INTRODUCTION
Avian chlamydiosis is a highly infectious, systemic, fetal and zoonotic disease of psittacine, wild and domestic birds (Andersen, 1997).Itis a reportable disease in many countries due to human affection and public health significance (Naveed et al., 2018; Hogerwerf et al., 2020).
Chlamydiosis in psittacine birds and in human was initially termed as psittacosis or parrot-fever, but a new term “ornithosis” has been introduced to describe the disease in domestic and wild birds (Meyer, 1941). Both terms have been considered as the same and the name “avian chlamydiosis” is more common (Andersen et al., 1997). The main cause of avian chlamydiosis is Chlamydia psittaci (C. psittaci), in addition, C. gallinacean, C. avium, C. ibidis and C. buteonisare new avian chlamydial species (Sachse et al., 2014, 2015; Cheong et al., 2019, Laroucau et al., 2019; Li et al., 2020). C. avium has been found mainly in pigeons and psittacines, while C. gallinacean has been reported in asymptomatic poultry flocks and linked to chlamydiosis in workers. It is interestingly to found these species not only in the same flock, but also in the same bird.
Chlamydophila is a relatively new genus name that divided Chlamydiaceae family to Chlamydia and Chlamydophila genera (Everett et al., 1999). Both genera contain 9 species like C. psittaci, C. pecorum, C. felis, C. caviae, C. abortus, C. pneumoniae, C. suis, C. trachomatis and C. muridarum (Laroucau et al., 2009a).
Considerable economic losses have been noted in outbreaks of chlamydiosis in ornamental and some domestic birds (Kaleta and Taday, 2003; Siraj et al., 2018). In addition, the disease is regarded as a potential zoonosis to human (Evans et al., 2011; Pal, 2017). Chlamydiosis in pet and domestic poultry is a systemic disease and represented in acute, sub-cute, chronic or subclinical forms (Taylor-Brown et al., 2015). Psittacosis in human causing atypical pneumonia, but the disease may be fetal if not treated (Bommana and Polkinghorne, 2019). Awareness among bird owners about the disease risk is very important (Overmars-Marx, 2019).
Treatment of Chlamydia infection in birds is the most important means of disease control (Rodolakis and Laroucau, 2015).There is no commercial vaccine for chalmydiosis till now. Quarantine and testing of imported birds are very critical to prevent introduction of the disease (Matsui et al., 2008).
Chlamydial infection is not only demonstrated in psittacines, but also in domesticated birds. So, great effort should be directed toward this important infection. Chlamydiosis is often accompanied with concurrent infections as well as several outbreaks. In addition, Human in contact with all types of living birds and with dead or slaughter carcasses are also susceptible and exposed to infection hazard. In Egypt, despite the rapid growth of psittacine bird’s populations, there are few available information about chalmydial infection in birds or human.
So, this review article gains more insight to avian chlamydiosis considering the incidence especially in Egypt and some Middle East countries, the causative agent, susceptibility, infection and transmission, the clinical picture in birds and human, diagnosis as well as prevention and control.
The History and Incidence of Avian Chlamydiosis
Avian chlamydiosis is a widely distributed disease all over the world. The disease has been recorded in several countries. The incidence of C. psittaci infection in different avian species and human either in Egypt or different Middle East countries (Saudi Arabia, Iran, Israel and Turkey) is present in Table 1. In these countries, the reported studies about avian chlamydiosis are few, so comprehensive studies about such infection are urgently required.
The Causative Agent
The cause of avian chlamydiosis is C. psittaci which is a Gram-negative obligatory intracellular coccoid bacterium. The organism is belonging to family Chlamydiacaeca, order Chlamydiales and genus Chalmydia. The phylogenic analysis of the 16S and 23S rRNA genes showed that order Chlamydiae contained two distinguished groups’ genera at the family level; Chlamydia and Chlamydophila (Everett et al., 1999; Geens et al., 2005). About 7 genotypes of C. psittaci was isolated from avian origin (A to F, E/B) and 2 mammalian (M56 and WC) that can be transmitted to human (Andersen and Vanrompay, 2003; Lent et al., 2012). These genotypes are host or species specific as genotypes A and F for pisttacine birds, B for pigeons and doves, C for ducks and geese, D for turkeys and E for pigeons, turkeys, ducks, ratites and sometimes human (Andersen, 1997; Andersen and Vanrompay, 2000; Meijer and Ossewaarde, 2002; Zhang et al., 2015; Wang et al., 2018). Genotype E/B was isolated from ducks (Pannekoek et al., 2010). Other genotypes (I, J, 1V, 6N, MatI16, R54, YP84 and CPX0308) are also demonstrated in birds. Genotypes WC and M56 are infecting their specific hosts (Piasecki et al., 2012).
Sachse et al. (2014) isolated C. avium strains from cloacal swabs and organs of pigeons and pisttacine birds suffered from respiratory signs and/or diarrhae. C. avium appears to be common among pigeons and psittacines in Europe (Sachse et al., 2015). A German study found C. aviumin 15% of breeder flocks of domesticated pigeons, and a French study detected it in 8% of urban pigeons. A little data is available bout this type of chalmydial infection in birds and human.
The first detection of C. gallinacea was in France where the organism has been isolated from poultry slaughterhouse workers with atypical pneumonia (Laroucau et al., 2009a). C. gallinacea can be regarded as a bacterium with the potential to infect humans and animals. From different European countries, China and Argentina, C. gallinacea has been isolated from chickens, turkeys and ducks (Frutos et al., 2015; Hulin et al., 2015; Guo et al., 2016). Experimental infection of chickens with C. gallinacea was done by Guo et al. (2016) and the results revealed only weight loss of the infected birds without obvious signs. Moreover, C. gallinacea has been found in asymptomatic chickens, guinea fowl, turkeys and ducks. The sequence analysis of C. gallinacea plasmid has been carried out with detection of specific virulent factor (Ho¨lzer et al., 2016). From wild birds in Korea, genetic variant strains of C. gallinacea have been detected that was differ from those of European and Chinese origin (Jeong et al., 2017). In China, mixed infections with C. gallinacea, C. psittaci and C. pneumoniae have been detected in apparently healthy dairy and beef cattle that was in close contact with poultry (Li et al., 2016).
Table 1: The incidence of avian chlamydiosis in Egypt and in some Middle East countries
| Country |
Findings |
Reference |
|
Egypt
|
Early in 1984, the incidence and public health importance of ornithosis and psittacosis in the imported and exported lovebirds had been demonstrated. |
Mousa (1984)
|
| A case report study was demonstrated in Egypt, where confirmed case of woman psittacosis had been confirmed. The history of exposure to diseased psittacine bird, clinical presentation and laboratory tests supported the diagnosis of such infection. |
Kay (1997)
|
|
It has been detected that C. psittaci antibodies were found in 20 out of 68 (29.91%) chicken’s serum samples using complement fixation test. Ten blood samples of the serologically positive cases were subjected The PCR results were positive for C. psittaci at 119 base pair.
|
Osman et al. (2007)
|
|
In a local commercial market in Egypt, 466 cloacal, 311 ocular and 205 nasal swabs were collected from diseased and apparently healthy turkeys, pigeons, ducks and chickens. Isolation of C. psittaci in embryonated chickens eggs revealed presence of the organism at incidences 74.5%, 79.2%, 5.6% and 17.5% in turkeys, pigeons, ducks and chickens; respectively.
|
El-Jakee et al. (2014)
|
|
Specimens including liver, lung, heart and spleen were taken from suspected C. psittaci infected chickens, ducks, turkeys and pigeons. The results of specimen’s inoculation in eggs and staining showed presence of C. psittaci in chickens (92%), ducks (88%), turkeys (76%) and pigeons (72%). The organism was identified in chickens and turkeys (91.6%) by PCR and (83.3%) using immunofluorescence, while in ducks and pigeons this percentage was 75%. Complement fixation test revealed C. psittaci in chickens, pigeons, ducks and turkeys in percentages 91.6%, 83.3%, 75% and 66.6%; respectively.
|
Hegazy et al. (2014)
|
|
The ompA gene PCR of C. psittaci has been demonstrated in 27 (52.94%) out of 51 faecal swabs and in 13 (54.17%) out of 24 conjunctival swabs in lovebirds. Egg inoculation and staining of negative PCR samples showed positive inclusions in 10 and 11 feacal and conjunctival swabs samples; respectively. Collectively, out of 51 and 37 feacal and conjunctival swabs, the incidence of positive C. psittaci samples were 72.55% and 79.17%; respectively.
|
Mostafa et al. (2015)
|
| One hundred and sixty asymptomatic Cattle Egret (n = 89) and Hoopoe (n = 71) were examined for chlamydiosis. The results showed that 96.4%, 81.8%, 89.1%, 80.0% and 58.2% of samples were positive for chlamydiosis using PCR, staining, florescence and complement fixation test; respectively among Hoopoe. The percentages were 90.6%, 77.4%, 83.0%, 75.5% and 66.0% respectively for the previous tests among Cattle Egret. |
El-Jakee et al. (2017)
|
|
The results of yolk sac inoculation, stained impression smears and PCR of specimens collected from four species of wild birds (50 native and 40 migratory quails, 30 doves and 25 tree sparrows) and four species of pet birds, (20 Budgerigars,10 cockatiels, 3 finches, 5 love birds) showed existence of C. psittaci in 80-100%, 85-100% and 80-100% in pet birds followed by wild birds 64-85%, 76- 95% and 80-90%; respectively. The pathogenicity test in 15 days old chickens and quails demonstrated that the most pathogenic strain was the pet bird’s one. The partial ompA gene sequence of C. psittaci strain was represented in a genotype A which had the highest identity (91.9-94%) with the previous similar strains of genotype A.
|
Hegazy et al. (2017)
|
|
In this study, sputum specimens collected from 70 Egyptians that were in contact with psittacine birds were screened for the presence of C. psittaci using chicken embryo and mice inoculation tests and a PCR assay. The evaluation of these tests revealed that PCR assay overtakes the inoculation tests and so holds better promise for routine surveillance psittacosis programs.
|
Mahmmod et al. (2018)
|
|
This experiment concluded that aqueous leaves neem extract (Azadirachta indica) especially in concertation of 8% induced an excellent as an anti-C. psittaci water medicament without side effects and it could be recommended for controlling chlamydiosis in broiler chickens.
|
Hegazy et al. (2018)
|
|
Saudi Arabia
|
It is the first report of chlamydiosis outbreak in captive breeding group of birds belonging to family Otididae. Birds showed peracute deaths, severe and variable signs, pathological and histological typical lesions. Stained impression smears of spleen showed typical inclusions with prevalence rate (80%) of anti- Chlamydia antibodies using a competitive enzyme immunoassay test. |
Greth et al. (1993)
|
|
Iran
|
Different species of exotic birds were subjected for C. psittaci examination. Conjunctiva, choana, and cloaca and/or droppings were inoculated in tissue culture for isolation followed microscopical examination of the organism. Typical chlamydial inclusion bodies detected in from a ring necked parakeet, an Alexandrine parakeet, an African grey parrot and a Timneh grey parrot.
|
Madani et al. (2011)
|
|
The average infection rate of C. psittaci was %52 (46 samples from 88 pigeon’s samples) after using of nested PCR.
