Advances in Animal and Veterinary Sciences
Research Article
The Preventive Effects of Cynara scolymus Leaf and Flower Extracts on Diethylnitrosamine/ Acetylaminoflourene Induced Nephrotoxicity in Male Wistar Rats
Lamiaa Nabil Bakry1, Abeer Mohamed Abd El-Hameed2, Sanaa Mahmoud Abd El-Twab1, Osama Mohamed Ahmed1, Adel Abel-Moneim1*
1Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Egypt; 2Chemistry Department, Faculty of Science, Taibah University, Saudi Arabia.
Abstract | The present investigation aims to estimate the preventive actions of Cynara scolymus (artichoke) extracts on diethylnitrosamine (DEN)/acetylaminofluorene (2AAF)-induced nephrotoxicity in Wistar male rats. Male rats were divided into 4 groups. The 1st group was kept as normal control, while the other 3 groups have been intraperitoneally injected with 150 mg DEN/kg body-weight (b.w.) twice for two weeks, followed by oral administration of 2AAF at 20 mg/kg b.w. four days/week for three weeks. One of the DEN/2AAF-administered groups was kept as a positive control and the two groups had been orally given artichoke leaf (ALE) and flower extracts (AFE) at a dose of 100 mg/kg b.w. Notably, DEN/2AAF induced kidney dysfunction was confirmed by histopathological alterations and elevations in the levels of serum urea, creatinine, and uric acid as well as renal lipid peroxidation. However, renal glutathione-peroxidase, superoxide-dismutase, reductase and glutathione-S-transferase activities and glutathione content were markedly decreased in DEN/2AAF-administered rats. Additionally, renal Bcl-2 and p53 mRNA gene-expressions were down-regulated. Treatment of DEN/2AAF-administered rats with ALE and AFE amended kidney histological perturbations, alleviate kidney function, oxidative stress biomarkers and up-regulated Bcl-2 and p53 mRNA gene-expressions. Thus, ALE and AFE successfully repressed DEN/2AAF-induced nephrotoxicity and nephocarcinogenicity through activation of the antioxidant defense system, and anti-apoptotic action mediated by Bcl-2 as well as reduction of the oxidative stress biomarkers.
Keywords | Nephrotoxicity, Diethylnitrosamine, Acetylaminofluorene, Cynara scolymus, Oxidative stress, Apoptotic actions
Received | July 21, 2020; Accepted | September 16, 2020; Published | September 20, 2020
*Correspondence | Adel Abdel-Moneim, Molecular Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Egypt. Salah Salem St., 62511, Beni-Suef, Egypt; Abeer Mohamed Abd El-Hameed, Chemistry Department, Faculty of Science, Taibah University, Saudi Arabia; Email: [email protected]; [email protected]; [email protected].
Citation | Bakry LN, El-Hameed AMA, El-Twab SMA, Ahmed OM, Abel-Moneim A (2020). The preventive effects of Cynara scolymus leaf and flower extracts on diethylnitrosamine/ acetylaminoflourene induced nephrotoxicity in male wistar rats. Adv. Anim. Vet. Sci. 8(s2): 74-81.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s2.74.81
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Bakry et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The kidney may be especially susceptible to toxic injury due to its high blood flow and/or its physiological and anatomical intricate nature (Schrier et al., 2004). Nephron toxicity may result from direct cytotoxic injury by environmental toxicants to the renal structures, from immunological processes, from indirect toxicity due to changes in kidney hemodynamics, or from the development of endogenous nephrotoxic compounds (Arany and Safirstein, 2003). The kidney tubules also were toxicity targets due partly to transporter expression that modulates the release and reabsorption of xenobiotics (George et al., 2017). Aminoglycosides, cephalosporin, cisplatin, amphotericin B and analgesics are common nephrotoxic xenobiotics that exercising its toxic effects through one or more common pathogenic pathways (Naughton, 2008).
