Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Antibiotic Residues and Associated Risk Factors in Milk in Chittagong, Bangladesh
Shaimaa Gouda1, Magdy Elgioushy2, Shimaa Ezzeldein3, Abdelmonem Abdallah1, Ahmed Abdelaal1*
1Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt; 2Department of Animal Medicine, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt; 3Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt.
Abstract | This study aimed to evaluate the role of ultrasonography in outlining the different etiologies of left abdominal distension in non-pregnant cattle. Sixty non-pregnant cows with a history of left abdominal distension that not responded to treatment were used in this study. Thorough clinical examination and biochemical analysis failed to locate the cause of distension. Four different abdominal disorders including 32 cases of traumatic reticuloperitonitis (TRP), 10 cases with left abomasal displacement (LDA), 12 with frothy tympany and 6 with a diaphragmatic hernia (DH) were identifed. All cases underwent to left laparotomy for confirmation and treatment. B-mode ultrasound was a quick, feasible, and non-invasive diagnostic tool that revealed subtle variations for different etiologies. In LDA-affected cows, the abomasum was imaged at the left paralumbar region with its heterogeneous content and echogenic folds. TRP-affected cows showed thickened echogenic reticular wall with hypoechoic effusions and echogenic fibrinous deposits. While in DH-affected cows, the echogenic reticular wall was seen inside the thoracic cavity at the level of the 4th intercostal space (ICS). It could be concluded that ultrasonography is a relatively accurate ancillary tool for assessing and differentiating the identified etiologies of abdominal distension in cows.
Keywords | TRP, Displaced abomasum, Frothy tympany, Diaphragmatic hernia, Ultrasound
Received | March 30, 2020; Accepted | June 04, 2020; Published | June 25, 2020
*Correspondence | Ahmed Abdelaal, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt; Email: [email protected]
Citation | Gouda S, Elgioushy M, Ezzeldein S, Abdallah A, Abdelaal A (2020). B. mode ultrasonographic imaging of 60 cattle with left abdominal distension. Adv. Anim. Vet. Sci. 8(7): 720-727.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.7.720.727
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Gouda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Abdominal distension is a common manifestation of many digestive and non- digestive disorders in cattle (Peek and Divers, 2018). Over distension of the rumen either with gases resulting from fermentation as in the case of tympany (bloat), or food as in case of impaction are the main causes of abdominal distension in ruminants (Radostits et al., 2007). Bloat may be primary or secondary in origin (Singh et al., 1993). Primary bloat (frothy tympany) is due to feeding of alfalfa hay and clover. Secondary bloat (free gas tympany) may be due to stenosis of the esophagus (Nishimura et al., 2014; Marzok et al., 2015), or due to traumatic reticulperitonitis (TRP), vagal indigestion, diaphragmatic hernia (DH) (Abdallah, 2012; Abdel-Raouf et al., 2012; Oman et al., 2016; Kumar, 2017). Abomasal displacement, volvulus, and torsion may be incriminated also in the induction of abdominal distension in cattle (Radostits et al., 2007).
Many non-gastrointestinal tract disorders may be implicated in the induction of abdominal distension. Urinary bladder rupture causing uroperitoneum (Braun and Nuss, 2015), uterine rupture (Kalita et al., 2018), as well as congestive heart failure can lead to abdominal distension (Buczinski et al., 2010).
Different diagnostic aids working together may help to differentiate causes of the abdominal distension including complete medical history and clinical examination (Majak et al., 2001), biochemical analysis of different blood parameters (Constable et al., 2016), abdominocentesis and intestinal biopsies (Fubini et al., 2016). Unfortunately, the absence of a specific clinical picture and similar findings in different abdominal disorders raised the attention for additional diagnostic procedures (Kurt and Cihan, 2013). The use of ultrasound is now well established as a non-invasive and relatively accurate diagnostic tool for different diseases across a range of animal species (Albury, 2015). Abdominal ultrasonography can be effective in the diagnosis and differentiation of various causes of abdominal distension in bovines (Nyland and Mattoon, 2002; Kurt and Cihan, 2013).
This study aimed to evaluate the diagnostic value of ultrasonography to differentiate between various etiologies of left abdominal distension in non-pregnant cows.
