Advances in Animal and Veterinary Sciences
Research Article
Effects of Rutin and Quercetin on Doxorubicin-Induced Renocardiotoxicity in Male Wistar Rats
Heba Uallah R. Mahmoud, Osama M. Ahmed, Hanaa I. Fahim, Noha A. Ahmed*, Mohamed B. Ashour
Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, P.O. Box 62521, Beni-Suef, Egypt.
Abstract | This study aims to assess the preventive effects of rutin and/or quercetin on doxorubicin-induced kidney and heart injuries and oxidative stress in male Wistar rats. The male Wistar rats, injected with doxorubicin at an intraperitoneal dose 2 mg/kg b.w. 2 days per week for 5 weeks, were orally treated with rutin and/or quercetin with dose level 50 mg /kg b.w. every other day for 5weeks. The treatments of doxorubicin-injected rats with rutin, quercetin and their combination resulted in a significant decrease in the elevated serum creatinine and urea levels reflecting an improvement in kidney function. Similarly, the elevated serum CK-MB and LDH activities were significantly ameliorated as a result of treatments of doxorubicin-injected rats, thereby manifesting an amendment of heart function. The treatments also led to the prevention of the elevated lipid peroxidation and amelioration of the lowered GPx, GST and SOD activities as well as GSH content in kidney and heart as a result of treatment of doxorubicin-injected rats with rutin and quercetin. Also, the treatments remarkably improved doxorubicin-induced deleterious histological alterations in kidney and heart. In conclusion, rutin and/or quercetin may have chemopreventive potentials against doxorubicin-induced nephrocardiotoxicity via suppression of oxidative stress and enhancement of antioxidant defense system.
Keywords | Rutin, Quercetin, Doxorubicin, Kidney, Heart, Oxidative stress
Received | December 05, 2019; Accepted | February 17, 2020; Published | March 20, 2020
*Correspondence | Noha A. Ahmed, Physiology Division, Zoology Department, Faculty of Science, Beni-Suef University, Salah Salim St., 62514, Beni-Suef, Egypt. Email: [email protected], [email protected]
Citation | Mahmoud HUR, Ahmed OM, Fahim HI, Ahmed NA, Ashour MB (2020). Effects of rutin and quercetin on doxorubicin-induced renocardiotoxicity in male wistar rats. Adv. Anim. Vet. Sci. 8(4): 370-384.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.370.384
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Mahmoud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cancer chemotherapy is considered to be a major treatment at late stages to avoid the remission of cancer after surgery (Poornima et al., 2014). It is a typical methodology of treatment for several types of cancer (Sahin et al., 2010).
Doxorubicin is one of the most effective anticancer chemotherapeutic belonging to anthracycline antibiotic group and it has a large spectrum of activity (Coldwell et al., 2010). It is being used alone or in combination to treat a variety of tumors, both hematological and solid, such as breast cancer (Jabłonska-Trypuc et al., 2017). Despite its therapeutic value, doxorubicin has significant cardiotoxicity (Kuznetsova et al., 2011), hepatotoxicity (Patel et al., 2010) nephrotoxicity (Mohan et al., 2010) and testicular toxicity (Trivedi et al., 2011) which limit its clinical application. It also inhibits cell proliferation, induces oxidative stress, inhibits topoisomerase II, and finally leads to the cell death mainly through apoptosis (Ta et al., 2008; Sathesh et al., 2010; Cao et al., 2013). Although inflammation, apoptosis, mitochondrial DNA damage, impairment of calcium metabolism and excessive production of free radicals may all contribute to a variable extent to the deterioration of organ function, the exact mechanism of doxorubicin-mediated multiple organ toxicity remains largely unknown (Gharanei et al., 2014). Doxorubicin tends to accumulate in mitochondria, causing changes in their structure and function. However, the main cause of doxorubicin side effects observed in human and experimental animals is an extremely high level of oxidative stress within the cell, eventually causing apoptotic cell death (Hozayen et al., 2013; Taskin and Dursun, 2015; Yapislar et al., 2016).
