Advances in Animal and Veterinary Sciences
Research Article
The Application of a Split-Conjugated Anti-Brucellosis Vaccine as a “Provocing Factor” for Identification of Brucellosis in Animals
Stepan Yurievich Veselovsky1 ,Valery Alexandrovich Agoltsov1, Davud Abdulsemedovich Devrishov 2, Olga Mikhailovna Popova1, Nataliya Victorovna Solotova1*
1Department of Veterinary Medicine, Saratov State Agrarian University named after N.I. Vavilov, Russia, 410012, Saratov, Teatralnaya square, 1; 2Moscow Academy of Veterinary Medicine and Biotechnology named after K.I. Scriabin “; Russia, 109472, Moscow, Academician Scriabin Street, 23.
Abstract | The split-conjugated vaccine against brucellosis of animals was tested as a provocateur in order to identify sick (latent) animals in both brucellosis safe and brucellosis poor farms. After administration of the test vaccine, antibodies remained in the blood of healthy animals for up to 2.5 months, then disappeared, and antibodies remained in the blood of sick animals after 3.5, 4.5 and 7.5 months and gradually disappearing over time. The split-conjugated animal brucellosis vaccine has a “provocative effect”, exacerbates the infectious process and thereby makes it possible to identify sick animals. We recommend the use of a split-conjugated animal brucellosis vaccine in brucellosis-poor households in order to detect latent animals and create humoral immunity in animals while maintaining antibodies in the blood of animals for 2.5 months. In the case of small detection of animals with brucellosis in the farm (from 1 to 5 animals), we recommend using the vaccine to detect animals with brucellosis and to create immunity in healthy animals twice with an interval of 3 months. If a large number of animals with brucellosis in the farm (from 6 to 20 and above goals) are detected, we recommend using the vaccine once after eliminating the sick livestock, followed by the use of the vaccine from the Brucella abortus 82 strain, which must be vaccinated after passing calving (May - July). The split-conjugated animal brucellosis vaccine can be used on both non-pregnant and pregnant cows, as it does not cause abortion.
Keywords | Animal brucellosis, Split-conjugated vaccine, Latent brucellosis, Exacerbation of the brucellosis infection process.
Received | October 30, 2019; Accepted | January 17, 2020; Published | May 02, 2020
*Correspondence | Nataliya Victorovna Solotova, Department of Veterinary Medicine, Saratov State Agrarian University named after N.I. Vavilov, Russia, 410012, Saratov, Teatralnaya square, 1; Email: [email protected]
Citation | Veselovsky SY, Agoltsov VA, Devrishov DA , Popova OM, Solotova NV (2020). The application of a split-conjugated anti-brucellosis vaccine as a “provocing factor” for identification of brucellosis in animals. Adv. Anim. Vet. Sci. 8(5): 524-530.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.524.530
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Solotova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Brucella as an optional intracellular pathogen establishes a close relationship with the host’s immune cells. Through the disruptive function of the immune system, the pathogen is able to support a chronic infection, which often makes treatment and diagnostics difficult. In recent decades, many studies have been conducted in an attempt to develop safe and effective animal brucellosis vaccines. There is no licensed vaccine for the prevention of human brucellosis. A human vaccine would be useful in protecting farmers, veterinarians, animal care workers, laboratory staff and the general population living in endemic areas of brucellosis (Dornless et al., 2015).
Studies aimed at developing the ideal vaccine against brucellosis in animals and humans have been being carried out since the beginning of the twentieth century (Blasco et al., 2016).
Since then, live as well as inactivated vaccines have been developed. Vaccination is the best economical measure to control brucellosis in endemic areas. Many countries have developed control measures to eradicate the disease in animals. These programs minimized economic losses due to abortion, infertility, weak offspring, and reduced milk production (Caminiti et al., 2016; Carvalho et al., 2016).
Currently, vaccination programs are based on the control of brucellosis mainly caused by B. melitensis and B. abortus (Ductoroy et al., 2014).