MATERIALS AND METHODS
Ethical approval
All procedures used in the present study were approved by the Committee of Animal Welfare and Research Ethics, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Animals
Sixty non-pregnant cows with a history of left abdominal distension, decreased feed intake, decreased milk production, and fecal abnormalities were referred to Veterinary Teaching Hospital, Zagazig University, Egypt during the period from January 2017 to January 2020. Diseased animals aged 3 to 8 years and weighed 350 to 600 kg. For comparison. Another 10 apparently healthy non-pregnant cows with similar weights and ages were obtained from Zagazig University farm to be a control group.
Clinical examination
Each animal was subjected to general physical examination with a special examination of the gastrointestinal system for both control and diseased animals. The physical parameters (rectal temperature, heart rate, respiration rate and the color of the mucous membrane) were recorded (Rosenberger, 1990). Special examination of the gastrointestinal system included observation of the abdominal contour, rumen consistency, rumen motility (ruminal contractions per two minutes) were performed. Withers pinch test and Knee/fist test were applied as physical pain tests for the anterior abdominal pain as described by (Cockcroft and Jackson, 2004). A metal detector was used (Hauptner-Herberholz, Germany) as a diagnostic tool for the presence of metallic foreign bodies. Moreover, simultaneous percussion and auscultation of the abdomen and rectal examination were also applied (Cockcroft and Jackson, 2004).
Blood sampling
Ten ml of blood samples were collected from the jugular vein of all diseased and control cows into test tubes without anticoagulant for biochemical analysis. Serum was extracted by centrifugation at 3000 rpm for 20 minutes within 2 hours of collection. The clear serum in the supernatant was collected in the Eppendorf tube and stored at -20 °C until evaluation.
Biochemical parameters analysis
All parameters were colorimetrically estimated using the commercial kits provided by (BioMérieux, Marcy, L’Etoile, France). Serum total protein and albumin were measured as previously described (Krohn, 2011). Total bilirubin level was estimated as previously described (Stockham and Scott, 2008). Serum cholesterol and HDL-cholesterol were measured through an enzymatic method (Allain et al., 1974; Finley et al., 1978). Serum malondialdehyde (MDA), catalase, and nitric oxide (NO) levels were measured as described in previous studies (Kei, 1978; Aebi, 1984; Pertileet al., 1995). Liver transaminases, serum copper, iron and zinc levels were assayed calorimetrically (Brenner and Harris, 1995; Atakisi et al., 2006; Senturket al., 2009; Moretti et al., 2015), respectively.
Ultrasonographic examination
Abdominal scanning was applied by ultrasound adopted from the protocol previously described (Braun, 2009). Firstly, the area of the ventral abdomen behind the xiphoid until the umbilical region was examined, then both lateral sides of the abdomen using ultrasound machine (Sonoscape, A5V, China) with 3.5 MHz convex transducer. The ultrasonographic findings (contractility, contour, and shape of the different body organs) were recorded. The ventral abdominal scanning was applied for the evaluation of the reticulum and abomasum. The left lateral scanning was applied for evaluation of the rumen, reticulum, and LDA, while right lateral scanning was applied for evaluation of the abomasum.
Exploratory laparotomy and laparorumenotomy
Left flank laparorumenotomy was applied to all TRP, tympany and DH cases, while left laparotomy was applied in LDA cases as a gold standard technique for diagnosis and also in the aim of treatment the affected cases. All operations were performed through left paralumbar fossa using standardized surgical procedures (Ducharme and Fubini, 2004).
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) version 17.0 (USA). Variables were checked for normal distribution using the Shapiro-Wilk test. Non-parametric distribution variables will be log-transformed before statistical analysis (Manikandan, 2010). The differences between groups were compared using one-way ANOVA with a post-hoc Tukey test. Results were expressed as a mean ±SD. P-values at < 0.05 were considered as statistically significant.
RESULTS
Clinical and ultrasonography findings showed that out of 60 cows with abdominal distension: 32 cows were diagnosed with TRP, 10 cows with LDA, 6 cows with DH and 12 cows with frothy tympany.
Clinical findings
The clinical findings of diseased and control cows were illustrated in Table 1. All admitted cases suffered left abdominal distension, inappetence, anorexia, depression and ruminal hypomotility to stasis. Ping sound, systemic and pain reactions were observed with variable percent among affected cases.