N-nitroso compounds (NOCs) were some of the main classes of carcinogens that are commonly found in the human environment and the food chain. Diethylnitrosamine (DEN) is an established powerful carcinogenic component. It is generated mostly through the metabolism of certain drugs (Verna et al., 1996) and exists in a large variety of foods, tobacco smoke and agricultural chemicals (El-Shahat et al., 2012). Moderate quantities of reactive oxygen species (ROS) were mainly formed by mitochondria and NAD (PH) oxidase under usual physiological circumstances. When ROS production exceeds the capacity of the cell’s endogenous antioxidant systems oxidative stress is produced. The excess production of ROS and/or depletion in the endogenous antioxidants represent a major contribution of DEN to induce renal damage (Mahmoud et al., 2015). DEN was reported to induce the generation of free radicals leading to oxidative stress and cell injury through its metabolized end product which plays a critical role in the pathophysiology of renal dysfunction (Abdel-Moneim et al., 2016). Hence, attention was focused on antioxidants in order to combat DEN- induced renal damage.
Artichoke, Cynara scolymus (C. scolymus), belonging to Apiaceae, is widely cultivated in Mediterranean countries and plays a vital role in human nutrition (Lattanzio et al., 2009). Artichoke significantly contributes to the Mediterranean agricultural economy, where over 60 % of the total global income is produced. Several previous studies have shown antioxidant, anticarcinogenic, hypolipidemic, antihyperglycemic, hepatoprotective activities of artichoke (Heidarian and Rafieian-Kopaei, 2013; Magielse et al., 2014). Although the most essential active ingredient in C. Scolymus, cynarin, occurs across the whole plant, the main levels are derived from the leaves. For such reason, leaves create a great majority of the natural medicines procured from this plant. Cynarin is present in the whole plant and is also known as one of the major active chemical compounds. Cynarin is technically a caffeo-lquinic acid and concentrates mainly on the leaves (Speroni et al., 2003).
With this background, medicinal plants possessing antioxidant activity are among the potential targets of research for their promising merit to be used in alleviating DEN-induced toxicity. Thus, the present investigation aims to determine the preventive activities of C. scolymus against DEN/2AAF-induced nephrotoxicity in male rats.
MATERIALS AND METHODS
Experimental animal and chemicals
Forty adult male albino rats of Wistar strain, weighing 100-120 g, have been purchased from Helwan Station for Experimental Animals, Helwan, Cairo, Egypt. They were housed in well-aerated stainless steel cages at normal temperature (20-25οC) and normal daily lighting cycle (12 hr dark and light cycle) and received standard diet and water ad libitum. Animal protocol was performed according to the guidelines of the Experimental Animal Ethics Committee of Faculty of Science, Beni-Suef University, Egypt (Ethical Approval Number: BSU/FS/2016/17). Diethylnitrosamine (DEN) and 2-acetylaminoflourene (2AAF) were obtained from Sigma Chemicals Co., St. Louis, MO, USA. All used chemicals in the study are of analytical grade.
Plant material and extraction preparation
C. scolymus was purchased from outlets selling products the Ministry of Agriculture, El Dokki, Giza, Egypt, and was authenticated by Botany Department, Faculty of Science, Beni-Suef University, Egypt. The artichoke (leaf and flower) were separately air-dried in a well-aerated shade area, crushed and extracted two times with 70% ethanol (1:2 w/v) at normal temperature (20-25 οC) for two days. The hydroethanolic extracts were then filtered, the solvent was removed under vacuum, using a rotary evaporator. The obtained extracts were Keeps at 4°C until used.
Experimental design
Animals were divided to four groups (n=10rats/group), designed as follow: The 1st group is being considered as normal control, whilst another group had been injected intraperitoneally with 150 mg DEN/kg body weight (b.w.) twice for two weeks, followed by orally ingested of 2AAF at 20mg/kg b.w. four days/week for three weeks. One of three groups administered by DEN/2AAF was retained as control, while the two others were orally given artichoke leaf extract (ALE) and flower extract (AFE) at a dose of 100 mg/kg b.w. (Michel and Remscheid, 2002), for 17 weeks.
The animals were sacrificed under slight anesthesia by the end of the 17th week. Blood samples were taken out of the jugular vein, coagulated, then centrifuged and the sera were kept at -20o C. Samples of the kidneys have been excised quickly and classified into three portions. One portion has been fixed for 48 hours in neutral formalin buffered and then transported to 70 % alcohol for histological investigation. The 2nd portion was utilized for qRT-PCR analysis. Otherwise, the 3rd one has homogenized in 10 ml sterilized saline solution (0.9% NaCl) to obtain 1% homogenate (w/v). Homogenates were centrifuged and supernatants were stored at -20oC for oxidative stress and antioxidant parameter estimations.
