Advances in Animal and Veterinary Sciences
Research Article
Effect of Kebar Grass (Biophytum petersianum) Extract on the Seminiferous Tubules in Male Mice (Mus musculus) Treated With 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)
Aldis Ingrid Rusyawardani1, Widjiati1, Suzanita Utama2, Chairul Anwar1, Lilik Maslachah3, Epy Muhammad Luqman1*
1Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia – 60115; 2Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia – 60115; 3Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia – 60115.
Abstract | The aim of this research was to analyze the effect of kebar grass on the seminiferous tubules diameter and epithelium thickness of mice exposed to 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD). Twenty five BALB/C mice were used with five treatments (4 mice each group): Negative Control (C-), Positive Control (C+) with TCDD 7µg/kgBW IP, and treatment groups that treated with TCDD 7µg/kgBW IP and Kebar Grass orally for 53 days were presented T1 0,045mg/gBW/day, T2 0,080mg/gBW/day, and T3 0,135mg/gBW/day. Results showed that TCDD decreased the diameter and epithelium thickness of seminiferous tubules, while kebar grass extract was proven to maintain diameters but could not maintain epithelium thickness of of the seminiferous tubules.
Keywords | Kebar grass, Seminiferous tubule, Tetrachlorodibenzo-p-dioxin (TCDD), Mice
Received | October 30, 2019; Accepted | April 13, 2020; Published | May 02, 2020
*Correspondence | Epy Muhammad Luqman, Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia – 60115; Email: [email protected]
Citation | Rusyawardani AI, Widjiati, Utama S, Anwar C, Maslachah L, Luqman EM (2020). Effect of kebar grass (biophytum petersianum) extract on the seminiferous tubules in male mice (mus musculus) treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd). Adv. Anim. Vet. Sci. 8(5): 519-523.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.519.523
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Luqman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
2,3,7,8 - tetrachlorodibenzo-p-dioxin (TCDD) compounds have a wide enough toxic level that they can cause damage to the body tissues (Pelclová et al., 2006). Observations from several studies proved that dioxin exposure provides many toxic effects on humans and experimental animals (Dorbrzynski et al., 2009). The male reproductive system is known to be sensitive to TCDD, decreased testis and accessory sex organ weights, decreased spermatogenesis, and reduced fertility. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is one of the causes of infertility and immune damage. The results of the study from Latchoumycandane and Mathur (2002) proved that TCDD administration can induce an oxidative stress in the testes. According to research that conducted by Jong et al., (2007) showed that TCDD could significantly reduce the diameter of tubules and tubular percentages containing spermatozoa.
A good compound to counteract the toxic effects of TCDD is an antioxidant kebar grass (Biophytum petersianum) is one of many types of herbs that grow wild. The usage of kebar grass as a fertile fertilizer had been carried out in Papua for a long time (Sembiring and Darwati, 2013). Research conducted by Lefaan (2014) showed that kebar grass contains three types of chemical compounds flavonoids, saponins, and tannins that have the potential to give effect to the process of spermatogenesis. The administration of 5% kebar grass infusion could significant increase spermatogenesis activity (Lefaan, 2014). With significantly increased spermatogenesis, it could increase the diameter and thickness of the seminiferous tubular epithelium (Lefaan, 2014). This improve that kebar grass is a potential material and good for male reproduction (Lefaan, 2014). Research on kebar grass extract so far is not widely known, therefore the researchers wanted to observe the effect of kebar grass extract to the diameter and thickness of seminiferous tubule epithelium in mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin to observe potential kebar grass in counteracting the toxic effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Materials and methods
Materials
The apparatus used in this study was a plastic enclosure and closed wire netting, a bottle of 80 ml of volume, a micropipette, a digital scale with an accuracy of 0.1 g. sterile glass bottle, sterile 1ml Eppendorf vial, feeding tube for giving the extract to the mice, 1 ml syringe, tweezers, blade, scalpel, and surgical scissors. The materials used in this study were male (Mus musculus) mice of BALB-C strain, kebar grass extract dose of 0.045 mg / gBB, 0.08 mg / gBB, and dose of 0.135 mg / gBB (Lefaan, 2014), solution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (Supelco brand no. catalog 48599), corn oil, aquadest, 10% formalin buffer, 70% alcohol, xylol, water-soluble paint, and canada balsam.
Methods
The samples in this study were 25 male mice (Mus musculus) 12 weeks old with the body weights between 25-35 grams obtained from Veterinaria Farma Surabaya with a replication of five groups. Experimental animals were obtained using simple random sampling procedure. There are five treatment groups (4 mice each group): Negative Control (C-) group which administered with physiological saline 0.9%, Positive Control (C+) with TCDD 7µg/kgBW IP, and treatment groups treated with TCDD 7µg/kgBW IP and Kebar Grass orally for 53 days is Treatment 1 (T1) 0,045mg/gBW/day, (T2) 0,080mg/gBW/day,and (T3) 0,135mg/gBW/day. Testes examination was conducted by hematoxylin and eosin (HE) staining (Hematoxylin Staining for Millicell®-HA, Merck, Germany). Three slices of each sample were observed and examined by microscope (Olympus® CX-41).