Biochemical findings
In comparison to controls, AST was significantly increased in DH cases. On the other hand, the serum levels of cholesterol and HDL-cholesterol were significantly (P<0.05) decreased and the serum levels of bilirubin, as well as zinc, were significantly (P<0.05) elevated in all groups of abdominal distension as compared to the controls Non-significant (P≥0.05) changes in serum total protein, cholesterol, HDL-cholesterol, bilirubin, ALT, NO, catalase, MDA, iron, copper, and zinc in all etiological groups of abdominal distension when compared with each othe (Table 2).
Ultrasonographic findings
In control cases, the reticular wall appeared as half-moon with 4 to 5 every 4 minutes biphasic contractions. The abomasum appeared as a heterogenic structure with echogenic abomasal folds, the cranial sac of rumen located caudally when the probe placed behind the xiphoid cartridge (Figure 1). As the probe located at the left flank region, the ruminal wall appeared as an echogenic wall with 0.3 to 0.5 cm in diameter located medial to the abdominal wall (Figure 2).
Ultrasonography of all TRP cases showed hyperechoic corrugated and thicker reticular wall than normal. In 27 cases, there were hyperechoic fibrin threads and exudate between the reticulum, rumen, and diaphragm that described as local TRP (Figure 3). Reticular abscesses were visualized in 5 diseased cows and appeared as a circumscribed structure with echogenic wall and anechogenic to hypoechogenic content between the reticulum and the abdominal wall (Figure 4).
Table 1: Clinical findings in control and cattle suffered left abdominal distension.
| Clinical signs |
Control (n=10)
|
Abdominal distension (n=60) | |||
|
LDA (n=10) |
TRP (n=32) |
DH (n=6) |
Tympany (n=12) |
||
| Body temperature °C | |||||
| >37.2 | 0 | 0 | 2 | 0 | 0 |
| 37.8-39 .2 | 10 | 10 | 7 | 5 | 12 |
| >39.2 | 0 | 0 | 23 | 1 | 0 |
| Respiratory rate/minute | |||||
| 15-30 | 10 | 7 | 4 | 2 | 0 |
| >30 | 0 | 3 | 28 | 4 | 12 |
| Heart rate/minute | |||||
| 60-80 | 10 | 7 | 4 | 2 | 0 |
| >80 | 0 | 3 | 28 | 4 | 12 |
| Conjunctival mucous membrane | |||||
| Bright rose red | 10 | 10 | 4 | 2 | 2 |
| Congested | 0 | 0 | 28 | 4 | 10 |
| Ruminal contractions/ 2 minutes | |||||
| 2-3 | 10 | 0 | 0 | 0 | 0 |
| Less than 2 | 0 | 8 | 25 | 2 | 9 |
| Absent | 0 | 2 | 7 | 4 | 3 |
| Positive pain tests | 0 | 0 | 22 | 3 | 0 |
| Pain reactions* | 0 | 0 | 16 | 1 | 0 |
| Inappetence | 0 | 8 | 25 | 4 | 11 |
| Anorexia | 0 | 2 | 7 | 2 | 1 |
| Depression | 0 | 10 | 32 | 6 | 0 |
| Left ping sound | 0 | 9 | 2 | 0 | 3 |
| Scanty hard faeces | 0 | 6 | 25 | 4 | 12 |
| Diarrhoea | 0 | 4 | 7 | 2 | 0 |
* Pain reaction refers to the presence of one or more of the following: stiffness in gait, abducted elbows or expiratory grunt
The abomasum displaced from its ordinary area seen from the ventral abdominal wall to the left region from 9th to 12th intercostal spaces between the abdominal wall and the rumen. The ultrasonographic findings of displaced abomasum were visualized as dorsal reverberation artifact (gas cap), moving ventrally, heterogenous moderately echogenic contents appeared with elongated, echogenic, sickle-shaped abomasal folds (Figure 5).
In cases of diaphragmatic hernia, the reticular wall was imaged at the left fourth and fifth ICS inside the thoracic cavity under the heart, the herniated reticular wall appeared as a thick and irregular echogenic structure (Figure 6).