Reactive oxygen species (ROS) are extremely harmful to organisms at high concentrations. Once the amount of ROS exceeds the defense mechanisms, a cell is claimed to be during a state of “oxidative stress”. The increased production of ROS throughout environmental stresses will cause a threat to cells by inflicting peroxidation of lipids, oxidization of proteins, harm to nucleic acids, enzymes inhibition, activation of programmed cell death (PCD) pathway and ultimately resulting in death of the cells (Mishra et al., 2011; Srivastava and Dubey, 2011; Mesquita and Leão, 2018). It was reported that oxidative stress may be implicated in doxorubicin-induced toxicity of different body organs including kidney and heart (Ahmed et al., 2017; Songbo et al., 2019; Ahmed et al., 2019). Thus, the use of antioxidant agents such as flavonoids in combination with doxorubicin may reduce or inhibits the doxorubicin-induced side effects.
Flavonoids and the other phenolic compounds are commonly known as plant secondary metabolites that hold an aromatic ring bearing at least one hydroxyl groups. More than 8000 phenolic compounds as naturally occurring substances from plants have been reported (Kumar and Pandey, 2013a; Ahmed et al., 2016). It is very interesting to note that half of these phenolic compounds are flavonoids presenting as aglycone, glycosides and methylated derivatives (Kumar and Pandey, 2013b). These phytochemical substances are presented in nutrients and herbal medicines, both flavonoids and many other phenolic components have been reported on their effective antioxidant, anticancer, antibacterial, cardioprotective, anti-inflammatory, immune system promoting and skin protective properties (Działo et al., 2016; Andreu et al., 2018; Meng et al., 2018).
Rutin (quercetin rutinoside) is considered to be a glycoside of the flavonoid quercetin. Rutin is used as a drug for protection of blood vessel and is an ingredient of various multivitamin preparations and herbal remedies. It can combine with cations, supplying nutrients from the soil to the cells in plants (Luo et al., 2008). In humans, it is a potent antioxidant where its actions include attaching to the iron ion Fe+2, preventing it from binding to hydrogen peroxide, which would otherwise create a highly-reactive free-radical that may damage cells (Patel et al., 2010). Rutin also shows anti-inflammatory activities in some animal and in vitro models (Guardia et al., 2001).
Quercetin may be a plant-derived aglycone form of flavonoid glycosides, has been used as a nutritionary supplement and should be useful against a lot of diseases. Some of the beneficial effects include cardiovascular protection, anticancer, antitumor, anti-ulcer, anti-allergy, anti-viral, anti-inflammatory, anti-diabetic, gastroprotective, antihypertensive, immunomodulatory and anti-infective properties (Lakhanpal and Rai, 2007). Epidemiological survey showed diet with more vegetables and fruits have protective effect against cancer. Quercetin has potential anticancer properties which include antiproliferative, growth factor suppression and antioxidant (Lamson and Brignall, 2000).
Therefore, this current study was conducted to assess the preventive and antioxidant effects of rutin and quercetin and their combination on doxorubicin-induced kidney and heart injuries in male Wistar rats.
MATERIALS AND METHODS
Experimental animals and housing
Fifty male Wistar rats weighing about 120-140 g (9-10 weeks old), were used as experimental animals in the present investigation. They were obtained from the Animal House of the National Research Center, Dokki, Giza, Egypt. Animals were acclimatized to laboratory conditions and under observation 10 days before the onset of the experiment to exclude any intercurrent infection. The animals were housed in polypropylene cages with good aerated stainless steel covers at normal atmospheric temperature (25±5ºC) and 12 hrs daily normal light-dark cycles. Rats were given access of water and supplied daily enough balanced standard diet ad libitum. All animal procedures are in accordance with the recommendations and guidelines of the Ethics Committee for Care and Use of Experimental Animals, Faculty of Science, Beni-Suef University, Egypt (Ethical Approval number: BSU/FS/2015/22). All efforts were done to minimize the pain, distress and discomfort.
Chemicals and drugs
The doxorubicin in the form adriamycin hydrochloride was obtained from Pharmacia Italia (Milan, Italy). Rutin and quercetin were obtained from Sigma Chemical Company (St. Louis, MO, USA). All other chemicals used for the investigation were ultra-pure and were commercially obtained.