In foreign countries, it is recommended to use only three live attenuated vaccines to fight infection caused by B. abortus in cattle: B. abortus 45/20, strain B. abortus 19 (S19) and B. abortus RB51 (Mansoori et al., 2016). B. abortus strain 45/20 was isolated after 20 passages (sequential reseeding of microorganism on various nutrient media or inoculation of pathogenic microbes from one infected animal to another) in guinea pigs. This R - strain form (rough strain) is used only as a thermally killed vaccine to avoid reversion to the virulent strain. This vaccine can be administered with an adjuvant to adult cattle. It does not interfere with serological diagnostics, and is safe for use in pregnant animals. A positive result in test mode was obtained in some countries (Khan and Zahoor, 2018; Li et al., 2017).
Another licensed live attenuated bovine brucellosis vaccine is B. abortus S19. This strain was isolated at the beginning of the twentieth century and subjected to attenuation by room temperature for one year [32]. B. abortus S 19 is effective in controlling bovine brucellosis (prevents abortion) and also reduces the prevalence of brucella in herds. However, due to the characteristic features of strain S19 and a strong antibody response against the O-side chain, it does not distinguish between infected and vaccinated animals. Competitive ELISA and radial immunodiffusion tests were used to allow differentiation between vaccinated or infected animals (Godfroid, 2017).
A low rate of abortion in livestock production and a significant increase in milk production during vaccination with B. abortus S19 have been reported (Figueredo et al., 2015).
Another live vaccine from strain B. abortus RB 51 is a spontaneous “rough” mutant obtained by subculturing a virulent strain of B. abortus 2308 on a medium containing rifampicin and penicillin [31]. Unlike strain 45/20, B. abortus RB 51 is very stable and is currently used in many countries instead of B. abortus S19. Rough deformation of RB51 is less virulent and does not cause a positive response in a typical serological diagnostic test (Saez et al., 2014).
Vaccination of pregnant cows with strain RB 51 can cause low abortion rates (less than 0.2%); however, it is safe at lower doses during pregnancy. The vaccine strain RB 51 can infect humans, but it is less virulent than strain S 19 (Saez et al., 2014).
Another live vaccine B. melitensis Rev. 1 is used to control brucellosis in small ruminants. This strain was developed by Herzberg and Elberg in the mid-1950s. The strain retains the general characteristics of Brucella species; it is resistant to streptomycin 2.5 μg / ml and susceptible to penicillin G, which makes it possible to differentiate from field strains [18]. Subcutaneous or conjunctival immunization with B. melitensis Rev. 1 provides sufficient immunity in small ruminants. B. melitensis Rev. 1 induces a positive antibody response in serological tests in vaccinated animals. However, B. melitensis Rev. 1 can infect people. Vaccination is recommended before the first pregnancy in 3 to 7 months to avoid abortion in pregnant animals.
Since B. melitensis can be isolated from cattle, some scientists have suggested using the live attenuated vaccine B. melitensis Rev. 1 for controlling the disease in cattle (Lopez-Santiago et al., 2019).
Compared to extensive research efforts to develop new vaccines against B. melitensis or B. abortus, a small study was conducted to protect pigs against B. suis. Oral or intramuscular vaccination with strain RB 51 killed by B. suis or purified with O-polysaccharide has been reported to be effective in protecting pigs infected by boars (Ivanov NP, 2006).
However, other studies have shown very little protection with strain RB 51 in immunized pigs against infection with the field strain (Mansoori et al, 2016; Li et al, 2017).
For the first time, immunization of animals with a vaccine from B. abortus 19S strain in 1953 was included in the system of anti-brucellosis measures of the USSR. This vaccine was used until 1970. During the application of the vaccine many households, and even entire areas, were cured of the disease (Hosein et al., 2018). However, in regions with a wide spread of brucellosis, the effectiveness of recreational activities was insufficient. This was primarily due to the high agglutinogenicity of the vaccine. Agglutinins and complement-binding antibodies remain in the body of immunized animals up to 5-8 years, which greatly complicates the differentiation of such animals from patients with brucellosis.