Table 2: Biochemical parameters of the control and cattle expressed left abdominal distension.
| Parameters | LDA (n=10) | TRP (n=32) | DH (n=6) | Tympany (n=12) | Control (n=10) |
| Total protein (g/dl) |
7.7±1.9abc |
7.4±.7abc |
8.7±.6 b |
7.8±.24abc |
7.05±.23c |
| Albumin (g/dl) |
2.4±0.55a |
2.9±.49ab |
3.4±.9bd |
3.7±.7cd |
4.2±.4c |
| HDL-Cholesterol (mg/dl) |
36.6±21.5a |
19±12.7a |
12.1±11.6a |
13.6±3.8a |
42±21b |
| Cholesterol (mg/dl) |
47±21a |
35±19.4a |
30±6.75a |
44.6±12.9a |
172±30b |
| Bilirubin |
0.98±.08a |
1.02±.07a |
1.26±.05a |
1.1±.09a |
0.6±.24b |
| AST(U/L) |
145.1±53.7ac |
101.5±13.6b |
151.3±37.7c |
86.6±34abd |
90.4±39.9ab |
| ALT(U/L) |
12.6±5.4ab |
14.2±8.5ab |
12.2±3.2ab |
8.5±6.1b |
17.3±6.5a |
| Nitric oxide (µmol/l) |
31.6±12.6a |
28.3±13.4a |
30.4±5.8a |
29.7±8.6a |
28.9±2.4a |
| Catalase (U/l) |
398.8±73a |
306.2±72.3a |
294.7±25.4a |
413± 14a |
343±123a |
| MDA (nmol/m) |
6.88±.77a |
6.62±1.11a |
6.8±.59a |
6.5±1.1a |
6.1±.59a |
| Fe (mg/dl) |
132.8±27.5a |
155.4±53.4a |
137.3±33.8a |
133.7±25.9a |
148.3±82.1a |
| Zinc(ug/L) |
0.089±.015a |
0.096±.02a |
0.11±.023a |
0.1±0.022a |
0.07±0.017b |
| Copper (ug/L) |
0.158±0.066a |
0.1±0.011a |
.094±.001a |
0.12±0.004a |
0.13±0.097a |
HDL-cholesterol: high-density lipoprotein cholesterol, AST: aspartate aminotransferase, ALT: alanine aminotransferase, MDA: serum malondialdehyde. For each group, different superscription considered significant at P < 0.05 (comparison of different groups of abdominal distension with each other’s and with control), results were expressed as mean ±SD.
In cases suffered from frothy tympany, the distended rumen could be visualized from the left flank region. On moving dorso-ventrally, the abdominal and ruminal walls appeared thinner than the control, with short reverberation artifacts indicating a small gas cap appeared due to mixing of the ruminal ingesta with the frothy trapped gases (Figure 7).
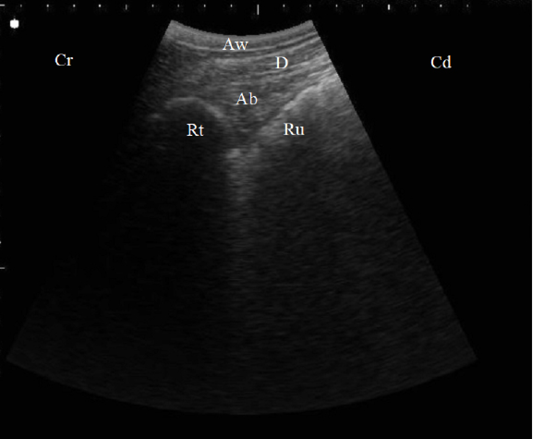
Figure 1: B. Mode ultrasonographic image in a healthy cow. Echogenic half-moon shape reticular wall (Rt) and the cranial dorsal ruminal sac (Ru) imaged from the left 6th ICS, the abomasum (Ab) appeared as a heterogenic structure with echogenic abomasal folds between the reticulum and the diaphragm (D), Aw: Abdominal wall, Cr: Cranial, Cd: Caudal.
Surgical findings
Exploratory laparotomy and laparorumenotmy were helpful in diagnosis confirmation of TRP, LDA, DH, and frothy tympany cases. In TRP cases, Abdominal exploration revealed adhesions between the reticulum, rumen, and diaphragm in 27 animals and intra-abdominal abscessation in 5 cases.