|
Mahzonieh et al. (2013)
|
|
Out of 253 clinical samples collected from 27 avian species with 7 orders, 22 (12.6%) were positive for C. psittaci ompA by a nested PCR. Twelve nested PCR-positive specimens were identified as genotype A from an African grey parrot and a lorikeet, genotype B from a rock dove and a canary, a third new restriction pattern from African grey parrots, and a fourth new restriction pattern from a ring-necked parakeet and an Alexandrine parakeet. The 3rd and 4th restriction patterns are suggested to be provisional genotypes I and J, respectively. The two new genotypes have the closest identity with C. psittaci genotype F and C. abortus, respectively.
|
Madani and Peighambari (2013)
|
|
Out of 270 blood, liver and muscle tissue from pigeons, C. psittaci was demonstrated in 16 (17.78%) blood, 14 (15.56%) liver and 5 (5.56%) muscles.
|
Khodadadi et al. (2015)
|
|
Nasal and cloacal swabs that collected from 11 species of Psittaciformes and 1 species of Columbiformes showed detection of C. psittaci in 37 (18.5%) out of 200 birds (18/37 symptomatic and 19/37 asymptomatic birds) by nested PCR. From 10 C. psittaci genotype A samples of cockatiels, ring-necked parakeet and African gray parrot, 6 samples were from asymptomatic and 4 from symptomatic birds. Genotype B was observed in 3 samples from symptomatic birds and pigeon, and provisional genotype I was found in one symptomatic cockatiel.
|
Mina et al. (2019)
|
|
Israel
|
About 62% of 37 examined people showed specific symptoms of C. psittaci, while 67% of diseased birds died. Acute C. psittaci infection was detected in 81% of patients (30/37). Diagnosis was confirmed in 22 patients by C. psittaci isolation and in 8 patients by positive IgM serology. All examined birds had microbiological evidence of C psittaci infection with typical post-mortem lesions.
|
Huminer et al. (1988)
|
|
Turkey
|
Fecal samples of pet birds were collected from shops and homes for detection of C. psittaci by isolation methods, staining, fluorescein-conjugated monoclonal antibody staining and PCR. In 96 fecal specimens, 33 (34.4%) were positive with PCR, 21 (21.9%) were positive by staining, and 29 (30.2%) were positive with fluorescein technique. As well, from 33 positive PCR, 28 samples were positive with fluorescein, and 20 specimens were positive with staining.
|
Celebi and Seyyal (2006)
|
| In this case, psittacosis has been diagnosed in a mother and her son with Friedreich ataxia, who raised two parrots. Pneumonia, central nervous system and liver affections were also detected in these patients. |
Ciftci et al. (2008)
|
Interestingly, new species of chlamydial infection; avian C. abortus has been molecularly characterized in Poland in 2017 from wildfowl (Szyman´ska-Czerwin´ska et al., 2017).Intermediate strain between C. psittaci and C. abortus has been also detectedin red-tailed hawk (Joseph et al., 2015). These novel species of Chalmydia may be acquired from feeding of these wild predator or scavenger birds on infected carcasses containing different Chalmydia strains.
The developmental cycle of Chlamydia has been described by Borel et al. (2018). The infectious agent of Chlamydia organisms is called LCL; Levinthal (Levinthal, 1930)-Coles (Coles, 1930) and Lillie (Lillie, 1930) that enter the host cells causing the infection (Meyer and Eddie, 1933). Once the infectious elementary body attaches to the cell membrane of the host’s cell, it enters and differentiates into non-infectious reticulate body forming vacuole. The reticulate body shows binary fission division and re-differentiates into elementary body again. Then the elementary body releases and infects another host cell. Development of what is called aberrant body occurs under stressors in the persistence state. The infective body can survive in droppings and equipment for up to 30 days.
Host Susceptibility
The highest incidence of chlamydial infections was recorded in Psittaciformes, Passeriformes, Galliformes, Columbiformes and Anseriformes. Avian chlamydiosis is a world-wild disease that affects more than 465 avian species including companion, domestic and wild birds (Tan et al., 1990) as well as 30 different order of birds (Kaleta and Taday, 2003). Pet birds, domestic poultry species (chickens, turkeys, ducks and geese) and wild birds can be infected with C. psittaci (Vanrompay et al., 1995a). Psittacidae (parrots, parakeets, cockatoos, cockatiels, amazon parrots and macaws) (Mousa, 1984; Smith et al., 2011) as well as Columbiformes (pigeons and doves) are particularly affected with chlamydiosis (Ghorbanpoor et al., 2015). Zoo, fancy or pet marked birds are very susceptible host to C. psittaci that can transmit the infection to human and other domestic birds (Feng et al., 2016; Siraj et al., 2018). Pal and Dahiya (1985) and Sachse et al. (2015) noted that captive parrots are the main global reservoir of C. psittaci.Wild birds have been shown susceptibility to C. psittaciwith persistent infection for long period (Roberts and Grimes, 1978; Brand, 1989; Holzinger-Umlauf et al., 1997; El-Jakee et al., 2014).
Several reports showed natural infections with C. psittaci in broiler, layer and breeder chickens (Durfee et al., 1975; Barr et al., 1986; Malkinson et al., 1987; Arzey and Arzey, 1990; Osman et al., 2007; Yang et al., 2007; Gaede et al., 2008; Zhang et al., 2008; Laroucau et al., 2009a; Robertson et al., 2010; Zhou et al., 2010; Dickx and Vanrompay, 2011; Zocevic et al., 2012; Yin et al., 2013; Lagae et al., 2014; Guo et al., 2016; Čechová et al., 2018), geese (Sadowski and Minta, 1979), ducks (Bracewell and Bevan, 1982; Farmer et al., 1982; Arzey et al., 1990; Newman et al., 1992; Hinton et al., 1993; Laroucau et al., 2009b, Lin et al., 2019), pigeons (Sachse et al., 2012; Zocevic et al., 2013; Sara et al., 2018) and turkeys (Hedberg et al., 1989; Newmann, 1989; Vanrompay et al., 1997b; Enany et al., 2009; Dickx and Vanrompay, 2011).
Young birds are more susceptible to infection than older birds. Adult birds may have sub-clinical disease, while young birds have acute infection (Herrmann et al., 2006).
It has been shown that mammals could be infected with avian C. psittaci strains. For example, C. psittaci has been detected in rabbits (Ni et al., 2015), goat and sheep (Osman et al., 2011), cattle (Reinhold et al., 2011; Li et al., 2016), dogs (Sprague et al., 2009), horses (Bocklisch et al., 1991; Henning et al., 2000; Szeredi et al., 2005; Theegarten et al., 2008; Gough et al., 2019) and pigs (Kauffold et al., 2006).
Mode of Infection and Transmission
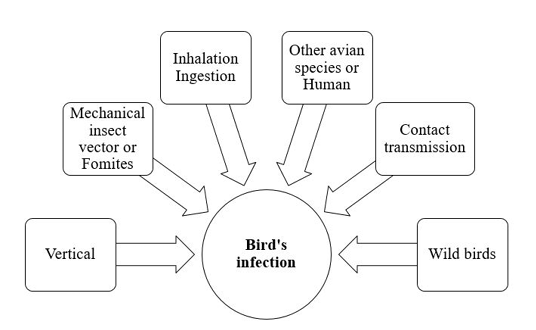
The dose and the virulence of the strain are critical for induction of chlamydial infection. The avian respiratory and intestinal organs are the main targets of C. psittaci (Rodolakis and Mohamad, 2010). Infection and transmission of C. psittaci infection in birds is illustrated in Figure 1.
Infection is usually occur through inhalation or ingestion of contaminated material (Burkhart and Page, 1971; Andersen and Vanrompay, 2003). As elementary bodies do not survive very long outside the host, so close contact with infected birds is very important for induction of infection. Infected birds may transmit chlamydial infection for other avian species and humans (Harkinezhad et al., 2009). Insects, mites and lice may help in mechanical transmission of chlamydiosis (Longbottom and Coulter, 2003; Cobb, 2011). Asymptomatic carrier birds act as a reservoirs and shed the pathogen for long time. Vertical transmission via contamination of egg shell surface has been experimentally documented in chickens, ducks, parakeets, seagulls and snow geese (Vanrompay et al., 1995a). This type of transmission induced high embryonic mortalities. Moreover, vertical mean of transmission creates a problem during preparation of live vaccines of C. psittaci due to contamination of the prepared biological vaccine product. Contact transmission of Chlamydia infection from infected parents to their offspring in the nest was reported in Columbiformes, cormorants, egret, herons, snow geese, gulls and shorebirds. This type of transmission from parent to young may occur via feeding, regurgitation, or contamination of the nest with infective exudates and droppings (Brand, 1989; Harkinezhad et al., 2009). Wild birds are important sources for transmission of chlamydiosis to domestic poultry (Andersen, 1991, 1997). Mechanical transmission through contaminated fomites with the bacterial elementary bodies has been recorded.
The shedding period of Chlamydia organisms from birds depends mainly on the pathogen’s strain and the host (Harkinezhad et al., 2009).Some apparently healthy and sub-clinically infected birds shed Chlamydia for long time (Longbottom and Coulter, 2003).The shedding rate can be exaggerated by transportation, overcrowdings, very high temperature and reproductive activities (Deschuyffeleer et al., 2012).
Clinical Signs
The incubation period of C psittaci in birds varies from 3 days to many weeks (Fudge, 1997). The severity of avian chlamydiosis depends mainly on species, age and immune-status of the birds and the virulence of the infective strain (Guzman et al., 2010).
Some avian species, especially older psittacine birds, may reveal sub-clinical asymptomatic chlamydial infection but shed the organism in the nasal secretions and feces for long time (Smith et al., 2005). Stressors as overcrowding, nutritional deficiency, transportation and temperature variations may transfer sub-clinical intermittent chlamydial infection to acute one.
The disease picture of chlamydiosis in psittacines has three forms; acute, sub-acute and chronic. Clinical signs often appear as fever, anorexia, greenish watery diarrhea, respiratory signs (sneezing, nasal and ocular discharge, sinusitis and dyspnea), dehydration, weight loss and lethargy (Andersen and Vanrompay, 2009).
The disease in turkeys is affected by the virulence of C. psittaci. Serovar D induces severe respiratory distress and high mortalities, while low virulence serotypes prompts anorexia and diarrhea (Vanrompay et al., 1995b).
It has shown that feral pigeons are carriers of C. psittaci as they shed the organism in the droppings, respiratory and conjunctival secretions without signs (Magnino et al., 2009). Nevertheless, concurrent diseases as trichomoniasis, salmonellosis, paramyxovirus and herpesvirus infection can induce diarrhea and respiratory disease condition (Longbottom and Coulter, 2003; Andersen and Vanrompay, 2009).
Although ducks are usually asymptomatic carriers, but they can transmit chlamydial infection to human and induce severe pneumonia (Laroucau et al., 2009b).
Natural infection of commercial chickens flocks with C. psittaci is not common, however, some experimentally infected birds showed signs and mortalities. Moreover, some human cases of chlamydiosis have been reported as a result of processing of sub-clinically infected chickens.
The mortality rate of chlamydiosis depends on the species of the affected birds, the virulence of the invading strain and the presence of secondary invaders of pathogens. Mortalities can reach to 50% or more in psittacine birds, however, less rate was seen in pigeons and it has been usually caused by secondary infections.In turkeys, infected cases showed mortalities ranged from 5% to more than 40%.