Since 1974, in Russia, vaccines from B. abortus strains 82 and 19 have been used in the general complex of anti-brucellosis measures. Since 1997 vaccines from B. abortus 75/79-AB and 17/100 strains were also used (Sklyarov et al., 2011).
Ivanov AV et al. (2009) found that in the fight against cattle brucellosis, the most effective vaccine strains are in the SR form, with weak agglutinogenic and pronounced immunogenic properties. The vaccine from the Brucella abortus 82 strain possesses all these properties (Ivanov AV and Salmakov, 2009; Ivanov AV, 2009; Ivanov AV and Yusupov, 2009).
A vaccine from the strain of Brucella abortus KB 17/100 was successfully tested for immunogenicity, and its prototype from the strain of Brucella abortus 45/20 is widely used in the countries of the Near and Middle East, Asia and Africa. Its main drawback is pronounced reactogenicity, its advantages are inagglutinogenicity, pronounced immunogenicity and the fact that in animals with a latent form of brucellosis it provokes the synthesis of antibodies detected by serological studies with standard antigens, which allows animals to be removed from the herd in a short time (Sklyarov et al., 2011).
Other researchers used an adjuvant, a vaccine against cattle brucellosis from the strain Brucella abortus KB 17/100. In a controlled epizootic experiment, the high epizootic efficacy of inactivated non-agglutinogenic adjuvant, a vaccine from the Brucella abortus strain KB 17/100, was established during the recovery of farms with cattle brucellosis (Khairov et al., 2005).
Unfortunately, the drug has reactogenic properties, manifested by a short-term increase in body temperature, swelling at the injection site and slight depression with a partial loss of appetite. All these signs gradually disappear, without harming the health of animals and without affecting their productivity. The introduction of adjuvant vaccines causes specific sensitization of the body of immunized animals. Due to the prospects of using the adjuvant vaccine from the strain Brucella abortus KB 17/100 as part of complex measures to combat cattle brucellosis, an adjuvant vaccine was used to immunize all cows and 3-6 month old heifers in 1998 in 5 districts of the Saratov region.
It has long been known that many vaccines have not only an immunostimulating effect, forming immunity, but also excite the whole animal and human organism (Hull and Schumaker, 2018), as a result of which antibodies from latent animals are released into the blood. Such action of vaccines is able to detect and thereby quickly eliminate many infectious diseases, including animal brucellosis. It is called a provocative action in the fight against infectious diseases (Novitsky, 2016). The elimination of brucellosis actually takes a lot of time, and often stretches over years, especially in places where the fight against brucellosis has not been carried out for a long time (Ivanov NP, 2006). There were cases when a double negative result for brucellosis took several years, which made it impossible to use vaccines in the fight against brucellosis, and contributed to the further spread of the infection. In addition, a long procedure for the elimination of brucellosis requires certain financial costs, patience and labor from both the veterinary services and the owners of animals (The concept of controlling the risks of the occurrence and spread of epizootic foci of zooanthroponosis: Method. Regulations, 2011).
So, the goal of research was to evaluate the “provocative” effect of the split-conjugated brucellosis vaccine when used in a brucellosis-poor farm.
Materials and methods
Serological studies of blood samples of vaccinated animals were carried out in the Republic of Kazakhstan, the Aktobe region, the Mugalzhar district in the farm «Kustabaev». 39 heads of cows aged 3 to 6 years were used for the experiment.
The split-conjugated animal brucellosis vaccine was administered subcutaneously in the middle third of the neck at a dose of 1.0 ml. Before use, the vaccine was mixed with a solvent (immunoprotector). The immunoprotector polypeptide-C was obtained from activated calf spleen cells, consisting of a mixture of peptide activators of B cells, and a technique was developed for its conjugation with brucella antigens. The injection site was trimmed and pretreated with a 70% alcohol solution.