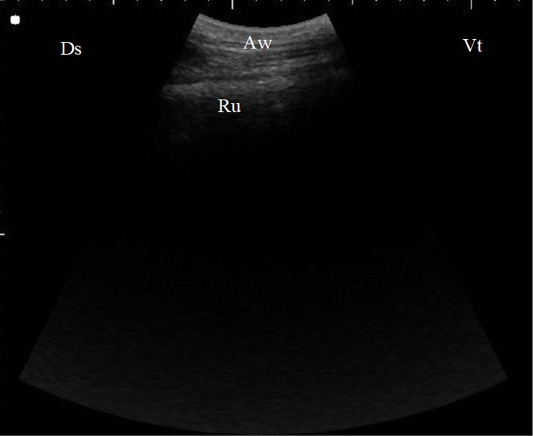
Figure 2: B. Mode ultrasonographic image of the rumen screened from the left flank region in a healthy cow. The ruminal wall (Ru) appeared as an echogenic wall with 0.3 to 0.5 cm in diameter located medial to the abdominal wall (Aw), Ds: Dorsal, Vt: Ventral.
In LDA cases, the abomasum was felt reclining on the dorsal rumen and distended, when the hand advanced over the dorsal rumen across to the dorsal left side of the abdomen. In all cases with LDA, the abomasopexy was performed successfully and correct the position of the displaced abomasum.
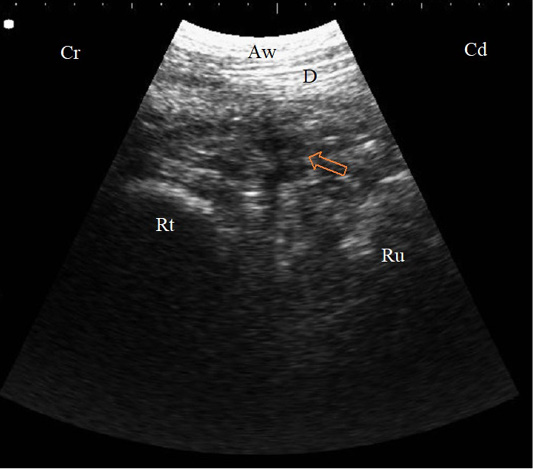
Figure 3: B. Mode sonogram in a case with localized traumatic reticulo-peritonitis. Thickened reticular wall (Rt) separated from the diaphragm (D) with an echogenic fibrinous mass and anechoic exudate (arrow) as imaged from the left 6th ICS, Aw: Abdominal wall, Cr: Cranial, Cd: Caudal.
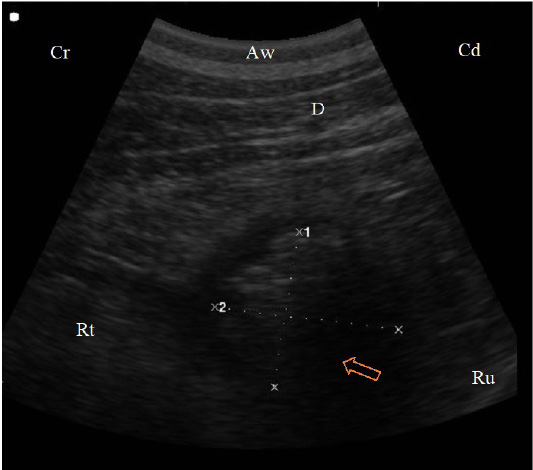
Figure 4: B. Mode sonogram in a case with reticular abscess (arrow) appeared as a circumscribed structure, 4.8 cm diameter, with echogenic wall and anechogenic to hypoechogenic content between the reticulum (Rt) and the diaphragm (D) imaged from the left 7th ICS, Aw: Abdominal wall, Cr: Cranial, Cd: Caudal.
In DH cases, a tear in the ventral 3rd of the diaphragm was located and the reticulum found herniated in the thoracic cavity. Metallic linear foreign bodies and other foreign objects (round, oval, or tiny metallic pieces; small stones and sand particles) were removed from the herniated reticulum. In all cases, adhesions were present between the herniated reticulum and the torn edges of the diaphragm and/or the pleura. The ruminal contents had the characteristic appearance of soft, foamy and porridge-like.