Doses and treatment
The animals were treated with doxorubicin by an intraperitoneal route. The chosen dose was adjusted to 2 mg/1ml saline (0.9% NaCl)/kg b.w. (Mandziuk et al., 2015) and was given 2 times per week for five weeks. Rutin and quercetin doses were adjusted to 50 mg/kg b.w (Suganya and Sumathi, 2016; Bo et al., 2018) and were orally given every other day for five weeks by oral gavage for five weeks. They were dissolved in 1% carboxymethyl cellulose (1% CMC) at concentration 50 mg rutin or quercetin/5 ml 1% CMC.
Animal grouping
The adult male Wistar rats used in the present experiment were divided into five groups (10 rats for each).
Group 1 (Normal group): Rats of this group were intraperitoneally injected with the equivalent volume of saline (1 ml/kg b.w.) every other day for five weeks. This group was also orally administered the equivalent volume of 1% CMC (5 ml/kg b.w.) every other day for five weeks.
Group 2 (Doxorubicin-injected control group): Rats of this group were intraperitoneally injected with doxorubicin at dose level 2 mg/kg b.w. (Mandziuk et al., 2015) 2 days per week for five weeks. This group was also orally administered the equivalent volume of 1% CMC (5 ml/kg b.w.) every other day for five weeks.
Group 3 (Doxorubicin-injected group treated with rutin): This group was intraperitoneally injected with doxorubicin like doxorubicin-injected control group and was orally treated (by oral gavage) with rutin at dose level of 50 mg /kg b.w. (Suganya and Sumathi, 2016) every other day for five weeks.
Group 4 (Doxorubicin-injected group treated with quercetin): Rats of this group were intraperitoneally injected with doxorubicin like doxorubicin-injected control group and were also orally treated with quercetin at dose level 50 mg/kg b.w. (Bo et al., 2018) every other day for five weeks.
Group 5 (Doxorubicin-injected group treated with rutin and quercetin): Rats of this group were intraperitoneally injected with doxorubicin like doxorubicin-injected control group and were also orally treated with mixture of rutin and quercetin at dose level 50 mg/kg b.w. every other day for five weeks.
Blood and tissue sampling
At the end of the experiment, rats were anesthetized by a diethyl ether inhalation anesthesia and blood sample from each rat was collected from jugular vein into centrifuge tube. The blood samples were left to clot at room temperature for 45 minutes and then centrifuged at 3000 r.p.m. for 15 minutes. The clear, non-heamolysed supernatant sera were quickly removed, divided into four portions for each individual animal and kept at -30ºC for various biochemical analyses. After sacrifice and dissection, one kidney and half of heart were removed quickly, separately homogenized by using Telfon homogenizer (Glas-Col, Terre Haute, USA), in isotonic solution (0.9% NaCl) at 10% concentration (w/v). The resultant homogenates were centrifuged at 3000 r.p.m. and the homogenate supernatants were aspirated and divided into three portions, and then kept in deep freezer at -30oC till used for determination of oxidative stress and antioxidant defense markers.
Biochemical investigations
Serum creatinine and urea levels were determined by kinetic method using kits obtained from Diamond Diagnostics (Egypt) according to Young (2001). CK-MB activity in serum was determined according to method of Young (1995) using reagent kits purchased from Spinreact (Spain). LDH activity in serum was determined according to method of Young (1990) using reagent kits purchased from Spectrum, Egypt. Kidney and heart malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and superoxide dismutase (SOD) levels were determined according to the methods of Preuss et al. (1998), Beutler et al. (1963), Matkovics et al. (1998), Mannervik and Gutenberg (1981), and Marklund and Marklund (1974), respectively.
Histopathological investigations
For histopathological examination, one half of kidney and half of heart from each animal were fixed in 10% neutral buffered formalin and transferred to the Pathology Department, National Cancer Institute, Cairo university, Egypt for processing, sectioning and staining with hematoxylin and eosin (H and E) according to the method of Banchroft et al. (1996).