Blood was examined in the district veterinary laboratory of the Mugalzhar region in the agglutination reaction (AR), rosbengal test (RBT) and complement fixation reaction (CFR). Assessment of the results of serological studies was carried out according to the «Instructions for the diagnosis of animal brucellosis” issued in September 29, 2003 No. 13-5-02 / 0850 [10].
Results were analyzed after 3,5, 4,5 and 7 months after vaccine injection.
Blood from vaccinated calves of 6 months of age, owned by Berezovskoye Ltd. in the Engels district of the Saratov region, was taken 15, 30 and 90 days after immunization (a brucellosis-free farm).
Blood tests for brucellosis were performed using the complement fixation reaction (CFR) and agglutination reaction (AR) in the Engels veterinary laboratory (Saratov region) for combating animal diseases.
Before vaccination, all animals underwent serological studies for brucellosis in AR and CFR. The results of preliminary studies were negative.
To verify the data obtained Student t-test was used, and the statistical significance level of cow parameters was (p≤0.05).
Experimental research, maintenance, care and euthanasia were carried out according to the requirements of the «European Convention for the Protection of Vertebrates used for experiments and other scientific purposes» (1986).
Results
The change in the number of animals that respond positively to brucellosis in AR and CFR during a triple study of blood samples after vaccination in a brucellosis-friendly farm is shown in Table 1.
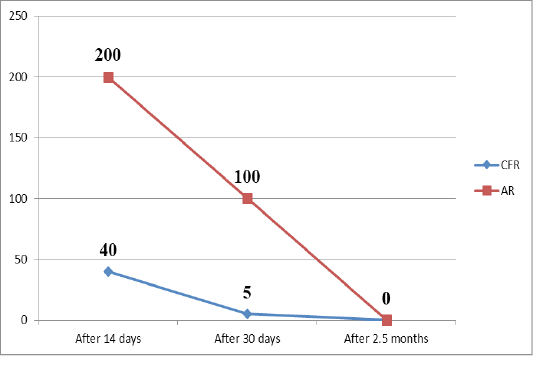
Table 1: Change in the number of animals that respond positively to brucellosis in AR and CFR during a triple study of blood samples after vaccination
A month after vaccination a blood test by serological reactions revealed that almost all blood samples in CFRs lack complement-binding antibodies. Agglutinins were present in the vast majority of cases in a titer of 1:100, sometimes 1:50, which is considered a dubious reaction. In only one sample in the CFR, questionable results were found (1/5). This indicates that complement-binding antibodies in the blood circulate for a short time, and after a month disappear from the blood.
When examining blood samples 2.5 months after vaccination, it was found that negative results for brucellosis were obtained in both AR and CFR. Only one test using CFR was doubtful (1/5), all the rest were negative (Veselovsky et al., 2018).
The results of serological studies of blood samples of cattle after vaccination with a test vaccine in a dysfunctional farm are presented in Table 2.
When examining animal blood samples in a brucellosis-poor farm, the following results were obtained:
• 3.5 months after vaccination, the appearance of agglutinin antibodies in the blood of the vast majority of animals was observed.
• The percentage of sick cattle was 79.5%, which indicates «excitation» of the immune system of animals for the vaccine.
• 4.5 months after vaccination a decrease in the number of animals responding positively to brucellosis to 56.3% was observed, which is predictable due to a decrease in the activity of the animal immune system over time.
• 7 months after vaccination, a decrease in animals positively responding to brucellosis was also noted to 45.5%.
All the data obtained indicate that after the exacerbation of the infectious process with the introduction of the vaccine, there is a gradual decrease in the animals that respond positively to brucellosis over time with the transition of the disease back to a chronic form. It should be noted that RBT for the diagnosis of brucellosis is used in Kazakhstan, but not in the Russian Federation. Nevertheless, with the help of RBT and CFR, all households in France were cured of brucellosis in a relatively short time (within 30 years).