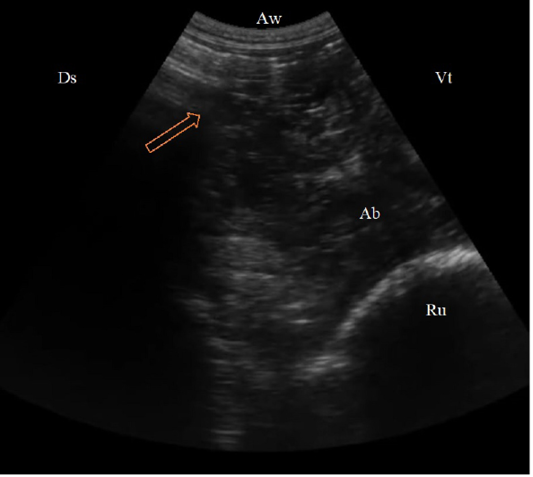
Figure 5: (B. Mode ultrasonographic image in a cow with left abomasal displacement. The abomasum displaced to the left side between the rumen (Ru) and the left abdominal wall as imaged from the left 11th ICS, the reverberation artifacts of the gas cap (arrow) appeared dorsally, followed by the hypoechoic abomasal contents (Ab), Aw: Abdominal wall, Ds: Dorsal, Vt: Ventral.)
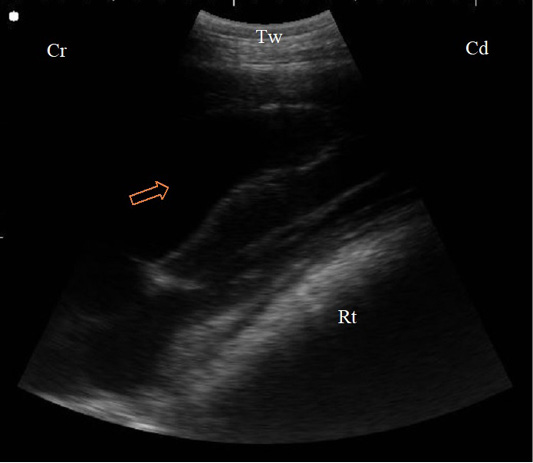
Figure 6: B. Mode sonogram in a case of diaphragmatic hernia imaged from the left 4th ICS. The reticular wall (Rt) compresses the left ventricle of the heart that appeared as echogenic wall with anechoic blood inside (arrow), Tw: Thoracic wall, Cr: Cranial, Cd: Caudal.
In frothy tympany cases, a huge quantity of frothy ruminal contents with non-perforated metallic foreign bodies consisting of nails and wires were detected and removed.
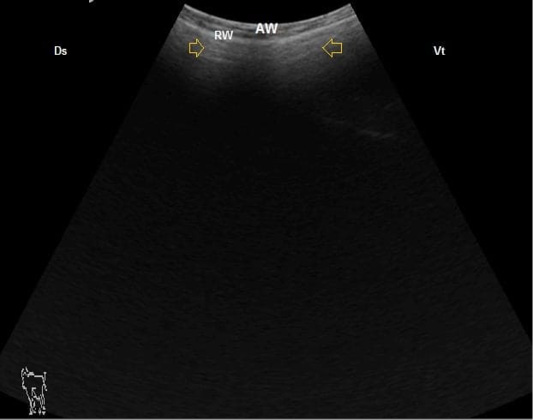
Figure 7: B. Mode ultrasonographic image of ruminal frothy tympany. The small gas gap appeared as reverberation artifact (arrows) medial to the ruminal wall (RW), AW: Abdominal wall, Ds: Dorsal, Vt: Ventral.
DISCUSSION
This study throws the light on the role of ultrasonography in diagnosis the most common surgical causes of left abdominal distension in the non-pregnant cows. In the present study, TRP, LDA, frothy tympany, and DH were the detected causes of left abdominal distension.
Regarding clinical findings of TRP-affected cows, there was an increase in body temperature in 71.8% of TRP cases, heart rate and respiratory rate in 87.5%. This might be due to the infectious agent as bacteria and it’s an endotoxin that leads to the release of cytokines (endogenous pyrogens) such as interleukin-1 causing increase synthesis of prostaglandins that results in elevated body temperature (Radostits et al., 2007). Decreased or absence of ruminal motility in DH cases were previously reported in buffaloes and cattle affected by diaphragmatic hernia (Misk, 2015). Left ping sound that detected in 9 (90%) of LDA-affected cattle, is coincided with that previously reported in LDA- affected cattle (Abdelaal, 2014), this sound is usually pathognomonic for LDA. Peritonitis and frothy tympany also implemented as causes of left ping sound (Constable et al., 2016). Therefore, it couldnot be diagnostic for LDA.