Biochemical investigations
Serum urea, creatinine and uric acid levels were performed by reagent kits from Biosystems (Spain). A supernatant sample was used for lipid peroxidation estimation (LPO) (Yagi, 1987) by use of prepared chemical reagents. Reduced glutathione (GSH) content (Beutler and Kelly, 1963) and activities of renal antioxidant enzymes including glutathione peroxidase (GPx) (Matkovics et al., 1998), superoxide dismutase (SOD) (Marklund and Marklund, 1974), and glutathione reductase (GR) (Goldberg and Spooner, 1983) were also estimated using prepared chemical reagents.
RNA isolation and quantitative qRT-PCR
Quantitative reverse reaction of transcriptase-polymerase chain (qRT-PCR) has been used to determine the impact of DEN/2AAF on kidney mRNA abundance of p53 and Bcl-2. Complementary DNAs (cDNAs) were produced from 2 μg RNA and processed via SYBR Green Master Mix (Thermo Fisher Scientific, USA) with Table 1. In addition, the 2-∆∆Ct method (Livak and Schmittgen, 2001) was then used to investigate the amplification results. The variables were standardized to β-actin and identified as % of control.
Histopathological study
Kidney tissues from each group were cut into smaller parts and fixed for 24 hours in neutral buffered formalin. The specimen was handled, sectioned with a microtome at a thickness of 4-5 μm and then stained with hematoxylin and Eosin (H and E) stain (Banchroft et al., 1996).
Statistical analysis
Results were analyzed and presented as mean ± standard error. Data were interpreted using statistical software IBM SPSS Statistics 20 (IBM Corporation, NY, USA). The data were analyzed using One-way analysis of variance (ANOVA). Values with P<0.05 were considered statistically significant.
RESULTS and Discussion
Effect on serum creatinine, urea and uric acid concentrations
The rats-administered DEN/2AAF revealed a noticeable (P˂0.05) increase in serum urea, creatinine and uric acid concentrations relative to the normal control rats. The administration of ALE and AFE to rats-administered DEN/2AAF induced a marked (P˂0.05) amelioration in serum creatinine, urea and uric acid urea as compared to DEN/2AAF-administered control group (Table 2).
Table 1: Primer pairs used for qPCR.
| Gene | Gen Bank accession number |
Sequence 5 ́-3 ́ |
| p53 | NM_022112 |
F: 5′ GCTGCCCTCCCTTCTCCTAG3' R: 5′ CCCCGACTTTGGAGTAGTCTGA3' |
| Bcl2 | NM009741 |
F: 5′ TAAGCTGTCACAGAGGGGCT3' R: 5′ TGAAGAGTTCCTCCACCACC3' |
|
β -actin |
NM _007393 |
F: 5'TCACTATCGGCAATGTGCGG-3′ R: 5' GCTCAGGAGGAGCAATGATG-3′ |
Table 2: Effect of C. scolymus leaf and flower hydroethanolic extract on serum creatinine, urea and uric acid levels in DEN/2AAF-administered rats.
| Groups | Uric acid (mg/dl) | Urea (mg/dl) | Creatinine (mg/L) | |
| Normal control |
2.533±0.056a |
32.0±1.571a |
0.622±0.028a |
|
| DEN/2AAF |
3.60±0.086c |
51.333±1.856b |
0.778±0.011b |
|
| leaf extract |
DEN/2AAF +C. scolymus |
2.783±0.114ab |
33.167±1.014a |
0.628±0.014a |
| flower extract |
2.818±0.098b |
34.667±1.687a |
0.617±0.009a |
|
Data are expressed as mean ± standard error. Number of animals in each group is six. For each parameter, means which have the same superscript symbol (s), are not significantly different. Percentage changes were calculated by comparing DEN/2AAF-administered control group with normal control group and comparing treated DEN/2AAF-administered groups with DEN/2AAF-administered control.