Pathology
Asymptomatically C. psittaci infected birds often have no post-mortem lesions.
The post-mortem lesions of the affected pet birds showed multifocal necrosis of liver and spleen with enlargement and fibrinous airsacculitis, perihepatitis, pericarditis and peritonitis (Mohan, 1984; Andersen, 1997).Generalized vascular congestion and enteritis may also observed. Challenging of broilers with C. psittaci induced septicemia, nephritis and thickening of the air sac (Zhou et al., 2010; Yin et al., 2013).
Turkeys affected with C. psittaci serovar D showed rhinitis, conjunctivitis, sinusitis, tracheitis, airsacculitis, pneumonia, pericarditis and enteritis.
Human Infection
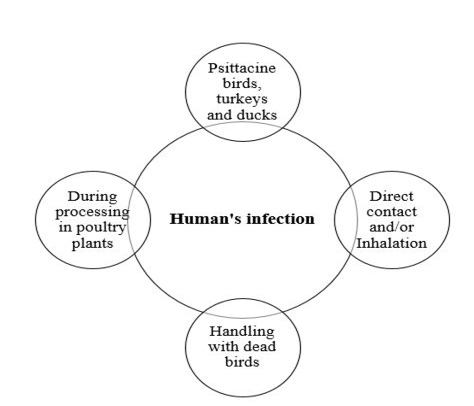
The different means of C. psittaci infection and transmission in human is represented in Figure 2. Psittacosis in humans contracted from turkeys and ducks is often as severe as at contracted from psittacine birds. Human gain C. psittaci infection through direct contact and/or inhalation of infected droplets in the respiratory exudates, droppings dust or feathers of infected living birds (Rzedzicki and Tokarzewski, 2001; Andersen and Vanrompay, 2003; Beeckman and Vanrompay, 2009; Harkinezhad et al., 2009;
West, 2011; Knittler and Sachse, 2015; Szyman´ska-Czerwin´ska and Niemczuk, 2016). Moreover, other potential zoonotic risk ofC. Psittaci may occur as a result of mouth-beak contact, bites of infected birds and practices involving handling of plumage or tissues of dead birds (Williams et al., 1998; Telfer et al., 2005; Beeckman and Vanrompay, 2009) as well as evisceration in slaughterhouses (Gaede et al., 2008; Dickx et al., 2010). The disease has been recorded amongst workers in poultry processing plants, Veterinarians and bird dealers (Trávnicek et al., 2002; Pal, 2013). Recently, it has been demonstrated that there is a possibility of human (especially Veterinarians) infection with C. psittaci from the direct contact with equine’s placenta (Chan et al., 2017; Jelocnik et al., 2017; Polkinghorne and Greub, 2017).
Psittacosis is a notifiable disease. The incubation period of psittacosis in human may be 1-2 weeks, with the possibility of longer incubation period. Human infected with C. psittaci show symptoms vary from asymptomatic infection to severe systemic disease with fever, headache, respiratory disease (sore throat, pharyngitis, cough, dyspnea and pneumonia), gastro-intestinal problems (abdominal pain, vomiting and diarrhea), hepatomegaly, splenomegaly and other complications like conjunctivitis, arthritis, endocarditis, encephalitis and fetal death (Pal, 2004; Petrovay and Balla, 2008; Beeckman and Vanrompay, 2009; Chau et al., 2015; DE Boeck et al., 2016; Radomski et al., 2016).
C. gallinacea has been discovered in poultry flocks and was associated with workers in abattoir had atypical pneumonia while C. avium has not been recorded in human.
Human-to-human transmission of psittacosis has been recorded (Hughes et al., 1997; Ito et al., 2002; McGuigan et al., 2012; Wallensten et al., 2014; Schlossberg, 2015).
Diagnosis
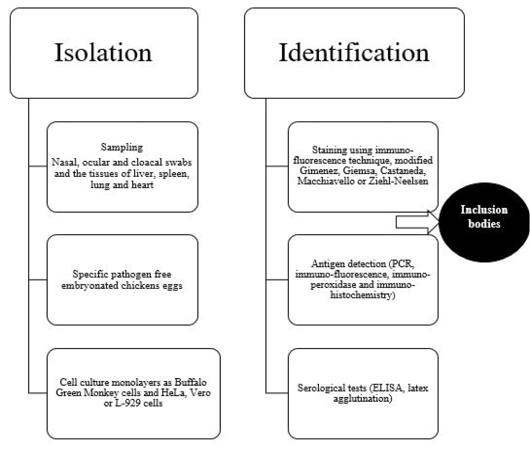
Diagnosis of avian chlamydiosis is based on the typical signs, the isolation and identification of the pathogen, the detection of Chlamydiae in tissues, or the demonstration of a four-fold increase in specific humoral antibodies (Vanrompay et al., 1995a). The different means of isolation and identification of Chlamydia organism is summarized in Figure 3.
Isolation of Chlamydia organism has been done in eggs or on tissue culture (gold standard) (Madani et al., 2011; Mostafa et al., 2015), although other methods are also used (OIE, 2000; Vanrompay, 2000). Isolation of C. psittaci is somewhat shows some difficulties as some affected birds have sub-clinical asymptomatic infection, the isolation techniques require specific cell cultures or specific pathogen free embryonated chicken eggs and the frequency of obtaining false-negative results is common due to intermittent shedding of C. psittaci in the droppings (Fudge, 1997; Balsamo et al., 2017). In addition, there is a risk health hazard to laboratory workers during isolation process (Spoorenberg et al., 2016) as some strains of C. psittaci have been categorized as a biosafety level 3 organism (Ortega et al., 2011).
Samples for C. psittaci isolation should be taken in acute conditions from nasal, ocular and cloacal swabs and the tissues of liver, spleen, lung and heart (Andersen, 1996). More than one type of Chlamydiae can be detected in one case. It is important to not collect the sample after antibiotic treatment of birds to avoid false negative results.Specific pathogen-free 6-7 day-old embryonated eggs are used mainly for primary isolation of C. psittaci (Messmer et al., 2000). Although inoculation of eggs is a standard method for detection of C. psittaci, this method requires long time at high temperature 39°C (Pearson et al., 1989; Bougiouklis et al., 2000; Condon and Oakey, 2007). Death of the embryo is usually occur within 3-10 days post-inoculation as well asvascular congestion of the yolk sac membranes may be also seen. Some cases require two additional blind passages to induce embryonic deaths or before considering the sample as negative.
Yolk sac suspension could be inoculated on cell culture monolayers as Buffalo Green Monkey cells and HeLa, Vero or L-929 cells, and then examined after staining using immuno-fluorescence technique for the presence of inclusion bodies (Vanrompay et al., 1992; Andersen, 1998; Yin et al., 2013).
Microscopic examination of the yolk sac, tissue culture or organs impression smears after staining with modified Gimenez, Giemsa, Castaneda, Macchiavello or Ziehl-Neelsen revealed presence of specific inclusion bodies (Sachse et al., 2009). Typical intracytoplasmic inclusion bodies appear as small, round or hat-shaped red dots against a bluish green background. In some virulent strains of C. psittaci, the inclusions break up and disperse in the cytoplasm (Trevejo et al., 1999).
As mentioned before, traditional standard isolation methods of C. psittaci need long period, require good sample quality as well as the risk of zoonotic transmission for microbiologist (Trevejo et al., 1999). Accordingly, Polymerase Chain Reaction (PCR) has been developed as a rapid, safe, simple and sensitive method for detection of C. psittaci (Hewinson et al., 1991; Kaltenboeck et al., 1991; Hewinson et al., 1997; Moroney et al., 1998; Olsen et al., 1998; McElnea and Cross, 1999; Messmer et al., 2000; Sachse et al., 2005, 2009). Mahmmod et al. (2018) evaluated the accuracy of different isolation and detection methods of C. psittaci and concluded that PCR assay outperforms chicken embryo and other inoculation tests as well as holds a better promise for surveillance programs for psittacosis. It is recommended that samples are taken on 3 consecutive days to detect intermittent shedding of the organism.
Furthermore, the different species of Chlamydia can be differentiated using DNA microarray hybridization tests. Immuno-fluorescence, immuno-peroxidase and immuno-histochemistry as immuno-staining methods have been used for detection of chlamydial antigens (Sachse et al., 2009). Monoclonal antibodies toward some chlamydial antigens as lipopolysaccharides or major outer membrane protein have been found to be more sensitive than histochemical methods of diagnosis (Borel et al., 2014).
Serological tests as elementary body agglutination test (Grimes et al., 1994) and latex agglutination (Arizmendi and Grimes, 1993) can be used for demonstration of antibodies against Chlamydia especially immunoglobulins IgM in recent infection. Different types of Enzyme Linked Immuno-sorbent Assay (ELISA) has been developed to detect C. psittaci infection (Evans et al., 1983; Ruppanner et al., 1984; Verminnen et al., 2006). Dickx et al. (2010) and Dickx and Vanrompay (2011) used C. psittaci recombinant major outer-membrane protein based antibody ELISA to examine broiler breeder, broiler and layer chicken farms in Belgium and demonstrated positive cases in percentages of 98, 95 and 95 in layers, broilers and broiler breeders, respectively. Conventional type of ELISA showed non-specific reaction and cross reactivity with other Gram-negative organisms (Andersen, 1998), but blocking ELISA revealed higher sensitivity (Gerlach, 1999). Complement fixation test detected four fold rise in Chlamydia antibody titer in paired samples. Indirect fluorescent antibody technique (Andersen, 1991) and PCR-restriction fragment length polymorphism (Vanrompay et al., 1997a) have been used also to identify Chlamydia serovar using specific monoclonal antibodies against outer membrane protein.
Prevention and Control Strategies
Prevention and eradication programs of avian chlamydiosis is difficult due to presence of large number of asymptomatic carrier hosts, the intermittent shedding of the pathogen, the endemic nature of the bacteria and the long survival time in the organic matter (Vanrompay et al., 2007).
Specific antibiotics including tetracyclines, macrolides (erythromycin and azithromycin) and fluoroquinolones prove their efficacy for the treatment of chlamydial infection. Tetracyclines group of medicaments is the most preferable group for the treatment of the affected birds with Chlamydia. Treatment should be maintained for long time, whenever, 45 days is recommended for the treatment of pet birds (Vanrompay et al., 1995a). Chlortetracycline is usually given in feed and the dose differs according to bird’s species and the type of feed (Gerlach, 1999). Chlortetracycline treatment has some disadvantages including less tendency of the birds to feed on the treated feed, insufficient blood level of the drug as well as destruction of natural gut microbiota (Gerlach, 1999). Other medicament like oxyteteracyline could be used intramuscularly especially for large size birds (Flammer et al., 1990). However, injection of muscle may induce necrosis at the inoculation site as well as prolonged withdrawal period with residual effect (Jawad et al., 2014). Doxycycline may be used either in feed (Gerlach, 1999) or in the drinking water (Flammer, 2000) with effective results especially after 45 days treatment period. Although tetracyclines inhibit the synthesis of chlamydial ribosomal proteins, prolonged administration of antibiotic arises the chance of drug resistance (Guzman et al., 2010; Rodolakis and Laroucau, 2015). Pet birds could be treated in feed with enrofloxacin (Gerlach, 1999). Azithromycin water treatment has been found to be effective for Cockatiels after 21 days treatment period (Guzman et al., 2010).Periodical sampling of the treated birds after each treatment to check the relapse and clearance of the bacteria.
