphic population of vimentin positive myoepithelial cells was reported. Glycogen-rich carcinoma with its described histologic patterns was similar to that reported in a Persian queen by Campos et al. (2016). Feline mucus producing tumors are rare, Sarli et al. (2006) reported a small percentage of this type of carcinoma in their study. One of our cases were diagnosed morphologically as lipid- rich carcinoma it was firstly reported in cats by Kamstock et al. (2005) and then by Gal et al. (2017). Despite the small number of collected samples, presence of such rare cases indicates their increasing incidence lately.
In our study, the sole benign tumor reported was fibroadenomatous hyperplasia (Viste et al., 2002; Zappulli et al., 2013; Filgueira et al., 2015) also reported another benign cases of ductal papillary adenomas and as well as some cases of fibroadenomatous hyperplasia.
Unlike the results of Morris (2013); Mills et al. (2015) and Cassali et al. (2018) that reported lobular hyperplasia and duct ectasia as a single lesions. Many of our cases showed those changes along with different patterns of malignant carcinomas.
In agreement with Mills et al. (2015); Sammarco et al. (2020) and Chen et al. (2020), FMT were usually associated with intense stromal fibrosis (scirrhous reaction) while, Morris (2013) reported that stromal fibrosis is uncommon in cats.
Necrosis, apoptosis and karyorrhexis were frequently observed in the examined cases, these results support the findings of Avci and Toplu (2012); Silva et al. (2017); Valentina et al. (2015); Chen et al. (2020) and Sammarco et al. (2020).
Our findings reported the presence of diffuse inflammatory reaction and hemorrhages associated with FMT which are consistent with the results of Mills et al. (2015); Valentina Zappulli et al. (2015); Silva et al. (2017); Senna et al. (2019); Avci and Toplu (2012) and Shafiee et al. (2013). Concerning digital microscopy, the overall evaluation of the trial as a diagnostic tool was satisfying; identifying the tumor type and the histologic pattern of each sample was easily done by all participant supporting the findings of Bertram et al. (2018) in their validation study on canine skin tumors. Some participants surprisingly added some missing features to the histologically described patterns giving a value to the second consultation process. Using WSI perfectly fitted the heterogenic nature of the examined FMT samples.
One advantage of such high quality WSI is being a corner stone in the process of digital pathology used in veterinary education (Dee and David, 2007).
One concerning factor was the complaint of increased file sizes that require time and advanced facilities for uploading, downloading and viewing of images, this disadvantage of WSI was previously reported in many studies (Huisman et al., 2010; Neel et al., 2007; Alcala-canto et al., 2012; Webster and Dunstan, 2014) suggesting the need for large spaces for storage and high speed processors (Al-janabi et al., 2012; Abels et al., 2019). Another addressed drawback was the poor color display on LCD screens, this issue was discussed in the study of Krupinski et al. (2012) who concluded that using color-managed/calibrated display for pathology images had no significant impact on diagnosis accuracy but had a positive impact on limiting diagnosis time which was a complaint of one participant.
Conclusions
Feline mammary gland tumors (FMT) are quite common among aged queens. Most of FMT are malignant and of epithelial origin (carcinomas). FMT are generally characterized by the presence of different histologic patterns predominately, tubulopapillary carcinoma. Rare FMT patterns were encountered in the collected samples including glycogen-rich mucinous and lipid-rich carcinomas could be considered as an indicator of their increased incidence lately. Digital microscopy as a tool for remote diagnosis and secondary consultation of FMT is adequate and non-inferior to normal light microscopy despite some limitations.
acknowledgements
Authors would like to thank the veterinary surgeons and practitioners who supplied us with biopsies and animals’ history. We would also thank our consulting pathologists who helped us in telediagnosis.
conflict of interest
Authors declare no conflict of interest.
authors contribution
I.S. and A.A. made the study design, M. A and A.A. carried out the practical part, M.A. made the scans, B.H., A. A. and M.A. wrote the manuscript draft, I.S supervised the work, revised and corrected the final draft. All authors read and approved the final manuscript.
References