The data were obtained by measuring the diameter and thickness of the seminiferous tubular epithelium using a light microscope connected to an optilab camera to facilitate the measurement process. Furthermore, the measurement of diameter and thickness of seminiferous tubules is done with the Image Raster application. Measurement of the thickness of the seminiferous tubular epithelium was calculated from the basement membrane to the lumen of the seminiferous tubules. Data were presented as means ± SE (standard error) and were analyzed using one way analysis of variance (ANOVA) with significant level set on P <0.05 and the differences among the groups were determined by Duncan’s multiple range test
RESULTS AND DISCUSSION
Table 1. showed a decrease in the mean diameter of seminiferous tubules in the positive control group compared to negative control. This is directly proportional to the decrease in the thickness of the seminiferous tubular epithelium in positive control. Furthermore, in groups T1, T2, and T3 there was a gradual increase in diameter and thickness of seminiferous tubular epithelium. The increase in diameter of seminiferous tubules that occurred in all treatment groups reached the highest size in the T3 group, which was 170.5 μm, which was close to the diameter size of the seminiferous tubules of the negative control group.
Table 1: The Mean Diameter and Thickness of Seminiferous Tubule Epithelium
| Treatment Groups |
Seminiferous Tubule (Mean ± SD) |
|
| Diameter | Epithel Thickness | |
| C- |
170,80b± 2,81 |
56,17a± 1,35 |
| C+ |
129,50a± 8,42 |
33,57e± 1,61 |
| T1 |
160,58ab± 30,50 |
43,80d± 1,46 |
| T2 |
168,79b± 11,00 |
48,49c± 1,08 |
| T3 |
170,5b± 0,65 |
52,15b± 0,86 |
Description: Different superscripts in the same column show significantly different (p <0.05).
Increased diameter of seminiferous tubules in the treatment group had the same pattern as the increase in the thickness of seminiferous tubular epithelium in the same treatment group, but the highest increase in the thickness of the seminiferous tubule epithelium in T3 was still significantly different from the thickness of the epithelium in the negative control group.
Mann Whitney test results showed that the diameter of seminiferous tubules had a significant difference (p <0.05) in the C+ group against T2, namely a significant decrease in diameter of the seminiferous tubules due to TCDD exposure and administration of kebar grass extract at dose 0.080 mg / g BB / day in the T2 group. A significant difference (p = 0.009) was also found in the C+ group against T3.
Duncan test showed significant changes (p <0.05) in all treatment groups. This can be seen by a significant decrease in the thickness of the seminiferous tubular epithelium in the positive control, whereas in the T1, T2, and T3 groups there was a significant increase, although in the T3 group it could not maintain the epithelial thickness as in the C-group (Figure 1).
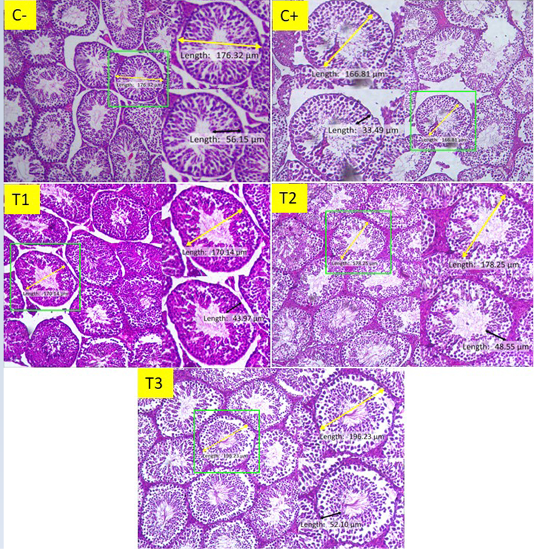
Figure 1: Histological features of seminiferous tubules of mice (Mus musculus) in groups C-, C +, T1, T2, and T3 (100 X, H&E stain). The yellow line shows the measurement of the diameter of the seminiferous tubule, while the black line shows the measurement of the thickness of the seminiferous tubular epithelium.
There is a difference in the picture that shows shrinkage in diameter and thickness of the seminiferous tubular epithelium in the positive control group compared to the other groups. In the image of the T3 group the size of the epithelium is close to the C- size, while the seminiferous tubules diameter in the T3 group image appears to exceed C-.
The diameter of the seminiferous tubules decreased due to TCDD exposure that with the Arylhidrocabon Receptor (AhR) which is the receptor base protein in the cytoplasm of leydig cells, then enters the nucleus and interacts with DNA, and attacks genes that control various biochemical reactions such as synthesis and metabolism enzymes, hormones, or growth factors (Winarti and Winarso, 2005). Inside the nucleus, TCDD ligands, AhR, and ARNT will bind to certain DNA, namely Dioxin-Responsive Enhancer Elements (DRE), the bond will change the expression of various genes including cytochrome P450 (Yin et al., 2012).