Statistical analysis
Results were expressed as mean ± standard error (SE). One-way ANOVA followed by LSD test were applied for data analysis using PC-STAT program (Rao et al., 1985). F-probability of ANOVA results expresses the general effect between groups at p<0.05, p<0.01 and p<0.001. For each parameter, LSD test was used to compare various groups with each other’s at p<0.05 and p<0.01.
RESULTS
Biochemical effects
The data describing the effect of rutin and/or quercetin on serum creatinine and urea levels in doxorubicin-administered rats are represented in Table 1. Serum creatinine and urea levels exhibited a highly significant elevation (p<0.01; LSD) in doxorubicin-injected control rats; the recorded percentage changes were 21.05% and 82.99% respectively as compared to normal control. The treatment of doxorubicin-injected rats with rutin and/or quercetin produced a highly significant decrease (p<0.01; LSD) in serum creatinine and urea levels. Quercetin was the most potent in decreasing the elevated creatinine and urea levels recording percentage decreases of 33.33% and 46.32% respectively (Table 1).
The data elucidating the effect of rutin and/or quercetin on serum CK-MB and LDH activities are represented in Table 2. Doxorubicin injection induced a significant increase (p<0.05; LSD) and a highly significant increase (p<0.01; LSD) in serum CK-MB and LDH activities recording percentage changes 19.29% and 33.20% respectively. On the other hand, the administration of rutin, quercetin and their combination to doxorubicin-injected rats produced a highly significant decrease (p<0.01; LSD) in serum CK-MB and LDH activities. It seemed that quercetin was the most potent in decreasing the elevated CK-MB and LDH activities recording percentages decreases of 27.60% and 35.63% respectively.
The data depicting the effect of rutin and/or quercetin on GSH content in kidney and heart are represented in Table 3. In the kidney, the treatment with quercetin produced a highly significant increase (P<0.01; LSD) recording percentage change of 48.25% while the treatment with rutin alone and with its combination with quercetin induced a non-significant increase (LSD; P>0.05) of GSH content recording percentage changes of 24.71% and 25.29% respectively. In heart, doxorubicin-injected group treated with quercetin and combination of rutin and quercetin caused a non-significant increase of GSH content (P>0.05) recording percentage increases of 9.18% and 8.17% respectively while the treatment with rutin alone induced produced a highly significant increase (P<0.01; LSD).
Data showing the effect of rutin and/or quercetin on kidney and heart LPO in doxorubicin-injected rats are represented in Table 4. The administration of doxorubicin to Wistar rats caused a highly significant elevation (P<0.01; LSD) of LPO in kidney and heart; the percentage changes marked an increase of 71.60%and 64.31% respectively as compared to normal rats. The kidney LPO exhibited a highly significant decrease (P<0.01; LSD) in doxorubicin-injected rats treated with rutin, quercetin and their combination recording percentage changes of -37.96%, -36.25% and -26.50% respectively. Similarly, doxorubicin-injected rats treated with rutin, quercetin and their combination exhibited a highly significant decrease in heart LPO; rutin is considered the most effective in decreasing the elevated LPO recording percentage amelioration of 52.32%.
Data describing the effect of rutin and/or quercetin on serum GPx activity in doxorubicin-injected rats are represented in Table 5. The administration of doxorubicin to Wistar rats caused a non-significant effect on kidney GPx activity, but it produced a highly significant decrease (P<0.01; LSD) in heart GPx activity recording percentage change of 9.41% as compared with normal control.
The treatment of doxorubicin-injected rats with rutin produced a highly significant increase (P<0.01; LSD) in kidney GPx activity recording percentage change of 7.51% while the treatment with quercetin and its combination with rutin induced a non-significant effect (P>0.05; LSD). On other hand, the treatment with rutin, quercetin and their combination all have no significant effect on heart GPx activity (P>0.05; LSD) as compared with doxorubicin-injected control rats.