It is very important to treat concomitant bacterial pathogens as Streptococcus and Lactobacilli that associated with Chlamydia infection. It has been shown that prolonged antibiotic treatment of chlamydial infection arising the problem of antibiotic resistance especially in case of preventive medication (Dugan et al., 2004; Di Francesco et al., 2008; Beeckman and Vanrompay, 2009) as well as persistence of the bacteria even after treatment.
Accordingly, new substances like phytobiotics may replace the usage of antibiotic for the treatment of chlamydiosis (Vanka et al., 2001). The effect of natural herbal plants extracts like aqueous neem on C. psittaci infection of broilers was studied (Hegazy et al., 2018). The results proved potent effect of 8% concentration of neem extract on C. psittaci without adverse effect on liver or kidneys tissues as it could be substitute oxytetracycline treatment.
There is no available commercial vaccine for prevention of Chlamydia infection in birds. The production of chalmydial vaccine depends mainly on the protective level of the prepared vaccine as well as the cost of production. In the studies of Vanrompay et al. (1999a,b) and Vanrompay et al. (2001), the gene encoding for the major outer membrane protein of C. psittaci serovar A was used for production of plasmid DNA vaccines in turkeys and the results showed promising protection. In addition, the demonstration of the outer membrane protein expression for at least 10 weeks past-vaccination with such vaccine was reported (Loots et al., 2006).
For prevention of the disease introduction, newly introduced birds or birds returned from shows or fairs should be quarantined for at least one month and observed for specific signs (Davies and Collins; 1995; De Freitas Raso et al., 2004; Dovcˇ et al., 2007; Matsui et al., 2008).Testing and isolation of birds from unknown sources before boarding are also important (Van Loock et al., 2005; Heddema et al., 2006). Mixing of birds from different sources should be prohibited (Sclossberg et al., 1993; Circella et al., 2011).
Thorough cleaning and disinfection using some lipid solvents disinfectants like 1:1000 quaternary ammonium compounds, formaldehyde, chlorophenols and 70% alcohol (Jencek et al., 2012). Wild birds and insects should be controlled. Hygienic disposal of dead birds is the must.
For Veterinarians, workers in the poultry farms and processing plants and pet bird handlers; protective clothes, gloves and filter mask should be wear. Keeping ventilation and continuous air disinfection should be considered to avoid aerosol contamination and transmission of Chlamydia (Deschuyffeleer et al., 2012).
As psittacosis is a reportable disease, so local public health authorities must be reported within 48 hours of the disease detection (Chau et al., 2015).In addition, oral tetracycline treatment of psittacosis in human (Aundria, 2011) can induce sub-clinical persistent disease and may provoke chronic infection with relapsing after the treatment course (Elwell et al., 2016).
CONCLUSION
From the previously mentioned information, it can be concluded that avian chlamydiosis causes severe losses especially in pet birds, besides its public health significance in human. So, it is very important to pay attention toward this disease regarding the epidemiology, the methods of diagnosis and the strategies of prevention and control.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Wafaa A. Abd El-Ghany collected the data, wrote and prepared the manuscript.
REFERENCES
• Andersen AA (1991). Serotyping of Chlamydia psittaci isolates using serovar-specific monoclonal antibodies with the micro-immunofluorescence test. J. Clin. Microbiol. 29(4): 707-711.https://www.ncbi.nlm.nih.gov/pubmed/1890172
• Andersen AA (1996). Comparison of pharyngeal, fecal, and cloacal samples for the isolation of Chlamydia psittaci from experimentally infected cockatiels and turkeys. J. Vet. Diagn. Invest. 8(4): 448-450. https://doi.org/10.1177/104063879600800407
• Andersen AA (1997).Two new serovars of Chlamydia psittaci from North American birds. J. Vet.Diagn. Invest. 9: 159-164.
• Andersen AA (1998). Chlamydiosis. In: A Laboratory Manual for the Isolation and Identification of Avian Pathogens, 4th ed. D.E. Swayne, J.R. Glisson, M.W. Jackwood, J.E. Pearson, and W.M. Reed (eds.). American Association of Avian Pathologists, University of Pennsylvania, New Bolton Center, Kennett Square, PA. pp. 81-88.
• Andersen AA, Vanrompay D (2000). Avian chlamydiosis. Rev. Sci. Tech. 19(2): 396-404.
• Andersen AA, Vanrompay D (2003). Avian chlamydiosis (psittacosis, ornithosis). Saif YM, ed. Diseases of Poultry, 11th edition, Iowa State University Press, Iowa, USA; 2003. p. 863-879.
• Andersen A, Vanrompay D (2009). Avian Chlamydiosis. In ‘Diseases of Poultry.’ (Eds YM Saif, AM Fadly, JR Glisson, LR McDougald, LK Nolan, DE Swayne.) Vol. 12 pp. 978-981.
• Andersen AA, Grimes JE,Wyrick PB (1997). Chlamydiosis (psittacosis, ornithosis). In: Diseases of Poultry, 10th ed. B.S. Calnek, H.J. Barnes, C.W. Beard, L.R. McDougald, and Y.M. Saif (eds.). Iowa State University Press, Ames, Iowa. pp. 333-349.
• Arizmendi F, Grimes JE (1993). Evaluation of latex agglutination for detecting chlamydial antibody activity in psittacine bird sera by comparison with direct complement fixation. J. Vet. Diagn. Invest. 5(2): 277-279.
• Arzey GG, Arzey KE (1990). Chlamydiosis in layer chickens. Aust. Vet. J. 67(12): 461. https://doi.org/10.1111/j.1751-0813.1990.tb03069.x
• Arzey KE, Arzey GG, Reece RL (1990). Chlamydiosis in commercial ducks. Aust. Vet.J.67(9): 333-334. https://doi.org/10.1111/j.1751-0813.1990.tb07817.x
• Aundria W (2011). A brief review of chlamydophila psittaci in birds and humans. J. Exot. Pet. Med. 20: 18-20. https://doi.org/10.1053/j.jepm.2010.11.006
• Balsamo G, Maxted AM, Midla JW, Murphy JM, Wohrle R, Edling TM, Fish PH, Flammer K, Hyde D, Kobayashi M, Oiulfstad B, Ritchie BW, Stobierski MG, Ehnert K, Tully Jr TN (2017). Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis). J. Avian Med. Surg. 31(3): 262-282.https://doi.org/10.1647/217-265
• Barr DA, Scott PC, OíRourke MD,Coulter RJ (1986). Isolation of Chlamydia psittaci from commercial broiler chickens. Aust. Vet. J. 63(11): 377-378.https://doi.org/10.1111/j.1751-0813.1986.tb02906.x
• Beeckman DSA, Vanrompay DCG (2009). Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 15(1): 11-17. https://doi.org/10.1111/j.1469-0691.2008.02669.x
• Bocklisch H, Ludwig C, Lange S (1991). Chlamydia as the cause of abortions in horses. Berl. Munch. Tierarztl. Wochenschr. 104(4): 119-124.
• Bommana S, Polkinghorne A (2019). Mini review: Antimicrobial control of chlamydial infections in animals: Current practices and issues. Front. Microbiol. 10: 113.https://dx.doi.org/10.3389%2Ffmicb.2019.00113
• Borel N, Polkinghorne A, Pospischil A (2018). A review on chlamydial diseases in animals: Still a challenge for pathologists?. Vet. Pathol. 55(3):374-390. https://doi.org/10.1177/0300985817751218
• Borel N, Frey CF, Gottstein B, Hilbe M, Pospischil A, Franzoso FD, Waldvogel A (2014). Laboratory diagnosis of ruminant abortion in Europe. Vet. J. 200(2): 218-229. https://doi.org/10.1016/j.tvjl.2014.03.015
• Bougiouklis P, Papaioannou N, Georgopoulou I, Iordanidis P,Vlemmas I, Lekkas S, Siarkou V (2000). Chlamydia-induced bilateral ectropion of the inferior eyelids in pigeons. Avian Dis. 44: 372-378. https://doi.org/10.2307/1592552
• Bracewell CD, Bevan BJ (1982). Chlamydia infection in ducks: preliminary communication. J. Roy. Soc. Med .75(4): 249-252. https://www.ncbi.nlm.nih.gov/pubmed/7069695
• Brand CJ (1989). Chlamydial infections in free-living birds. J. Am. Vet. Med. Assoc. 195: 1531-1535.
• Burkhart RL, Page LA (1971). Chlamydiosis (ornithosis-psittacosis). In: Infectious and Parasitic Diseases of Wild Birds. J.W. Davis, R.C. Anderson, L. Karstad, and D.O. Trainer (eds.). Iowa State University Press, Ames, Iowa. pp. 118-140.
• Čechová L, Halánová M, Babinská I, Danišová O, Bartkovský M, Marcinčák S, Marcinčáková D, Valenčáková A, Čisláková L (2018). Chlamydiosis in farmed chickens in Slovakia and zoonotic risk for humans. Ann. Agr. Env. Med.25(2): 320-325. https://doi.org/10.26444/aaem/82948
• Celebi BS, Seyyel Ak (2006). A Comparative study of detecting Chlamydophila psittaci in pet birds using isolation in embryonated egg and polymerase chain reaction. Avian Dis. 50: 489-493.
• Chan J, Doyle B, Branley J, Sheppeard, V, Gabor, M, Viney K, Quinn H, Janover O, Mccready M, Heller J (2017). An outbreak of psittacosis at a veterinary school demonstrating a novel source of infection. One Health. 3:29-33. https://doi.org/10.1016/j.onehlt.2017.02.003
• Chau S, Tso EY, Leung WS, Fung KS (2015). Three cases of atypical pneumonia caused by Chlamydophila psittaci. Hong Kong Med. J. 21: 272-275. https://doi.org/10.12809/hkmj144321
• Cheong HC, Lee CYQ, Cheok YY, Tan GMY, Looi CY, WongF (2019). Chlamydiaceae: Diseases in primary hosts and zoonosis. Microorganisms. 7(5): 146. https://dx.doi.org/10.3390%2Fmicroorganisms7050146
• Ciftci B, Guler ZM, Aydogdu M, Konur O, Erdogan Y (2008). Familial outbreak of psittacosis as the first Chlamydia psittaci infection reported from Turkey. Tuberk Toraks. 56(2): 215-220.
• Circella E, Pugliese N, Todisco G, Cafiero MA, Sparagano OA, Camarda A (2011).Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Exp. Appl. Acarol. 55(4): 329-338. https://doi.org/10.1007/s10493-011-9478-9
• Cobb SP (2011). The spread of pathogens through trade in poultry meat: overview and recent developments. Rev. Sci. Tech. 30: 149-64. https://doi.org/10.20506/rst.30.1.2026
• Coles AC (1930). Micro-organisms in psittacosis. Lancet i: 1011.