Table 2: Serological studies of blood samples of cattle after vaccination with a test vaccine in a dysfunctional farm
| Results after vaccination | Animals examined, heads | Positive reaction - heads / % | ||
| AR | CFR | RBT | ||
| After 3.5 months | 39 | 31/79.5 | 22/56.4 | 29/74.4 |
| After 4.5 months | 32 | 20/62.5 | 16/50 | 18/56.3 |
| After 7 months | 33 | 17/51.5 | 18/54.5 | 15/45.5 |
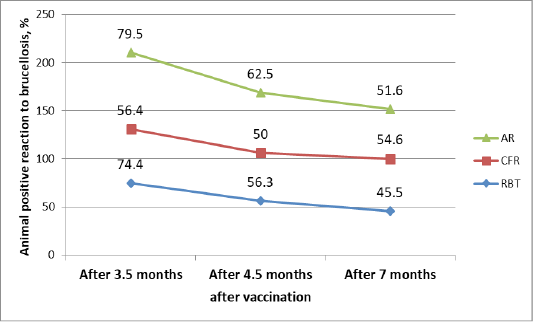
Table 3: Dynamics of a positive reaction to cattle brucellosis after vaccination in the study of blood samples by various serological reactions %
Table 3 shows the dynamics of changes in the number of animals with brucellosis using various serological reactions. In CFR, there was a fluctuation in animals that respond positively to brucellosis (56.4%), then a decrease in such animals to 50%, and then again an increase to 54.6%. In RBT, a gradual decrease in animals responding positively to brucellosis is from 74.4% to 45.5%. In AR, the number of brucellosis-infected animals gradually decreased from 79.5% to 51.6% over time.
Discussion
Dzhupina S.I. determined that in farms poor for brucellosis, many sick animals are not detected by generally accepted serological reactions. Such animals are involved in the spread of infection and the maintenance of epizootic situation, both of the farm itself and of the farms located nearby (Dzhupina et al., 2013). We agree with the opinion of Dzhupina S.I., since latent forms of brucellosis can release the causative agent of the disease into the environment and thereby infect new animals.
Other researchers recognized this feature as well. Having analyzed research works on small doses vaccination of cattle with the vaccine from strain 19, P.S. Ulasevich and V.A. Romakhov (Ulasevich and Romakhov, 1983) (noted that this method allowed to identify hidden carriers of the causative agent of brucellosis using serological studies carried out after vaccination of animals. In our case, small doses of the vaccine provoked the appearance of antibodies in the blood of animals and thereby found latent animals.
A.S. Mangazeeva together with I.A. Kosilov, as well as our experiment, prove that in herds with a natural chronic course of brucellosis, the secondary administration of antigen to hidden carriers of the infection pathogen provokes the synthesis of antibodies captured by antigens in AR and CFR (Kosilov et al, 1999; Mangazeeva, 1976) The same results were obtained by V.I. Kudla (Kudla, 1987) during the study of ram infectious epididymitis. Double administration of the vaccine we tested 3 months after the first injection will also allow to identify more animals with brucellosis, since repeated administration of the vaccine additionally stimulates the release of the pathogen of brucellosis into the bloodstream and, as a result, the formation of anti-brucellosis antibodies. Also, adjuvants, such as calcium hydroxyappatite, together with the vaccine, extended the immunity in animals, as shown in our previous experiments (Agoltsov et al., 2019).
Novitsky A.A. studied the antiepizootic efficacy of the combined use of brucellosis chemical vaccine (BCV) and vaccine from strain B. abortus 82 when stopping the focus of brucellosis infection. He proved that the provocative property of BCV allowed to additionally isolate animals positively responding to brucellosis on 21 day after its use. Further studies of the livestock after 2 and 3 months showed a marked decrease in epizootic tension. Three months later, animals with brucellosis infections were no longer recorded. Thus, as well as in our experiment, it was possible in a shorter time to heal the farm from cattle brucellosis (Novitsky, 2016).