Data elucidating the effect of rutin and/or quercetin on serum of GST activities in doxorubicin-injected rats are represented in Table 6. The administration of doxorubicin to Wistar rats caused a highly significant decrease (P<0.01; LSD) kidney and heart GST activity; the recorded percentage changes were -41.05% and -34.50% respectively. The treatment doxorubicin-injected rats with rutin induced a non-significant effect (P>0.05; LSD) on heart GST activity and the recorded percentage change 15.86% and produced a highly significant increase (P<0.01; LSD) in kidney GST activity with a recorded percentage change 39.11%. On the other hand, the treatment with quercetin induced a highly significant increase (P<0.01; LSD) in kidney and heart GST activity and the recorded percentage changes were 70.23% and 49.70% respectively. The treatment with a combination of rutin and/or quercetin induced a highly significant increase (P<0.01; LSD) in kidney and heart GST activity and the recorded percentage changes were 55.13% and 57.50% respectively.
Data describing the effect of rutin and/or quercetin on serum of SOD activity in doxorubicin-injected rats are represented in Table 7. The administration of doxorubicin to Wistar rats caused a highly significant decrease (P<0.01; LSD) in heart SOD activity; the recorded percentage was -5.45% while it produced a non-significant effect (P>0.05; LSD) on kidney SOD activity as compared with normal control. The treatment of doxorubicin-injected rats with rutin induced a highly significant increase (P<0.01; LSD) in kidney SOD activity recording percentage change of 17.62%, but it produced a non-significant increase (P>0.05; LSD) in heart SOD activity. On the other hand, the treatment with quercetin induced a highly significant increase (P<0.01; LSD) in heart SOD activity recording percentage change of 11.24% while it caused a non-significant increase (P>0.05; LSD) in kidney SOD activity. The treatment with combination of rutin and quercetin induced a highly significant increase (P<0.01; LSD) in kidney and heart SOD activity; the recorded percentage changes were 18.67% and 16.21% respectively as compared to doxorubicin-injected control rats.
Table 1: Effect of rutin and quercetin on serum parameters related to kidney functions in doxorubicin-injected rats.
| Parameter group | Creatinine (mg/dL) | %Change | Urea (mg/dL) | %Change |
| Normal control |
0.57± 0.01 b |
37.16 ±1.68b |
||
| Doxorubicin-injected control group |
0.69 ±0.01a |
21.05% |
68.00 ±8.62a |
82.99% |
| Doxorubicin-injected group treated with rutin |
0.56 ± 0.01b |
-18.84% |
47.31 ±2.48b |
-30.43% |
| Doxorubicin-injected group treated with quercetin |
0.46 ±0.02c |
-33.33% |
36.50 ±5.68b |
-46.32% |
| Doxorubicin-injected group treated with rutin and quercetin |
0.50 ±0.03bc |
-27.53% |
44.00 ±3.94b |
-35.29% |
| F-probability |
|
P< 0.001 |
|
P< 0.01 |
| LSD at the 5% level |
|
0.082 |
|
14.932 |
| LSD at the 1% level |
|
0.110 |
|
20.202 |
Data are expressed as mean± standard error. Number of detected samples in each group is six. Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
Table 2: Effect of rutin and quercetin in serum parameters related to heart functions in doxorubicin-injected rats.
| Parameter group | CK-MB ( U/L) | % Change | LDH (U/L) | % Change |
| Normal control |
66.00 ±2.68b |
1559.00 ±64.76b |
||
| Doxorubicin-injected control group |
78.73 ±3.27a |
19.29% |
2076.63 ±117.54a |
33.20% |
| Doxorubicin-injected group treated with rutin |
65.50 ±1.67b |
-16.80% |
1446.75 ±30.27bc |
-30.33% |
| Doxorubicin-injected group treated with quercetin |
57.00 ±3.87b |
-27.60% |
1336.63 ±33.25c |
-35.63% |
| Doxorubicin-injected group treated with rutin and quercetin |
57.50 ±4.09b |
-26.96% |
1500.12 ±32.69bc |
-27.76% |
| F-probability |
|
P<0.001 | P<0.001 | |
| LSD at the 5% level |
|
9.435 | 189.254 | |
| LSD at the 1% level |
|
12.765 | 256.045 | |
Data are expressed as mean± standard error. Number of detected samples in each group is six. Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
Table 3: Effect of rutin and quercetin on kidney and heart GSH contents in doxorubicin-injected rats.