• Condon K, Oakey J (2007). Detection of Chlamydiaceae DNA in Veterinary specimens using a familyspecific PCR. Lett.Appl. Microbiol.45: 121-127. https://doi.org/10.1111/j.1472-765X.2007.02169.x
• Davies A, Collins T (1995). Respiratory Chlamydia: the management of an outbreak. Public Health. 109(3): 207-211. https://doi.org/10.1016/s0033-3506(05)80054-x
• DE Boeck C, Dehollogne C, Dumont A, Spierenburg M, Heijne M, Gyssens I, Vander Hilst J, Vanrompay D (2016). Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in the Netherlands. Epidemiol. Infect. 144(8): 1710-1716. https://doi.org/10.1017/s0950268815003106
• De Freitas Raso T, Godoy S, Milanelo L, De Souza C, Matuschima E, Araújo J, Pinto A (2004). An outbreak of chlamydiosis in captive blue fronted Amazon parrots (Amazona aestiva) in Brazil. J. Zoo Wildl. Med. 35(1): 94-96.www.jstor.org/stable/20460364.
• Deschuyffeleer TPG, Tyberghien LFV, Dickx VLC, Geens T, Saelen JMMM, Vanrompay DCG, Braeckman, LACM (2012). Risk assessment and management of Chlamydia psittaci in poultry processing plants. Ann. Occup. Hyg. 56: 340-349. http://dx.doi.org/10.1093/annhyg/mer102
• Dickx V, Vanrompay D (2011). Zoonotic transmission of Chlamydia psittaci in a chicken and turkey hatchery. J. Med. Microbiol. 60: 775-779. https://doi.org/10.1099/jmm.0.030528-0
• Dickx V, Beeckman DS, Dossche L, Tavernier P, Vanrompay D (2010).Chlamydophila psittaci in homing and feral pigeons and zoonotic transmission. J. Med. Microbiol. 59: 1348-1353. https://doi.org/10.1099/jmm.0.023499-0
• Di Francesco A, Donati M, Rossi M, Pignanelli S, Shurdhi A, Baldelli R, Cevenini R (2008). Tetracycline-resistant Chlamydia suis isolates in Italy. Vet. Rec.163: 251- 529.https://doi.org/10.1136/vr.163.8.251
• Dovcˇ A, Slavec B, Lindtner-Knific R, Zorman-rojs O, Račnik J, Golja J, Vlahović K (2007). Study of Chlamydophila psittaci outbreak in budgerigars. Bull. Vet. Inst. Pulawy. 51: 343-346.
• Dugan J, Rockey D, Jones L, Andersen AA(2004). Tetracycline resistance in Chlamydia suismediated by genomic islands inserted into the chlamydial inv-like gene. Antimicrob. Agents Chemother. 48: 3989-3995. https://dx.doi.org/10.1128%2FAAC.48.10.3989-3995.2004
• Durfee PT, Pullen MM, Currier RWII, Parker RL (1975). Human psittacosis associated with commercial processing of turkeys. J. Am. Vet. Med. Assoc. 167(9): 804-808.
• El-Jakee JK, El-Hariri MD, El-Shabrawy MA, Gaber ES (2017). Isolation and identification of Chlamydophila in poultry species in Egypt. Int. J. Chem. Tech. Res. 10(5): 520-526.
• El-Jakee JK, Osman KM, Ezzeldeen NA, Ali HA, Mostafa ER (2014).Chlamydia species in free-living Cattle Egret (Bubulcus ibis) and Hoopoe (Upupa epops) in Egypt. Int. J. Vet. Sci. Med. 2(1): 1-6.https://doi.org/10.1016/j.ijvsm.2013.12.002
• Elwell C, Mirrashidi K, Engel J (2016).Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 14: 385-400. https://doi.org/10.1038/nrmicro.2016.30
• Enany ME, Mousa HA, Salem HAS (2009). Investigations on the prevalence of chlamydiosis in turkey flocks in Egypt with special emphasis on immunopathological characterization of Chlamydophila psittaci .Global Vet. 3(5): 424-428.
• Evans DW, Müller-Loennies S, Brooks CL, Brade L, Kosma P, Brade H, Evans SV (2011). Structural insights into parallel strategies for germline antibody recognition of lipopolysaccharide from Chlamydia. Glycobiology. 21(8): 1049-1059.https://doi.org/10.1093/glycob/cwr041
• Evans RT, Chalmers WSK, Woolcock PR, Farmer H, Taylor-Robinson D (1983). An enzyme-linked immunosorbent assay (ELISA) for the detection of chlamydial antibody in duck sera. Avian Pathol. 12: 117-124. https://doi.org/10.1080/03079458308436153
• Everett KDE, Bush RM, Andersen AA (1999). Amended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49(Pt2): 415-440. https://doi.org/10.1099/00207713-49-2-415
• Farmer H, Chalmers WS, Woolcock PR (1982).Chlamydia psittaci isolated from the eyes of domestic ducks (Anas platyrhynchos) with conjunctivitis and rhinitis. Vet. Rec. 110(3): 59. https://doi.org/10.1136/vr.110.3.59
• Feng Y, Feng YM, Zhang ZH, Wu SX, Zhong DB, Liu CJ (2016). Prevalence and genotype of Chlamydia psittaci in faecal samples of birds from zoos and pet markets in Kunming, Yunnan, China. J. Zhejiang Univ. Sci. B 17(4): 311. https://dx.doi.org/10.1631%2Fjzus.B1500091
• Flammer K (2000). Preliminary notes on treatment of chlamydiosis with doxycycline medicated water. Proceedings of the Annual Conference of Association of Avian Veterinarians, August 30 - September 1, 2000, Portland, Oregon, USA. pp. 3-5.
• Flammer K, Aucoin DP, Whitt DA, Styles DK (1990). Potential use of long-acting injectable oxytetracycline for treatment of chlamydiosis in Goffin’s cockatoos. Avian Dis. 34(1): 228-234. https://doi.org/10.2307/1591359
• Frutos MC, Monetti MS, Vaulet LG, Cadario ME, Fermepin MR, Ré VE, Cuffini CG (2015). Genetic diversity of Chlamydia among captive birds from central Argentina. Avian Pathol. 44(1): 50-56. https://doi.org/10.1080/03079457.2014.993593
• Fudge AM (1997). A review of methods to detect Chlamydia psittaci in avian patients. J. Avian Med. Surg. 11: 153-165.https://www.jstor.org/stable/30133120
• Gaede W, Reckling KF, Dresenkamp B, Kenklies S, Schubert E, Noack U, Irmscher HM,Ludwig C, Hotzel H, Sachse K (2008).Chlamydophila psittaci infections in humans during an outbreak of psittacosis from poultry in Germany. Zoonoses Public Health. 55: 184-188. https://doi.org/10.1111/j.1863-2378.2008.01108.x
• Geens T, Desplanques A, Van Loock M, Bönner BM, Kaleta EF, Magnino S, Andersen AA, Everett KDE,Vanrompay D (2005). Sequencing of the Chlamydophila psittaci OmpA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. J. Clin. Microbiol. 43(5): 2456- 2461. https://dx.doi.org/10.1128%2FJCM.43.5.2456-2461.2005
• Gerlach H (1999).Chlamydia. In: Avian medicine: Principles and Application. B.W. Ritchie, G.J. Harrison, and L.R. Harrison (eds.). HBD International Inc., Delray Beach, Florida. pp. 984-996.
• Ghorbanpoor M, Bakhtiari NM, Mayahi M, Moridveisi H (2015). Detection of Chlamydia psittacifrom pigeons by polymerase chain reaction in Ahvaz. Iran. J. Microbiol. 7: 18-22.https://www.ncbi.nlm.nih.gov/pubmed/26644869
• Gough SL, Carrick J, Raidal SL, Keane S, Collins N, Cudmore L, Russell CM, Raidal S, Hughes KJ (2019). Chlamydia psittaci infection as a cause of respiratory disease in neonatal foals. Equine Vet. J. https://doi.org/10.1111/evj.13170
• Greth A, Andral B, Gerbermann H, Vassant M, Gerlach H, Launary F (1993). Chlamydiosis in captive group of Houbara Bustards (Chlamydia undulata). Avian Dis. 34(4):1117-1120.
• Grimes JE, Tully TN, Arizmendi F, Phalen DN (1994). Elementary body agglutination for rapidly demonstrating chlamydial agglutinins in avian serum with emphasis on testing cockatiels. Avian Dis. 38(4): 822-831.https://doi.org/10.2307/1592120
• Guo W, Li J, Kaltenboeck B, Gong J, Fan W, Wang C (2016).Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Sci. Rep. 6: 19638. https://dx.doi.org/10.1038%2Fsrep19638
• Guzman DS, Diaz-Figueroa O, Tully TJ, Ciembor P, Morgan T, Walden M, Poston RP, Flammer K, Mitchell MA, Ritchie B (2010). Evaluating 21- day doxycycline and azithromycin treatments for experimental Chlamydophila psittaci infection in cockatiels. J. Avian Med. Surg. 24(1): 35-45.https://doi.org/10.1647/2009-009r.1
• Harkinezhad T, Geens T, Vanrompay D (2009). Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet. Microbiol.135:68-77.https://doi.org/10.1016/j.vetmic.2008.09.046
• Hedberg K, White KE, Forfang JC, Korlath JA, Friendshuh KA, Hedberg CW, MacDonald KL, Osterholm MT (1989). An outbreak of psittacosis in Minnesota turkey industry workers: implications for modes of transmission and control. Am. J. Epidemiol. 130(3): 569-577. http://doi.org/10.1093/oxfordjournals.aje.a115371
• Heddema ER, van Hannen EJ, Dium B, de Jongh BM, Kaan JA, van Kessel R, Lumeij JT, Visser CE, Vandenbroucke-Grauls CMJE (2006). An outbreak of psittacosis due to Chlamydophila psittaci genotype A in a veterinary teaching hospital. J. Med. Microbiol. 55(11): 1571-1575. https://doi.org/10.1099/jmm.0.46692-0
• Hegazy AM, El-Sisi MA, Hadia AA, Hala MNT (2014). Incidence of Chlamydophila psittaci in domestic birds in Sharkia governorate, Egypt. Zag. Vet. J. 42: 67-76.
• Hegazy AM, El-Sisi MA, Hassanin O, Tolba HMN, Baz HA (2017). Prevalence of Chlamydophila psittaci in some wild and pet birds. Zag. Vet. J. 45(3): 206-217.
• Hegazy AM, Tolba HMN, Abd EL-Samie LK, Abdelaziz AM, Ali AMA (2018). Effect of the medicinal plant (Azadirachta indica) on Chlamydophila psittaci infection in broiler chickens. Slov. Vet. Res. 55 (Suppl 20): 85-93. https://doi.org/10.26873/SVR-633-2018
• Henning K, Sachse K, Sting R (2000). Demonstration of Chlamydia from an equine abortion. Dtsch. Tierarztl. Wochenschr.107(2):49-52.