Increased cytochrome P450 can affect on leydig cells through a receptor called Androgen Receptor (AR) which plays a role in the maturation of the steroidogenesis pathway (Chang et al., 2004). However, an increase in cytochrome P450 results in the formation of cytochrome P450 bonds with Androgen Receptor (AR) which could reduce the action of AR on leydig cells and increase ROS formation in cells so that death occurs in leydig cells (Karmilawati, 2013) so that it can reduce testosterone levels that are indispensable in spermatogenesis process regulation. Testosterone is bound by a special protein, Androgen Binding Protein (ABP) to be carried into the seminiferous tubules (Hayati, 2011). The decrement in the testosterone could induce reduction in the number of spermatogenic cells, which could reduce the diameter and thickness of the seminiferous tubular epithelium. Cytochrome P450 bond with Androgen Receptor (AR) is known to increase Reactive Oxygen Species (ROS) production, so that it can trigger an increase in superoxid radicals which could cause membrane lipid peroxidase (Dobrzyński et al., 2009).
The diameter of seminiferous tubules in the negative control group was given 1% Natrium– Carboxymethyle Cellulose (CMCNa) solution, positive control exposed to TCDD and given 1% CMCNa solution, T1 exposed to TCDD and given kebar grass extract dose of 0.045mg/g BB/day, T2 exposed to TCDD and given Kebar grass extract dose of 0.080mg/g BB/day, and T3 exposed to TCDD and given kebar grass extract at a dose of 0.1350mg / g BB / day showed a significant difference in the positive control group compared with negative control. This was caused by exposure to TCDD in the positive control group, while the negative control was only given CMC Na 1%. According to Choi et al. (2007) demonstrated that TCDD could induce a significant decrease in testicular weight, seminiferous tubule size, number of spermatozoa, number of germ cells and sertoli cell index. The decrease in diameter of the seminiferous tubules is caused by a decrease in the number of spermatozoa cells in tubules in accordance with the opinions of Anindita and Sutyarso (2012) who found that the diameter of the seminiferous tubules is influenced by the number of spermatozoa production. This is reinforced by the research of Gulkesen et al. (2002) who proved that small testes or those that do not produce spermatozoa have decreased diameter of seminiferous tubules.
This study proved that in the positive control group was a decrease in the diameter of the seminiferous tubules than negative control. In contrast to the positive control group, there was a significant increase in T2 and T3 groups against positive controls. That improvement had been occurring because the activity of kebar grass extract at a dose 0.080 and 0.135mg/g BB/day, respectively. The results of the analysis of the chemical composition contained in kebar grass include crude fiber, ash content, fat, protein, carbohydrates, tannins, flavonoids, antioxidants, vitamin A, vitamin E, iron, calcium and phosphorus. The last two elements together with magnesium function in the formation of nucleoproteins which are responsible for cell formation and reproduction processes (Unitly and Inara, 2011).
Vitamin E that contained in kebar grass can inhibit the oxidation reaction by binding to the free radical, vitamin E formed in the process of breaking the free radical reaction could be re-functioned as an antioxidant (Pavlovic et al., 2005). Vitamin E can reduce TCDD-induced AhR activation so that it can be used as prevention and treatment of acute or chronic TCDD intoxication (Chang et al., 2009). This can be strengthened by research conducted by Wati et al. (2014) which proved that vitamin E has an antagonistic effect on TCDD toxicity in the process of spermatogenesis.
In this study, there was a significant decrease in the positive control group in thickness of the seminiferous tubular epithelium when compared to negative controls due to TCDD exposure. Whereas in T1, T2, and T3 there was a significant increase in thickness of the seminiferous tubular epithelium each group.This proves that the administration of kebar grass extract at a dose of 0.045mg/g BB/day in group T1 can significantly increase the thickness of the seminiferous tubular epithelium, but it has not been able to increase the diameter significantly. In the T2 and T3 groups there was a significant increase in epithelial thickness when compared with positive controls. The three treatment groups differed significantly with C- which proved that all three doses can maintain the thickness of the seminiferous tubular epithelium.
Kebar grass contains active ingredients of saponins, flavonoids, and tannins (Lefaan, 2014). Saponins included in the steroid group compound in an acidic stomach will break the sugar portion, so that it can have an effect for increasing the in free sterols. These sterol compounds are the basic ingredients of testosterone (pregnenolone) (Winarni, 2007). From this explanation it can be seen that saponins can increase testosterone levels which can accelerate the process of spermatogenesis so that it can increase the thickness of the seminiferous tubular epithelium.
CONCLUSION
Various doses of kebar grass extract in mice (Mus musculus) that exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can maintain the diameter of the seminiferous tubules but cannot maintain the thickness of the seminiferous tubular epithelium.
Acknowledgements
The authors express sincere thanks to the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for funding research and Dean Faculty of Veterinary Medicine for providing all necessary facilities and fund for conducting research work.
Conflict of Interest
Authors declare that they have no conflict of interest.
Authors Contribution
All authors contributed equally to the manuscript.
REFERENCES