| Parameter group | Kidney GSH (nmole/100 mg tissue) | % Change | Heart GSH (nmole/100 mg tissue) | % Change |
| Normal control |
117.30 ± 7.25a |
73.98 ±3.25a |
||
| Doxorubicin-injected control group |
64.98 ± 5.52c |
-44.60% |
56.54 ±2.01b |
-23.57% |
| Doxorubicin-injected group treated with rutin |
81.04 ±5.09bc |
24.71% |
71.66 ±2.93a |
26.73% |
| Doxorubicin-injected group treated with quercetin |
96.34 ± 4.27b |
48.25% |
61.73 ±2.04b |
9.18% |
| Doxorubicin-injected group treated with rutin and quercetin |
81.42 ±7.57bc |
25.29% |
61.17 ±2.51b |
8.17% |
| F-probability |
|
P<0.001 |
|
P<0.001 |
| LSD at the 5% level |
|
17.702 |
|
7.572 |
| LSD at the 1% level |
|
23.949 |
|
10.245 |
Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
Histological effects
Histological changes in kidney of normal control, doxorubicin-injected rats, doxorubicin-injected rats treated with rutin, quercetin and their combination were depicted in Figures 1a to 5d. Control group given 10% vehicle showed normal kidney architecture (Figure 1a, 1b) comprised of normal structure of glomerulus, proximal convoluted tubules, with their brush border and distal convoluted tubules.
On the other hand, the kidney of doxorubicin-injected rats exhibited marked changes like dilation and congestion of renal blood vessel (Figure 2a), vacuolation of endothelial lining glomerular tuft and perivascular oedema (Figure 2b) and showed peritubular mononuclear inflammatory cells infiltration (Figure 2c). Kidney sections of doxorubicin-administered rats treated with rutin showed focal mononuclear inflammatory cells infilteration (Figure 3a) and showed no hisopathological changes (Figure 3b and 3c).
Table 4: Effect of rutin and quercetin on kidney and heart LPO in doxorubicin-administered rats.
| Parameter group | Kidney LPO (nmole MDA/ 100 mg tissue/ hr) | % Change | Heart LPO (nmole MDA/100 mg tissue/ hr) | % Change |
| Normal control |
17.36 ±1.52c |
15.97 ±1.06bc |
||
| Doxorubicin-injected control group |
29.79 ±0.94a |
71.60% |
26.24 ±1.60a |
64.31% |
| Doxorubicin-injected group treated with rutin |
18.48 ±0.48bc |
-37.96% |
12.51 ±1.58c |
-52.32% |
| Doxorubicin-injected group treated with quercetin |
18.99 ±0.35bc |
-36.25% |
18.86 ±0.62b |
-28.12% |
| Doxorubicin-injected group treated with rutin and quercetin |
21.90 ±1.83b |
-26.50% |
17.38 ±0.96b |
-33.76% |
| F-probability | P<0.001 | P<0.001 | ||
| LSD at the 5% level | 3.434 | 3.579 | ||
| LSD at the 1% level | 4.646 | 4.842 | ||
Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
Table 5: Effect of rutin and quercetin on kidney and heart GPx activity in doxorubicin-injected rats.
| Parameter group | Kidney GPx(mU/100 mg tissue) | % Change | Heart GPx (mU/100 mg tissue) | % Change |
| Normal control |
130.81 ±1.94b |
137.99 ±1.72a |
||
| Doxorubicin-injected control group |
133.16 ±1.26b |
1.79% |
125.00 ±1.30bc |
-9.41% |
| Doxorubicin-injected group treated with rutin |
143.17 ±1.87a |
7.51% |
127.14 ±2.27bc |
1.71% |
| Doxorubicin-injected group treated with quercetin |
134.04 ±1.61b |
0.66% |
122.24 ±2.61c |
-2.21% |
| Doxorubicin-injected group treated with rutin and quercetin |
135.69 ±1.84b |
1.89% |
131.67 ±4.18ab |
5.33% |
| F-probability |
|
P< 0.001 |
|
P< 0.01 |
| LSD at the 5% level |
|
5.034 |
|
7.620 |
| LSD at the 1% level |
|
6.810 |
|
10.309 |
Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
Table 6: Effect of rutin and quercetin on kidney and heart GST activity in doxorubicin-administered rats.