Table 3: Effect of C. scolymus leaf and flower extract on kidney LPO, GSH content and GPx, GST, GR and SOD activities in DEN/2AAF-administered rats.
| Groups | SOD (U/mg protein) | GR (U/mg protein) | GST (U/mg protein) | GPx (U/mg protein) | GSH (mmol/mg protein) | LPO (nmol/mg protein/hr) | |
| Normal control |
2.98±0.289c |
66.75±4.315b |
137.60±8.264b |
84.417±5.997c |
52.617±2.461c |
3.187±0.50a |
|
| DEN/2AAF |
0.983±0.093a |
27.55±3.465a |
76.54±6.993a |
30.62±1.995a |
22.133±1.456a |
33.050±2.848c |
|
| leaf extract |
DEN/2AAF+ C. scolymus |
2.205±0.105b |
57.020±5.502b |
132.967±5.555b |
61.45±5.506b |
40.22±2.271b |
13.143±1.797b |
| flower extract |
2.14±0.256b |
60.617±5.584b |
124.617±6.4b |
62.1±5.669 b |
38.8±4.747b |
13.31±2.338b |
|
Effect on kidney antioxidant defence enzymes and oxidative stress biomarkers
DEN/2AAF administration to normal rats has recorded a significant (P<0.05) elevation in kidney LPO products as well as a significant (P<0.05) decrease in reduced GSH level (Table 3). Treatment of rats administered DEN/2AAF with both tested hydroethanolic extracts prevented this elevation in LPO to a great extent (P<0.05). In contrast, the treatment of DEN/2AAF-administered rats with ALE and AFE markedly (P<0.05) elevates GSH content compared to DEN/2AAF-administered control rats.
The administration of DEN/2AAF induced a marked (P<0.05) decline in GPx, GST, GR and SOD activities when compared to the normal rats. Oral gavage of ALE and AFE to DEN/2AAF-administered animals improved significantly (P<0.05) the activities of GPx, GST, GR and SOD (Table 3).
Effect on P53 and BCL2 mRNA gene expressions
DEN/2AAF-administration caused a significant (P˂0.05) and a non-significant (P>0.05) down-regulation of renal Bcl-2 and p53 mRNA gene expression level, respectively relative to the normal control rats (Figure 1). Also, the administration of ALE and AFE revealed a noticeable up-regulation of Bcl-2 mRNA expressions (Figure 1B). In addition, both extracts induced a non-significant change of p53 mRNA expressions (Figure 1A).
Histological examination of the kidney
Histological analysis of kidney samples of normal control animals revealed the normal histological architecture of cortex and medulla. The glomerulus surrounded with Bowman’s capsule forming the Malpigian corpuscle, both proximal and distal tubules are seen (Figure 2A, B). Proximal tubules have cuboidal epithelial cells and brush border and distal tubules with thinner epithelial cells and no brush border. Also, normal renal tubules appear in the corticomedullary junction. On the other hand, administration of DEN/2AAF causes histological changes including congestion in the intertubular blood vessels (Figure 2C), hyperplastic proliferation of the glomerulus and congested renal artery in the cortical region (Figure 2D).
The administration of ALE and AFE to DEN/2AAF-administered groups exhibit noticeable amelioration in kidney histological architecture and integrity. Very mild congestion in the intertubular blood vessels, renal corpuscles as a result of the treatment with ALE (Figure 2E, F) and disappearance of congestion observed result of treatment with AFE (Figure 2G, H).
The data of the current investigation showed that the administration of DEN/2AAF has caused kidney dysfunction confirmed by elevated levels of the kidney function biomarkers creatinine, urea and uric acid as previously recorded (Ahmed et al., 2015; 2016; Abdel-Moneim et al., 2016). Creatinine is produced from the breakdown of creatine phosphate in the muscle tissue and is commonly released by the body at a relatively constant level, based on the muscle mass (Zuo et al., 2008). Serum creatinine level is the most commonly used indirect indicator of glomerular filtration rate (Dickey et al., 2008). Thus, its abnormal elevation is a marker of kidney failure (Nenad et al., 2008). The higher concentration of blood urea is proportional directly to the severity of kidney injury (Dickey et al., 2008). Uric acid is generated from the oxidation of xanthine and hypoxanthine by the enzyme xanthine oxidoreductase (Wu and Wu, 2008). Additionally, serum uric acid has been identified as a risk factor for new onset of renal disease (Kanda et al., 2015).