The non-significant change of the total protein in cows with TRP comparing with controls is coincided with (Braun et al., 2018) who concluded that plasma protein concentration is a non-specific biomarker for TRP in cattle. However, many researchers documented a significant increase in serum TP in cattle with TRP and buffaloes (Gokce et al., 2007; Abdallah, 2012; Abdel-Raouf et al., 2012). The significant increase in serum TP in cattle with DH is in line with that previously recorded in buffaloes with DH (Abdelaal et al., 2014), they attributed this increase to the chronic inflammatory conditions associated with the process of reticular herniation through the diaphragm into the thoracic cavity. The significant decrease in serum albumin in TRP–affected cattle could be a result of inflammation, in such condition the synthesis of proteins by the liver is geared towards the development of positive acute-phase proteins and albumin synthesis is reduced as a negative acute-phase protein (Ellah et al., 2018).
The significant decrease in serum cholesterol and HDL–cholesterol in all etiological groups of abdominal distension as compared to control is comparable to previous results that attributed the decrease in HDL and HDL-cholesterol in LDA–affected cows to impairment in lipid metabolism (Durgut et al., 2016).
The non-significant change in AST activity documented in TRP, LDA, and frothy tympany affected-cattle compared to controls is nearly in parallel with that previously reported in buffaloes with TRP (Ellah et al., 2018) and cattle with LDA (Abdelaal, 2014). In contrast to our results, a significant increase in serum AST in LDA-affected cattle was previously mentioned and might be due to protein breakdown in the synthesis of glucose (Basiri et al., 2013). The significant increase in serum AST in cattle with DH reported in this study was previously reported in sheep with DH that could be attributed to diaphragmatic damage (Simsek et al., 2018).
The non-significant difference between the levels of oxidative biomarkers (NO, CAT, MDA) and the control is comparable to results of a recent study which concluded that the nitric oxide is not a specific marker for diffuse peritonitis in buffaloes (Elgioushy et al., 2019), in contrast to another study in which the nitric oxide considered as a sensitive biomarker for the diagnosis of inflammatory conditions in TRP- affected cattle (Atakisi et al., 2010). In this study, the non-significant difference between the levels of oxidative biomarkers, serum cholesterol, and HDL–cholesterol of different etiological classes with each other makes these biomarkers not specific for differentiating the identified causes of abdominal distension.
In this study, ultrasonography was successfully used for differential diagnosis of TRP, LDA, and DH. Ultrasonography has been reported as an important diagnostic tool for the investigation of diseases of the digestive system in cattle and the study of complicated cases of traumatic reticuloperitonitis and its sequelae (Braun et al., 2018). However, it is not possible to sonographically examine reticular contents, including foreign bodies, because of the presence of gas-mixed contents (Kumar et al., 2017). The ultrasonographic picture of the different causes of the abdominal distension agrees with those reported in buffaloes with complicated TRP either with or without an affected heart (Khalphallah et al., 2015). Furthermore, the changes in the contour of the reticulum in TRP cases in our study were depended on the severity of the inflammatory changes as recently reported (Braun et al., 2018).
Abdominal ultrasonography was a simple, non-invasive means of confirming a clinical suspicion of LDA. Imaging the abomasum dorsally between the rumen and left body wall in conjunction with medial displacement of the rumen has been reported as ultrasonographic evidence of LDA in adult cattle (Braun et al., 1997), similar ultrasonographic abnormalities were readily identifiable in the cows suffered LDA in our study. Considering its utility in diagnosing LDA in beef calves, ultrasonography may obviate the need for exploratory surgery in cases with subtle clinical signs (Oman et al., 2016).
Ultrasonography was previously proven to be diagnostic in cases of DH in cows and buffaloes (Saini et al., 2007; Abdelaal et al., 2014), with better and appropriate diagnosis using the right caudal thoracic approach (Kumar et al., 2017), as the reticulum frequently herniated to the right part of the thoracic cavity at the right 4th to 5th ICS (Saini et al., 2001), also the reticular wall could be visualized at the level of the left 4th ICS (Abdelaal et al., 2014) as we reported in this study. Our results could be limited to the fact that abdominal ultrasound has a limited ability as a confirmative diagnostic tool in frothy tympany cases.
CONCLUSION
Ultrasonography is well suited as a rapid, non-invasive and reliable ancillary tool that enables practitioners to outlines the different etiologies of left abdominal distension in cattle.
AUTHORS’ CONTRIBUTION
All auhtors contribute to this research equally. They made available relevant literatures and conducted examinations as well. All authors participated in draft and revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interests
The authors have declared no conflict of interest.
REFERENCES