| Parameter group | Kidney GST (mU/100 mg tissue) | % Change | Heart GST (mU/100 mg tissue) | % Change |
| Normal control |
106.54 ±3.28a |
97.20 ±2.89a |
||
| Doxorubicin-injected control group |
62.80 ±4.39c |
-41.05% |
54.54 ±4.14c |
-43.88% |
| Doxorubicin-injected group treated with rutin |
87.36 ±3.37b |
39.11% |
71.19 ±7.08bc |
30.50% |
| Doxorubicin-injected group treated with quercetin |
106.91 ±8.92a |
70.23% |
81.66 ±7.27ab |
49.70% |
| Doxorubicin-injected group treated with rutin and quercetin |
97.42 ±9.02ab |
55.13% |
92.31 ±10.16a |
69.22% |
| F-probability |
|
P<0.001 |
|
P<0.01 |
| LSD at the 5% level |
|
18.550 |
|
19.845 |
| LSD at the 1% level |
|
25.097 |
|
26.848 |
Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
The treatment of doxorubicin-injected rats with quercetin produced congestion of renal intertubular blood vessels (Figure 4a), vacuolation of endothelial lining glomerular tuft (Figure 4b) and showed no hisopathological changes (Figure 4c). The treatment of doxorubicin-administered rats with a combination of rutin and quercetin exhibited more improvement of the kidney histological changes as compared with doxorubicin-injected control. It exhibited no histopathological changes (Figure 5a, 5b, and 5c) and showed congestion of renal blood vessels (Figure 5d).
Table 7: Effect of rutin and quercetin on kidney and heart SOD activity in doxorubicin-injected rats.
| Parameter group | Kidney SOD (mU/100 mg tissue) | % Change | Heart SOD (mU/100 mg tissue) | % Change |
| Normal control |
123.94 ±3.19b |
133.32 ±2.25a |
||
| Doxorubicin-injected control group |
117.19 ±2.84b |
-5.45% |
112.35 ±2.52c |
-15.73% |
| Doxorubicin-injected group treated with rutin |
137.84 ±2.97a |
17.62% |
119.26 ±1.52bc |
6.15% |
| Doxorubicin-injected group treated with quercetin |
124.44±4.51b |
5.81% |
124.98 ±3.18ab |
11.24% |
| Doxorubicin-injected group treated with rutin and quercetin |
139.07 ±6.41a |
18.67% |
130.56 ±6.56a |
16.21% |
| F-probability |
|
P<0.01 |
|
P<0.001 |
| LSD at the 5% level |
|
12.262 |
|
10.656 |
| LSD at the 1% level |
|
16.589 |
|
14.417 |
Means, which have the same superscript symbol(s), are not significant different. Percentage changes were calculated by comparing doxorubicin-injected control group with normal control and doxorubicin-injected groups treated with rutin and/or quercetin with doxorubicin-injected control group.
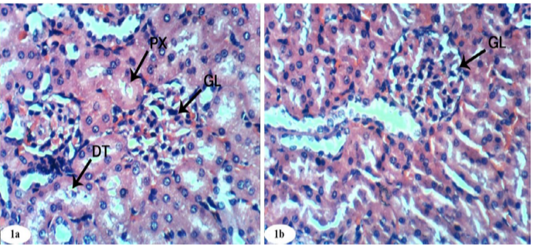
Figure 1: Photomicrographs of normal kidney sections showing glomeruli (GL), proximal convoluted tubule (PX), and distal convoluted tubules (DT) (Photomicrographs 1a and 1b). (H and E X 400).