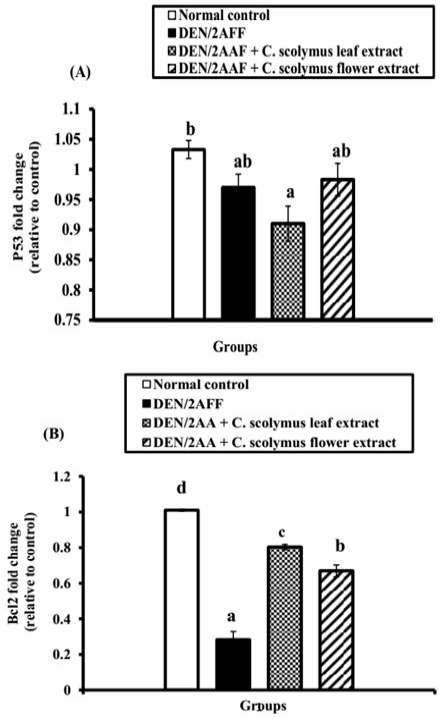
Figure 1: Effect of C. scolymus leaf and flower hydroethanolic extracts on kidney (A) p53 and (B) Bcl-2 mRNA gene expressions.
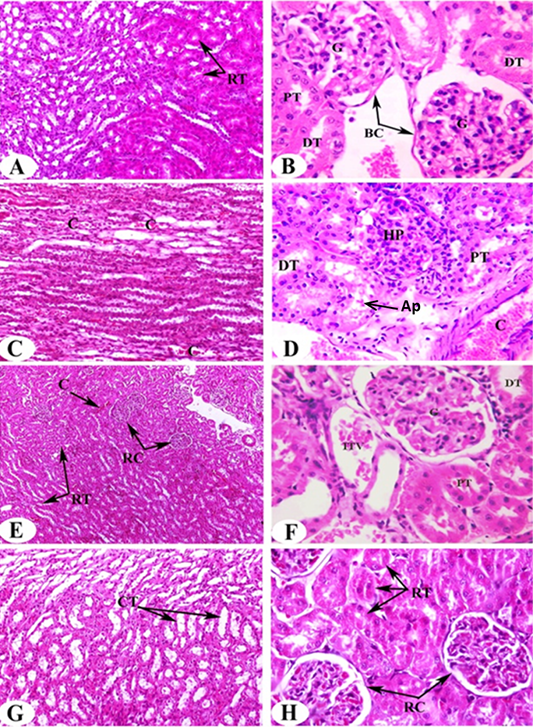
Figure 2: Photomicrographs of H and E stained kidney sections of rats in the experimental groups. Photomicrographs (A, B) clearly shows typical glomerulous histological structure (G), proximal (PT) and distal tubules (DT) in normal rat. Photomicrographs of kidney sections of DEN/2AAF show congestion (C) in the intertubular blood vessels of renal medulla (Photomicrograph C), hyperplastic proliferation of the glomerulus (HP), apoptotic cells (Ap) and congested renal artery (C) in the cortical region (Photomicrograph D). Photomicrograph of kidney section of DEN/2AAF-administered rat treated with C. scolymus leaf extract showing nearly normal renal tubules (RT) (Photomicrograph E), glomerulus (G), proximal (PT), distal tubule (DT) and inter-tubular vein (ITV) (Photomicrograph F). Photomicrograph of kidney section of DEN/2AAF-administered rat treated with C. scolymus flower extract showing nearly normal collecting tubules (CT) (Photomicrograph G) (X 200), renal tubules (RT) and corpuscles (RC) (Photomicrograph H) (X 400).
However, the DEN/2AAF rats treated with ALE and AFE clearly exhibited a significantly decrease in the concentration of urea, creatinine and uric acid suggesting Reno protective effect of the two extracts. This finding was parallel with the results of the previous study (Isoda et al., 2014).