• Herrmann B, Persson H, Jensen JK, Joensen HD, Klint M, Olsen B (2006). Chlamydophila psittaci in Fulmars, the Faroe Islands. Emerg. Infect. Dis. 12: 330-332. https://dx.doi.org/10.3201%2Feid1202.050404
• Hewinson RG, Griffiths PC, Bevan BJ, Kirwan SES, Field ME, Woodward MJ, Dawson M (1997). Detection of Chlamydia psittaci DNA in avian clinical samples by polymerase chain reaction. Vet. Microbiol. 54(2): 155-166.https://doi.org/10.1016/S0378-1135(96)01268-0
• Hewinson RG, Rankin SES, Bevan BJ, Field M, Woodward MJ (1991). Detection of Chlamydia psittaci from avian field samples using the PCR. Vet. Rec. 199: 129-130.https://doi.org/10.1016/s0378-1135(96)01268-0
• Hinton DG, Shipley A, Galvin JW, Harkin JT, Brunton RA (1993). Chlamydiosis in workers at a duck farm and processing plant. Aust. Vet. J. 70(5): 174-176.https://doi.org/10.1111/j.1751-0813.1993.tb06123.x
• Hogerwerf L, Roof I, de Jong MJK, Dijkstra F, van der Hoek W (2020). Animal sources for zoonotic transmission of psittacosis: a systematic review. BMC Infect. Dis. 20(1): 192. https://dx.doi.org/10.1186%2Fs12879-020-4918-y
• Ho¨lzer M, Laroucau K, Creasy HH, Ott S, Vorimore F, Bavoil PM, Marz M, Sachse K (2016).Whole-genome sequence of Chlamydia gallinacea type strain 08-1274/3. Genome Announc. 4(4): e00708–e00716.https://dx.doi.org/10.1128%2FgenomeA.00708-16
• Holzinger-Umlauf HAM, Marschang RE, Gravendyck M, Kaleta EF (1997). Investigation on the frequency of Chlamydia sp. infections in tits (Paridae). Avian Pathol. 26: 779-789. https://doi.org/10.1080/03079459708419252
• Hughes C, Maharg P, Rosario P, Herrell M, Bratt D, Salgado J, D Howard (1997). Possible nosocomial transmission of psittacosis. Infect. Control Hosp. Epidemiol. 18(3):165-168.https://doi.org/10.2307/30141976
• Hulin V, Oger S, Vorimore F, Aaziz R, de Barbeyrac B, Berruchon J, Sachse K, Laroucau K (2015). Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathog. Dis. 73(1): 1-11.https://doi.org/10.1093/femspd/ftv005
• Huminer D, Samra Z, Weisman Y, Pitlik S (1988). Family outbreaks of psittacosis in Israel. Lancet 2(8611): 615-618.
• Ito I, Ishida T, Mishima M, Osawa M, Arita M, Hashimoto T, Kishimoto T (2002). Familial cases of psittacosis: possible person-toperson transmission. Intern. Med. 41(7): 58-583.https://doi.org/10.2169/internalmedicine.41.580
• Jawad Z, Younus M, Rehman MU, Munir R, Maqbool A, Shahzad W, Masood S, Muhammad K (2014). Effect of Azadirachta indica on the hepato-renal functions in broilers chickens. J. Anim. Plant Sci. 24: 1012-1018. http://www.thejaps.org.pk/docs/v-24-4/07.pdf
• Jelocnik M, Branley J, Heller J, Raidal, S, Alderson S, Galea F, Gabor M, Polkinghorne A (2017). Multilocus sequence typing identifies an avian-like Chlamydia psittaci strain involved in equine placentitis and associated with subsequent human psittacosis. Emerg. Microbes Infect. 6(2): e7. https://dx.doi.org/10.1038%2Femi.2016.135
• Jencek J, Beaufrère H, Tully T, Garner M, Dunker F, Baszler T (2012). An outbreak of Chlamydophila psittaci in an outdoor colony of Magellanic penguins (Spheniscus magellanicus). J. Avian Med. Surg.26(4):225-231. https://doi.org/10.1647/2010-046r1.1
• Jeong J, An I, Oem JK, Wang SJ, Kim Y, Shin JH, Woo C, Kim Y, Jo SD, Son K, Lee S, Jheong W (2017). Molecular prevalence and genotyping of Chlamydia spp. in wild birds from South Korea. J. Vet. Med. Sci. 79(7): 1204-1209.https://dx.doi.org/10.1292%2Fjvms.16-0516
• Joseph SJ, Marti H, Didelot X, Castillo-Ramirez S, Read TD, Dean D (2015). Chlamydiaceae genomics reveals interspecies admixture and the recent evolution of Chlamydia abortus infecting lower mammalian species and humans. Genome Biol. Evol. 7(11): 3070-84. https://dx.doi.org/10.1093%2Fgbe%2Fevv201
• Kaleta EF, Taday EM (2003). Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 32: 435-462. https://doi.org/10.1080/03079450310001593613
• Kaltenboeck B, Kousoulas KG, Storz J (1991). Detection and strain differentiation of Chlamydia psittaci mediated by a two-step polymerase chain reaction. J. Clin. Microbiol. 29: 1969-1975. https://www.ncbi.nlm.nih.gov/pubmed/1774323
• Kauffold J, Melzer F, Henning K, Schulze K, Leiding C, Sachse K (2006). Prevalence of Chlamydiae in boars and semen used for artificial insemination. Theriogenology. 65: 1750-1758.https://doi.org/10.1016/j.theriogenology.2005.10.010
• Kay, RS (1997). Psittacosis in Egypt: A case study. J. Travel. Med. 4: 48-49.
• Khodadadi M, Hemmatinezhad B, Doosti A, Khamesipour F, Awosile B (2015). Molecular detection and prevalence of Chlamydophila psittaci in the blood, liver and muscle tissue of urban pigeons (Columba livia domestica) in Iran. Kafkas Universitesi Veteriner Fakultesi Dergisi, 21: 265-269.
• Knittler M, Sachse K (2015).Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog. Dis. 73(1):1-15.https://doi.org/10.1093/femspd/ftu007
• Lagae S, Kalmar I, Laroucau K, Vorimore F, Vanrompay D (2014). Emerging Chlamydia psittaci infections in chickens and examination of transmission to humans. J. Med. Microbiol.63: 399-407. https://doi.org/10.1099/jmm.0.064675-0
• Laroucau K, de Barbeyrac B, Vorimore F, Clerc M, Bertin C, Harkinezhad T, Verminnen K, Obeniche F, Capek I, Bebear C, Durand B, Zanella G, Vanrompay D, Garin-Bastuji B, Sachse K (2009b). Chlamydial infections in duck farms associated with human cases of psittacosis in France. Vet. Microbiol. 135(1-2): 82-89. http://dx.doi.org/10.1016/j.vetmic.2008.09.048
• Laroucau K, Vorimore F, Aaziz R, Berndt A, Schubert E, Sachse K (2009a). Isolation of a new chlamydial agent from infected domestic poultry coincided with cases of atypical pneumonia among slaughterhouse workers in France. Infect. Genet. Evol. 9(6): 1240-1247. https://doi.org/10.1016/j.meegid.2009.08.005
• Laroucaua K, Vorimorea F, Aaziz R, Solmonsonb L, Hsiac RC, Bavoil PM, Fache P, Hölzer M, Wuenschmannb A, Sachse K (2019). Chlamydia buteonis, a new Chlamydia species isolated from a red-shouldered hawk. Syst. Appl. Microbiol. 42: 125997. https://doi.org/10.1016/j.syapm.2019.06.002
• Lent SV, Piet JR, Beeckman D, Arie van der E, Nieuwerburgh FV, Bavoil P, Myers G, Vanrompay D, Pannekoek Y (2012). Full genome sequences of all nine Chlamydia psittaci genotype reference strains. J. Bacteriol. 194: 6930-6931.https://dx.doi.org/10.1128%2FJB.01828-12
• Levinthal W (1930). Die A¨ tiologie der Psittakosis (article in German). Klin. Wochschr. 9:654.
• Li Z, Liu P, Hou J, Xu G, Zhang J, Lei Y, Lou Z, Liang L, Wen Y, Zhou J (2020). Detection of Chlamydia psittaci and Chlamydia ibidis in the endangered Crested Ibis (Nipponia nippon). Epidemiol. Infect. 148:e1. https://dx.doi.org/10.1017%2FS0950268819002231
• Li J, Guo W, Kaltenboeck B, Sachse K, Yang Y, Lu G, Zhang J, Luan L, You J, Huang K, Qiu H, Wang Y, Li M, Yang Z, Wang C (2016). Chlamydia pecorum is the endemic intestinal species in cattle while C. gallinacea, C. psittaci and C. pneumoniae associate with sporadic systemic infection. Vet. Microbiol. 193:93-99. https://doi.org/10.1016/j.vetmic.2016.08.008
• Lillie RD (1930). Psittacosis: Rickettsia-like inclusions in man and experimental animals. US. Pub. Health Repts. 45: 773-778.
• Lin W, Chen T, Liao L, Wang Z, Xiao J, Lu J, Song C, Qin J, Chen F, Chang Y-F, Xie Q (2019). A parrot‐type Chlamydia psittaci strain is in association with egg production drop in laying ducks. Transbound Emerg. Dis. 66: 2002-2010.https://doi.org/10.1111/tbed.13248
• Longbottom D, Coulter LJ (2003). Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128: 217-244.https://doi.org/10.1053/jcpa.2002.0629
• Loots K, Loock MV, Vanrompay D, Goddeeris BM (2006). CpG motifs as adjuvant in DNA vaccination against Chlamydophila psittaci in turkeys. Vaccine. 24: 4598-4601.https://doi.org/10.1016/j.vaccine.2005.08.042
• Madani A, Peighambari M (2013). PCR-based diagnosis, molecular characterization and detection of atypical strains of avian Chlamydia psittaci in companion and wild birds. Avian Pathol. 42(1): 38-44. https://doi.org/10.1080/03079457.2012.757288
• Madani A, Peighambari M, Barin A (2011). Isolation of Chlamydophila psittaci from pet birds in Iran. Iran. J. Vet. Med. 5(2): 95-98.https://dx.doi.org/10.22059/ijvm.2011.23104
• Magnino S, Haag-Wackernagel D, Geigenfeind I, Helmecke S, Dovc A, Prukner Radovcic, E, Residbegovic E, Ilieski V, Laroucau K, Donati M, Martinov S, Kaleta EF (2009). Chlamydial infections in feral pigeons in Europe: Review of data and focus on public health implications. Vet. Microbiol. 135(1-2): 54-67. https://doi.org/10.1016/j.vetmic.2008.09.045
• Mahmmod YS, Mweu MM, Abou Elez RMM, Tolba HMN, Elsohaby I (2018). Bayesian evaluation of the performance of three diagnostic tests for Chlamydophila psittaci in humans. J. Zoonotic Dis. 3(1): 22-32.
• Mahzonieh, M, Khoei HH, Shamsabad MG, Heidari F (2013). Prevalence of Chlamydia psittaci in pigeons in Chaharmahal va Bakhtiari and Yazd provinces of Iran, by nested-PCR, 2012. Iran. J. Med. Microbiol. 7(1): 1-6.
• Malkinson M, Machany S, Aronovici A, Davidov K, Weisman Y (1987). Mixed infection with Chlamydia psittaci, fowlpox virus and Haemophilus gallinarum in broiler breeder chicks. Vet. Rec. 120(19): 461-462.