Conclusions
1. After administration of the test vaccine, antibodies remain in the blood of healthy animals for up to 2.5 months, and then disappear, and antibodies are detected in the blood of animals that respond positively to brucellosis after 3.5 months and gradually their number decreases.
2. The split-conjugated animal brucellosis vaccine has a “provocative effect”, exacerbates the infectious process, and thereby makes it possible to identify sick animals.
Recommendations.
The use of a split-conjugated animal brucellosis vaccine in farms with dysfunctional brucellosis is recommended in order to:
1. Detect latent (patients with a latent form of brucellosis) animals for immediate vaccine administration after the identification (for cattle). 14 days after the introduction of the vaccine, blood should be examined for brucellosis by all of the above mentioned reactions. If positively reacting animals are detected, record the inventory numbers and antibody titers of such animals and again examine them with serological reactions after 30 days. Compare the results of serological blood tests with previous ones; record the inventory numbers of animals and antibody titers. Examine the blood of all animals in the farm after 2.5 months for brucellosis by serological reactions, including RBP. Consider animals positively responding to brucellosis 2.5 months after vaccination as sick and eliminate them within 15 days. If necessary and if suspected of still having brucellosis-infected animals on the farm, re-vaccination with a split-conjugated animal brucellosis vaccine should be carried out and a triple blood test of all farm animals for brucellosis should be repeated.
2. To create the humoral immunity of the animal organism to the administered vaccine.
In the case of small detection of animals with brucellosis in the farm (from 1 to 5 animals), we recommend using the vaccine both to identify animals with brucellosis and to create immunity for healthy animals. The scheme is as follows: after isolation of positively reacting animals, they should be eliminated within 15 days, and the entire cattle stock of the farm should be vaccinated with a split-conjugated animal brucellosis vaccine. 2.5 months after vaccination, re-examine the blood of healthy animals for brucellosis with serological reactions. Consider positively reacting animals as patients with brucellosis and eliminate them within 15 days. Re-examine the blood of farm animals after 14 days. Upon receipt of a negative result for brucellosis, re-vaccinate all animals with a vaccine. After 3 months, again examine the blood of animals for brucellosis twice with an interval of 14 days. By this time, animals with brucellosis should not remain on the farm and the vaccine, when applied twice, will form a humoral immunity for brucellosis in animals, associated with the formation of specific antibodies and the creation of immune memory in animals. In order to prevent brucellosis, the vaccine should be used every 6 months.
In case of large detection of sick animals (from 6 to 20 and above) in the farm, the vaccine is recommended to use once after eliminating the sick livestock.
The scheme is as follows: 2.5 months after vaccination, re-examine the blood of healthy animals for brucellosis with serological reactions. Consider positively reacting animals as patients with brucellosis and eliminate them within 15 days. Re-examine the blood of animals in the farm after 14 days and, if necessary, every 2 weeks until a double negative result for brucellosis is obtained. The purpose of the vaccine: to detect and eliminate the sick (latent) animals in the shortest possible time. After the identification and elimination of all animals suffering from brucellosis, the whole cattle of the farm should be inoculated with the vaccine from the strain Brucella abortus 82 from the moment of calving (May - July). Examine blood according to the instructions for brucellosis after 10 months. The split-conjugated animal brucellosis vaccine can be administered to animals at any time of the year because it does not have abortogenic properties.
Acknowledgments
The authors are thankful to the Rector of Saratov State Agrarian University and to the individual farmer I. Kustabaev, farm «Kustabaev» the Republic of Kazakhstan, Aktobe region, Mugalzhar district for supporting with all essential requirements for present research.
Conflict of Interest
The authors have not declared any conflict of interests.
Authors contribution
Valery Alexandrovich Agoltsov, Olga Mikhailovna Popova designed the experiment. Stepan Yurievich Veselovsky conducted the statistical analysis, as well as conducting the experimental measurement, Davud Abdulsemedovich Devrishov and Nataliya Victorovna Solotova contributed by writing the article and reviewing it thoroughly before submission.
References