Increased ROS and decreased cellular antioxidants are signs of DEN/2AAF-induced renal damage (Rehman et al., 2013). The kidneys are susceptible to ROS injury caused by the accumulation of long-chain polyunsaturated fatty acids present in renal lipid composition (Ozbek, 2012). Also, ROS react with different tissue molecules resulting in renal, liver and other tissue dysfunction and damage (Shaban et al., 2014). In the current study, DEN/2AAF-administration induces a significant elevation in renal LPO. This is due to DEN is degraded and connect with renal tubular epithelium and is modified into active electrophilic species following α or β-hydroxylation (Marnett, 2002), producing unstable hydroxyalkyl compounds that are subsequently transformed to alkyl carbonium ions that linked to DNA leading to the formation of adducts and initiate superoxide radicals via LPO of phospholipid membrane fatty acids (Pracheta et al., 2011). Peroxidation of the cellular and the mitochondrial membrane resulted in a loss of cell integrity, an increase in membrane permeability and alternative homeostasis of Ca+2 that leads to cell death caused by changes in the internal membrane potential (Shaban et al., 2014). The kidney damage, however, is a result of adverse impact of DEN itself and/or its metabolites, including ethylcarbonium ions, NO and ROS (Bishayee et al., 2010). The current data are in harmony with previous studies of Zhang et al. (2012), Hamouda et al. (2015), Abdel-Moneim et al. (2016) and Ahmed et al. (2019) who reported that MDA level was elevated significantly due to DEN administration in rats compared to normal control.
Regarding increased ROS and MDA, GSH concentration and GPx, GST, GR, and SOD activities were declined in DEN/2AAF-administered rats. Such antioxidants provide protection from renal dysfunction caused by ROS activities. Thus, inhibiting ROS production and enhancing defenses against cellular antioxidants will reduce DEN/2AAF-induced renal dysfunction. The antioxidant defense mechanism works via enzymatic (SOD, GPx, GST and GR) and non-enzymatic constituents (Chen et al., 2012). SOD, the 1st line of enzymatic antioxidant defense, is contributed to the removal of superoxide radical toward less toxic hydrogen peroxide and molecular oxygen. In addition, GPx provides higher activity in scavenging reactive species and detoxifies peroxides and hydroperoxides which tend to GSH oxidation (Usunomena et al., 2012). GST enhances the combination of thiol functional groups of GSH with electrophilic xenobiotics, resulting in the removal and/or conversion of xenobiotic-GSH conjugate (Rao et al., 2006). Furthermore, GR is the enzyme that regenerates GSH in an NADPH-dependent reaction. Circu and Aw (2011) reported that GSH, the most important non-enzymatic antioxidant defense, plays a vital role in reducing oxidative stress through neutralizing hydroxyl radicals, suppressing LPO and removing H2O2, (Blair, 2006). Additionally, decline in GSH may result in disrupted cell defense versus free radical-induced cellular damage leading to the death of the cells (Srivastava and Shivanandappa, 2010). The observed depression of antioxidant enzyme activities together with reduced glutathione contents reflecting the weakness of antioxidant protection pathways to resolve the ROS influxes by DEN/2AAF administration. Also, it leads to the accumulation of free radicals and facilitates the enhancement of LPO in kidney tissues. Herein, the observed decrease in antioxidants (SOD, GPx, GST, GR, and GSH) provided strong evidence that oxidative stress is involved in nephrotoxicity induced by DEN/2AAF-administration. This can be attributed to overproduction of ROS developed from DEN metabolism which causes a decrease in the production of renal antioxidant enzymes. The obtained data run in parallel with those of previous investigators. Khan et al. (2001) reported that DEN administration decreases the activities of antioxidant enzyme and depletes GSH levels in renal tissues. Also, the studies of Mahmoud et al. (2015), Abdel-Moneim et al. (2016) and Ahmed et al. (2016) revealed that DEN-administration decline kidney GSH concentration and activities of GPx, GST, GR and SOD.