• Matsui T, Nakashima K, Ohyama T, Kobayashi J, Arima Y, Kishimoto T, Ogawa M, Cai Y, Shiga S, Ando S, Kurane I, Tabara K, Itagaki A, Nitta N, Fukushi H, Matsumoto A, Okabe N (2008). An outbreak of psittacosis in a bird park in Japan. Epidemiol. Infect. 136(4): 492-495. https://dx.doi.org/10.1017%2FS0950268807008783
• McElnea CL, Cross GM (1999). Methods of detection of Chlamydia psittaci in domesticated and wild birds. Aust. Vet. J. 77(8): 516-521.https://doi.org/10.1111/j.1751-0813.1999.tb12123.x
• McGuigan CC, McIntyre PG, Templeton K (2012). Psittacosis outbreak in Tayside, Scotland, December 2011 to February 2012. Euro. Surveill. 17(22): pii¼20186. https://doi.org/10.2807/ese.17.22.20186-en
• Meijer A, Ossewaarde JM (2002). Description of a wider diversity within the order Chlamydiales than currently classified. International Chlamydia Conference, Antalya, Turkey, P: 16-21.
• Messmer T, McNulty MS, Ritchie BW, Moroney MJF (2000). A tale of discrimination: Differentiation of Chlamydiaceae by polymerase chain reaction. Semin. Avian Exot. Pet Med. 9(1): 36-42.https://doi.org/10.1016/S1055-937X(00)80014-6
• Meyer KF (1941). Phagocytosis and immunity in psittacosis. Schweizerische. Medizinische. Wochenschrift. 71: 436-438.
• Meyer KF, Eddie B (1933). Latent psittacosis infections in shell parakeets. Proc. Soc. Exptl. Biol. Med. 30: 484-488.
• Mina A, Fatemeh A, Jamshid R (2019). Detection of Chlamydia psittaci genotypes among birds in Northeast Iran. J. Avian Med. Surg. 33(1): 22-28. https://doi.org/10.1647/2017-334
• Mohan R (1984). Epidemiologic and laboratory observations of Chlamydia psittaci infection in pet birds. J. Am. Vet. Med. Assoc. 184: 1372-1374.
• Moroney JF, Guevara R, Iverson C, Chen FM, Skelton SK, Messmer TO, Plikaytis B, Williams PO, Blake P, Butler JC (1998). Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clin. Infect. Dis. 26: 1425-1429.https://doi.org/10.1086/516368
• Mostafa ER, Elhariri M, Ali HA, Jakee JKE (2015).Emergence of Chlamydia psittaci in lovebirds: A new potential risk factor of chlamydiosis. Int. J. Adv. Res. Biol. Sci. 2(11): 1-9.
• Mousa HAA (1984). Incidence and public health importance of ornithosis and psittacosis in imported and exported love birds. M.V.Sc. Thesis, Fac.Vet. Med., Cairo Univ., Egypt.
• Naveed A, Abdullah S, Naveed R, Naveed MA (2018). Chlamydia psittaci: An omitted pathogen at the human-animal interface. Ann. Virol. Res. 4(1): 1033.
• Newmann JA (1989). Chlamydia spp. infection in turkey flocks in Minnesota. J. Am. Vet. Med. Assoc. 195(11): 1528-1530.
• Newman CPSTJ, Palmer SR, Kirby FD, Caul EO (1992). A prolonged outbreak of ornithosis in duck processors. Epidemiol. Infect. 108: 203-210. https://dx.doi.org/10.1017%2Fs0950268800049657
• Ni X, Qin S, Lou Z, Ning H, Sun X (2015). Seroprevalence and risk factors of Chlamydia infection in domestic rabbits (Oryctolagus cuniculus) in China. Biomed. Res. Int. 2015: 460473. https://doi.org/10.1155/2015/460473
• Office International des Epizootiesc (2000). Manual of standards diagnostic tests and vaccines. Chapter 2.7.4. Avian Chlamydiosis.
• Olsen B, Persson K, Broholm KA (1998). PCR detection of Chlamydia psittaci in faecal samples from passerine birds in Sweden. Epidemiol. Infect. 121(2): 481- 484. https://dx.doi.org/10.1017%2Fs0950268898001320
• Ortega N, Apaza D, Gonzalez F, Salinas J, Caro MR (2011). Occurrence of Chlamydiaceae in non-symptomatic free-living raptors in Spain. Eur. J. Wildl. Res. 57: 122-128.https://doi.org/10.1007/s10344-011-0538-6
• Osman KM, Ali HA, ElJakee JA, Galal HM (2011). Chlamydophila psittaci and Chlamydophila pecorum infections in goats and sheep in Egypt. Rev. Sci.Tech. 30(3): 939-948.https://doi.org/10.20506/rst.30.3.2088
• Osman W A, El-Naggar AL, Gooda ASA, Mahmoud MA (2007). Detection of Chlamydophila psittaci in chickens by complement fixation test and polymerase chain reaction. J. Vet. Med. Res. 17: 35-38. https://dx.doi.org/10.21608/jvmr.2007.77891
• Overmars-Marx T (2019). Increasing awareness about psittacosis among bird owners. Eur. J. Public Health. (29)4.ckz186.503. https://doi.org/10.1093/eurpub/ckz186.503
• Pal M (2004). Chlamydiosis: An anthropozoonosis. Vet. World 3: 5-7.
• Pal M (2013). Public health concern due to emerging and reemerging zoonoses. Int. J. Livest Res. 3(1): 56-62.
• Pal M (2017). Chlamydophila Psittaci as an emerging zoonotic pathogen of global significance. Int. J. Vaccines Vaccin. 4(3): 80. https://doi.org/10.15406/ijvv.2017.04.00080
• Pal M, Dahiya SM (1985). Occurrence of chlamydial infection in parrots. Indian J. Anim. Res. 19: 69-72.
• Pannekoek Y, Dickx V, Beeckman DSA, Jolley KA, Keijzers WC, Vretou E, Maiden MCJ, Vanrompay D, Ende VDA (2010). Multi locus sequence typing of Chlamydia reveals an association between Chlamydia psittaci genotypes and host species. PloS One. 5: e14179.https://doi.org/10.1371/journal.pone.0014179
• Pearson JE, Gustafson GA, Senne DA and Peterson LA (1989). Isolation and identification of Chlamydia psittaci from pet birds. J. Am. Vet. Med. Assoc.195:1564-1567.
• Petrovay F, Balla E (2008). Two fatal cases of psittacosis caused by Chlamydophila psittaci. J. Med. Microbiol. 57: 1296-1298.https://doi.org/10.1099/jmm.0.2008/001578-0
• Piasecki T, Chrzastek K, Wieliczko A (2012). Detection and identification of Chlamydophila psittaci in asymptomatic parrots in Poland. BMC Vet. Res.8: 233-238.https://doi.org/10.1186/1746-6148-8-233
• Polkinghorne A, Greub G (2017). A new equine and zoonotic threat emerges from an old chlamydial pathogen, Chlamydia psittaci. Clin. Microbiol. Infect. 23(10):693-694.https://doi.org/10.1016/j.cmi.2017.05.025
• Radomski N, Einenkel R, Müller A, Knittler MR (2016). Chlamydia-host cell interaction not only from a bird’s eye view: some lessons from Chlamydia psittaci. FEBS Lett. 590(21): 3920-3940. https://doi.org/10.1002/1873-3468.12295
• Reinhold P, Sachse K, Kaltenboeck B (2011). Chlamydiaceae in cattle: commensals, trigger organisms, or pathogens? Vet. J. 189: 257-267. https://doi.org/10.1016/j.tvjl.2010.09.003
• Roberts JP, Grimes JE (1978). Chlamydia shedding by four species of wild birds. Avian Dis. 22(4): 698-706.https://www.jstor.org/stable/1589647
• Robertson T, Bibby S, O’Rourke D, Belfiore T, Agnew-Crumpton R, Noormohammadi AH (2010). Identification of Chlamydial species in crocodiles and chickens by PCR-HRM curve analysis. Vet. Microbiol. 145: 373-379.https://doi.org/10.1016/j.vetmic.2010.04.007
• Rodolakis A, Mohamad KY (2010). Zoonotic potential of Chlamydophila. Vet. Microbiol. 140(3-4): 382-391. https://doi.org/10.1016/j.vetmic.2009.03.014
• Rodolakis L, Laroucau K (2015). Chlamydiaceae and chlamydial infections in sheep or goats. Vet. Microbiol. 181: 107-118.https://doi.org/10.1016/j.vetmic.2015.07.010
• Ruppanner R, Behymer DE, DeLong WJ, Franti CE, Schulz T (1984). Enzyme immunoassay of Chlamydia in birds. Avian Dis. 28(3): 608-615.https://doi.org/10.2307/1590229
• Rzedzicki J, Tokarzewski S (2001). Birds as a potential source of human infection by Chlamydiae. Medycyna weterynaryjna. 57(7): 459-463.
• Sachse K, Hotzel H, Slickers P, Ellinger T, Ehricht R (2005). DNA microarray-based detection and identification of Chlamydia and Chlamydophila spp. Mol. Cell Probes. 19: 41-50. https://doi.org/10.1016/j.mcp.2004.09.005
• Sachse K, Kuehlewind S, Ruettger A, Schubert E, Rohde G (2012). More than classical Chlamydia psittaci in urban pigeons. Vet. Microbiol. 157(3-4): 476-480. https://doi.org/10.1016/j.vetmic.2012.01.002
• Sachse K, Laroucau K, Vanrompay D (2015). Avian Chlamydiosis. Curr. Clin. Microbiol. Rpt. 2(1): 10-21.https://doi.org/10.1007/s40588-014-0010-y
• Sachse K, Vretou E, Livingstone M, Borel N, Pospischil A, Longbottom D (2009). Recent developments in the laboratory diagnosis of chlamydial infections. Vet. Microbiol. 135(1-2):2-21. https://doi.org/10.1016/j.vetmic.2008.09.040
• Sachse K, Laroucau K, Riege K, Wehner S, Dilcher M, Creasy HH, Weidmann M, Myers G, Vorimore F, Vicari N, Magnino S, Liebler-Tenorio E, Ruettger A, Bavoil PM, Hufert FT, Rosselló-Móra R, Marz M (2014). Evidence for the existence of two new members of the family Chlamydiaceae and proposal of Chlamydia avium sp. nov. andChlamydia gallinacea sp. nov. Syst. Appl. Microbiol. 37(2): 79-88. https://doi.org/10.1016/j.syapm.2013.12.004
• Sachse K, Laroucau K, Vorimore F, Magnino S, Feige J, Muller W, Kube S, Hotzel H, Schubert E, Slickers P, Ehricht R (2009). DNA microarray-based genotyping of Chlamydophila psittaci strains from culture and clinical samples. Vet. Microbiol. 135(1-2): 22-30. https://doi.org/10.1016/j.vetmic.2008.09.041
• Sadowski JM, Minta Z (1979). Chlamydiosis of the air sacs in geese. Bull. Vet. Inst. Pulawy. 23(3-4): 111-115.
• Sara AB, Röring RE, Heijne M (2018). Chlamydia psittaci and C. avium in feral pigeon (Columba livia domestica) droppings in two cities in the Netherlands. Vet. Q. 38(1): 63-66.https://dx.doi.org/10.1080%2F01652176.2018.1482028
• Schlossberg D (2015). Psittacosis (due to Chlamydia psittaci). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2171-2173.
• Sclossberg D, Delgado J, Moore M, Wishner A, Mohn J (1993). An epidemic of avian and human psittacosis. Arch. Intern. Med. 153(22): 2594-2595.https://doi.org/10.1001/archinte.1993.00410220106012
• Siraj I, Rahman SU, Ahsan Naveed Anjum Fr, Hassan S, Zahid Ali Tahir (2018). Prevalence of Chlamydia Psittaci in domesticated and fancy birds in different regions of district Faisalabad, Pakistan. United J. Microbiol. Infec. Dis. 1(2): 1-5.