Elevated antioxidant enzyme activities and increased GSH concentration concomitant with reduced LPO products, showed in the current study, following artichoke leaf and flower hydroethanolic extract Co-administration involves an increase in antioxidant activity and a reduction in the peroxidation of membrane lipids owing to free radical scavenging properties of artichoke hydroethanolic extracts (Lattanzio et al., 2009). Nephroprotective potential of C. scolymus may be ascribed to the existence of tannins, flavonoids, total phenols, β-carotene in plant extract that have a strong ability to scavenge free radicals like superoxide, hydroxyl and other free radicals (Lattanzio et al., 2009). The obtained results were in parallel with El‐Boshy et al. (2017) who stated that hydroalcoholic extract of artichoke leaf was capable of alleviating oxidative stress by reducing LPO and elevating antioxidant (SOD, GPx, CAT, and GSH) biomarkers indicating the renal protective impacts of ALE against DEN-induced oxidative stress. Also, ALE was effective in attenuating the elevated MDA level and reduced GSH content in renal tissues of ethyleneglycol-induced urolithiasis in rats (Jaleel et al., 2016). It was concluded that ALE consumption reduced MDA level and elevated SOD, GPx and CAT activity in aging rats’ model induced by d‐galactose.
Histopathological changes provided additional evidence of DEN’s nephrotoxic effect. Our findings showed hyperplastic proliferation of the glomerulus, congested renal artery in the cortical region, hyperemic medullary rays, sever congestion appear in the intertubular blood vessels of the renal cortex and medulla in addition to moderate vascular degeneration in the renal tubules of DEN/2AAF-administered rats. The present findings are in parallel with Ahmed et al. (2016) who reported that DEN caused renal injury confirmed by focal fibrosis, necrosis and focal intertubular inflammatory cell infiltration. On the other hand, the administration of artichoke extract to DEN/2AAF- administered group revealed somewhat normal histological structures. Interestingly, DEN/2AAF-administered rats treated with artichoke leaf hydroethanolic extract showed increased blood flow and/or dilation of renal intertubular blood vessels. The histological findings consistent with the obtained biochemical outcomes confirm renoprotective effect of artichoke hydroethanolic extracts against DEN/2AAF-induced nephrotoxicity in rats.
Regarding apoptosis, the current investigation showed a non-significant change of kidney proapoptotic protein, p53, and a significant decrease in the mRNA expression of anti-apoptotic mediators, Bcl-2, in the kidney of DEN/2AAF-administered rats compared to the normal group. This led us to suggest that DEN/2AAF may have apoptotic effects on the kidney mediated by a decline in Bcl-2 expression. The evidence for rise in the apoptosis was supported by the presence of apoptotic cells in the histological photomicrograph of kidney of DEN/2AAF-administered rats. The Bcl-2 is an anti-apoptotic family of proteins that controls cell death by neutralizing pro-apoptotic proteins and determining the apoptosis potential of a cancer cell (Tse et al., 2008). Numerous cancer cells evade apoptosis by modulating the Bcl-2 and Bcl-xL expressions (Danial and Korsmeyer, 2004), but our study revealed that DEN/2AAF-administration down-regulated Bcl-2 expression relative to the normal group. Otherwise, ALE and AFE produced non-significant change in p53. Bcl-2 mRNA expression level, on the other hand, significantly increased when compared to DEN/2AAF-administered control group reflecting the anti-apoptotic activities of both extracts.
CONCLUSIONS and Recommendations
Our findings demonstrate that artichoke hydroethanolic extract administration attenuated DEN/2AAF-induced nephrotoxicity in Wistar rats possibly through inhibition of oxidative stress and activation of the antioxidant defense system together with the anti-apoptotic action mediated through stimulation of Bcl-2. Thus, Artichoke (leaf and flower) extracts can behave as antioxidants agents. Accordingly, more trials are needed to evaluate the efficacy and safety of these extracts in humans beings.
ACKNOWLEDGEMENTS
The authors are thankful to Dr. Rasha Rashad Ahmed, Professor of Molecuolar Cell Biology, Faculty of Science, Beni-Suef University, and Dr. Mahmoud Badawey El-begawey, Professor of Pathology, Faculty of Veterinary Medicine, Beni-Suef University, Egypt for their fruitful directions and helping in histopathological investigations.
Author’s Contribution
Adel Abdel-Moneim, Osama Mohamed Ahmed, Sanaa Mahmoud Abd El-Twab conceived and designed the study. Lamiaa Nabil Bakry conducted the experiments and analysed the data. Adel Abdel-Moneim, Osama Mohamed Ahmed, Sanaa Mahmoud Abd El-Twab, Abeer Mohamed Abd El-Hameed drafted the manuscript, made critical contribution to the discussion and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors have declared no conflicts of interest.
REFERENCES