• Smith KA, Bradley KK, Stobierski MG, Tengelsen LA (2005). Compendium of measures to control Chlamydophila psittaci (formerly Chlamydia psittaci) infection among humans (psittacosis) and pet birds, 2005. J. Am. Vet. Med. Assoc. 226: 532-539.https://doi.org/10.2460/javma.2005.226.532
• Smith KA, Campbell CT, Murphy J, Stobierski MG, Tengelsen LA (2011). Compendium of measures to control Chlamydophila psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2010. J. Exot. Pet Med. 20(1): 32-45.
• Spoorenberg SM, Bos WJ, van Hannen EJ, Dijkstra F, Heddema ER, van Velzen-Blad H, Heijligenberg R, Grutters JC, de Jongh BM (2016).Chlamydia psittaci: a relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth. J. Med. 74(2): 75-81. http://hdl.handle.net/10029/621726
• Sprague LD, Schubert E, Hotzel H, Scharf S, Sachse K (2009). The detection of Chlamydophila psittaci genotype C infection in dogs. Vet. J. 181(3): 274-279. https://doi.org/10.1016/j.tvjl.2008.04.002
• Szeredi L, Hotzel H, Sachse K (2005). High prevalence of chlamydial (Chlamydophila psittaci) infection in fetal membranes of aborted equine fetuses. Vet. Res. Commun. 29(suppl 1): 37-49. https://doi.org/10.1007/s11259-005-0835-1
• Szyman´ska-Czerwin´ska M, Niemczuk K (2016). Avian chlamydiosis zoonotic disease. Vector Borne Zoonotic Dis. 16(1):1-3.https://doi.org/10.1089/vbz.2015.1839
• Szyman´ska-Czerwin´ska M, Mitura A, Niemczuk K, ZarębaK, Jodełko A, Pluta A, Scharf S, Vitek B, Aaziz R, Vorimore F, Laroucau K, Schnee C (2017). Dissemination and genetic diversity of chlamydial agents in Polish wildfowl: isolation and molecular characterisation of avian Chlamydia abortus strains. PLoS One. 12(3): e0174599.https://doi.org/10.1371/journal.pone.0174599
• Tan T, Alan JH, Ian EA, Gareth EJ (1990). Protection of sheep against Chlamydia psittaci infection with a subcellular vaccine containing the major outer membrane protein. Infect. Immun. 58(9): 3101-3108. https://www.ncbi.nlm.nih.gov/pubmed/2387636
• Taylor-Brown A, Vaughan L, Greub G, Timms P, Polkinghorne A (2015). Twenty years of research into Chlamydia-like organisms: a revolution in our understanding of the biology and pathogenicity of members of the phylum Chlamydiae. Pathog. Dis. 73: 1-15. https://doi.org/10.1093/femspd/ftu009
• Telfer BL, Moberley SA, Hort KP, Branley JM, Dwyer DE, Muscatello DJ, Correll PK, England J, McAnulty JM (2005). Probable psittacosis outbreak linked to wild birds. Emerg. Infect. Dis.11: 391-397. https://dx.doi.org/10.3201%2Feid1103.040601
• Theegarten D, Sachse K, Mentrup B, Fey K, Hotzel H, Anhenn O (2008). Chlamydophila spp. infection in horses with recurrent airway obstruction: similarities to human chronic obstructive disease. Respir. Res.9: 14. https://doi.org/10.1186/1465-9921-9-14
• Trávnicek M, Cisláková L, Deptuła W, Stosik M, Bhide MR (2002). Wild pigeons and pheasants - a source of Chlamydophila psittaci for humans and animals. Ann. Agric. Environ. Med. 9(2): 253-255.
• Trevejo RT, Chomel BB, Kass PH (1999). Evaluation of the polymerase chain reaction in comparison with other diagnostic methods for the detection of Chlamydia psittaci. J. Vet. Diagn. Invest. 11(6): 491-496.https://doi.org/10.1177/104063879901100602
• Vanka A, Tandon S, Rao SR, Udupa N, Ramkumar P (2001).The effect of indigenous Neem Azadirachta indica (correction of Adirachta indica) mouth wash on Streptococcus mutans and lactobacilli growth. Indian J. Dent. Res. 12:133-144.https://doi.org/11808064
• Van Loock M, Verminnen K, Messmer TO, Volckaert G, Goddeeris BM, Vanrompay D (2005). Use of a nested PCR-enzyme immunoassay with an internal control to detect Chlamydophila psittaci in turkeys. BMC Infect. Dis.5:76.https://doi.org/10.1186/1471-2334-5-76
• Vanrompay D (2000). Avian Chlamydial Diagnostics. In: Laboratory Medicine: Avian and Exotic Pets. Ed A.M. Fudge. Philadelphia, Saunders, pp. 99-110.
• Vanrompay D, Butaye P, Sayada C, Ducatelle R, Haesebrouck F (1997a). Characterization of avian Chlamydia psittaci strains using omp1 restriction mapping and serovar-specific monoclonal antibodies. Res. Microbiol. 148(4): 327-333.https://doi.org/10.1016/s0923-2508(97)81588-4
• Vanrompay D, Butaye P, Van Nerom A, Ducatelle R, Haesebrouck F (1997b). The prevalence of Chlamydia psittaci infections in Belgian commercial turkey poults. Vet. Microbiol. 54(1): 85-93. https://doi.org/10.1016/s0378-1135(96)01224-2
• Vanrompay D, Cox E, Vandenbussche G, Goddeeris B (1999a). Protection of turkeys against Chlamydia psittaci challenge by gene gun-base DNA immunizations. Vaccine. 17(20-21): 2628-2635. https://doi.org/10.1016/s0264-410x(99)00053-5
• Vanrompay D, Cox E, Volckaert G, Goddeeris B (1999b). Turkeys are protected from infection with Chlamydia psittaci by plasmid DNA vaccination against the major outer membrane protein. Clin. Exp. Immunol. 118(1): 49-55.https://dx.doi.org/10.1046%2Fj.1365-2249.1999.01024.x
• Vanrompay D, Cox E, Kaiser P, Lawson S, Van Loock M, Volckaert G, Goddeeris B (2001). Protection of turkeys against Chlamydophila psittaci challenge by parenteral and mucosal inoculations and the effect of turkey interferon-gamma on genetic immunization. Immunology. 103: 106-112. https://dx.doi.org/10.1046%2Fj.1365-2567.2001.01215.x
• Vanrompay D, Ducatelle R, Haesebrouck F (1992). Diagnosis of avianchlamydiosis: specificity of the modified Gimenez staining on smears and comparison of the sensitivity of isolation in eggs and three different cell cultures. Zentralbl. Veterinarmed. B. 39(2): 105-112. https://doi.org/10.1111/j.1439-0450.1992.tb01144.x
• Vanrompay D, Ducatella R, Haesebrouck F (1995a). Chlamydia psittaci infections: a review with emphasis on avian chlamydiosis. Vet. Microbiol. 45(2-3): 93-119. https://doi.org/10.1016/0378-1135(95)00033-7
• Vanrompay D, Harkinezhad T, van de Walle M, Beeckman D, van Droogenbroeck C, Verminnen K, Leten R, Martel A, Cauwerts K (2007). Chlamydophila psittaci transmission from pet birds to humans. Emerg. Infect. Dis. 13: 1108-1110.https://dx.doi.org/10.3201%2Feid1307.070074
• Vanrompay D, Mast J, Ducatelle R, Haesebrouck F, Goddeeris B (1995b). Chlamydia psittaci in turkeys: pathogenesis of infections in avian serovars A, B and D. Vet. Microbiol. 47: 245-256. https://doi.org/10.1016/0378-1135(95)00125-5
• Verminnen K, Van Loock M, Hafez HM, Ducatelle R, Haesebrouck F, Vanrompay D (2006). Evaluation of a recombinant enzyme-linked immunosorbent assay for detecting Chlamydophila psittaci antibodies in turkey sera. Vet. Res.37: 623-632. https://doi.org/10.1051/vetres:2006023
• Wallensten A, Fredlund H, Runehagen A (2014). Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January–February 2013. Euro. Surveill. 19(42). pii: 20937. https://doi.org/10.2807/1560-7917.ES2014.19.42.20937
• Wang X, Zhang NZ, Ma CF, Zhang XX, Zhao Q, Ni HB (2018). Epidemiological investigation and genotype of Chlamydia exposure in pigeons in three provinces in Northern China. Vector Borne Zoonotic Dis. 18(3): 181-184. https://doi.org/10.1089/vbz.2017.2214
• West A (2011). A brief review of Chlamydophila psittaci in birds and humans. J. Exot. Pet Med. 20(1): 18-20. https://doi.org/10.1053/j.jepm.2010.11.006
• Williams J, Tallis G, Dalton C, Ng S, Beaton S, Catton M, Elliott J, Carnie, J (1998). Community outbreak of psittacosis in a rural Australian town. Lancet. 351: 1697-1699. https://doi.org/10.1016/S0140-6736(97)10444-5
• Yang J, Yang Q, Yang J, He C (2007). Prevalence of avian Chlamydophila psittaci in China. Bull. Vet. Inst. Pulawy. 51: 347-350.
• Yin L, Kalmar I, Lagae S, Vandendriessche S, Vanderhaeghen W, Butaye P, Cox E, Vanrompay D (2013). Emerging Chlamydia psittaci infections in the chicken industry and pathology of Chlamydia psittaci genotype B and D strains in specific pathogen free chickens. Vet. Microbiol. 162: 740-749. https://doi.org/10.1016/j.vetmic.2012.09.026
• Zhang F, Li S, Yang J, Pang W, Yang L, He C (2008). Isolation and characterization of Chlamydophila psittaci isolated from laying hens with cystic oviducts. Avian Dis. 52: 74-78.https://doi.org/10.1637/8019-051207-Reg
• Zhang NZ, Zhang XX, Zhou DH, Huang SY, Tian WP, Yang YC, Zhao Q, Zhu XQ (2015). Seroprevalance and genotype of Chlamydia in pet parrot in China. Epidemiol. Infect. 143(1): 55-61.https://doi.org/10.1017/s0950268814000363
• Zhou J, ChangQing Q, GuoZhen L, Xiaoan C, FuYing Z, XiaoWei G, GuangHua W (2010). Isolation of Chlamydophila psittaci from laying hens in China. Vet. Res. 3: 43-45.http://dx.doi.org/10.3923/vr.2010.43.45
• Zocevic A, Vorimore F, Marhold C, Horvatek D, Wang D, Slavec B, Prentza Z, Stavianis G, Prukner-Radovcic E, Dovc A, Siarkou VI, Laroucau K (2012). Molecular characterization of atypical Chlamydia and evidence of their dissemination in different European and Asian chicken flocks by specific real-time PCR. Environ. Microbiol. 14(8): 2212-2222.https://doi.org/10.1111/j.1462-2920.2012.02800.x
• Zocevic A, Vorimore F, Vicari N, Gasparini J, Jacquin L, Sachse K, Magnino S, Laroucau K (2013). A real-time PCR assay for the detection of atypical strains of Chlamydiaceae from pigeons. PLoS One 8(3): e58741. https://doi.org/10.1371/journal.pone.0058741