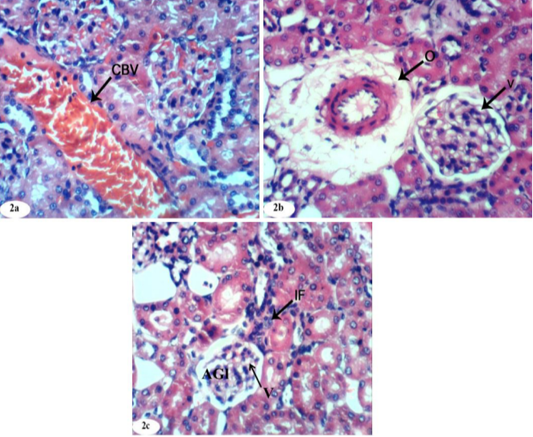
Figure 2: Photomicrographs of kidney sections of doxorubicin-injected group showing dilation and congestion (CBV) of renal blood vessel (Photomicrographs 2a), vacuolation (V) of endothelial lining glomerular tuft, perivascular oedema (O) (Photomicrograph 2b), peritubular mononuclear inflammatory cells infiltration (IF) and atrophied glomeruli (Photomicrograph 2c). (H and E X 400).
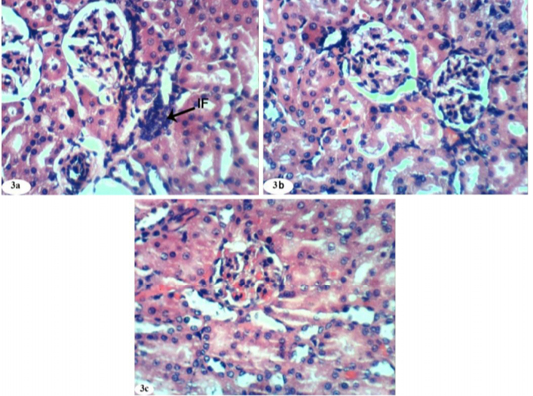
Figure 3: Photomicrographs of kidney sections of doxorubicin-injected group treated with rutin showing mild focal mononuclear inflammatory cells infiltration (IF) (Photomicrograph 3a), improvement and nearly normal structure of the renal tissue (Photomicrographs 3b and 3c). (H and E X 400).
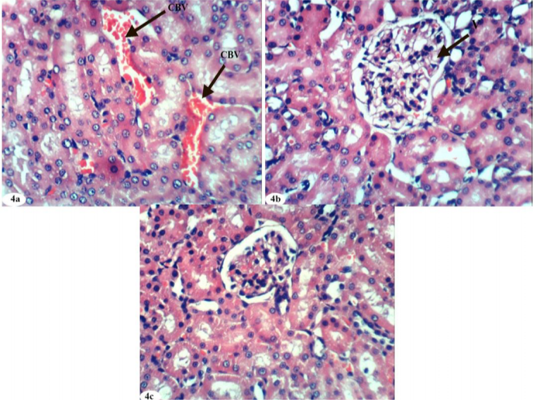
Figure 4: Photomicrographs of kidney sections of doxorubicin-injected group treated with quercetin showing mild congestion (CBV) of intertubular blood vessels (Photomicrograph 4a), vacuolation (V) of endothelial lining glomerular tuft (Photomicrograph 4b) and nearly normal structure of the renal tissue (Photomicrograph 4c). (H and E X 400).
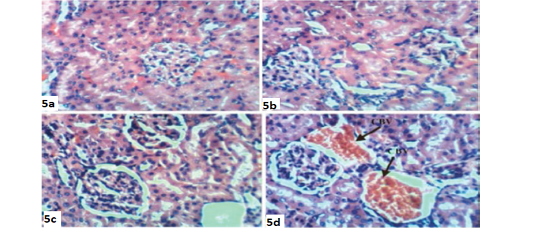
Figure 5: Photomicrographs of kidney sections of doxorubicin-injected group treated with combination of rutin and quercetin showing improvement and nearly normal structure of the renal tissue (Photomicrographs 5a,5b and 5c) and congestion (CBV) of renal blood vessels (Photomicrograph 5d). (H and E X 400).
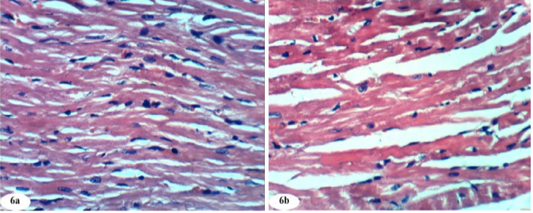
Figure 6: Photomicrographs of normal heart section showed normal histological architecture (photomicrographs 6a and 6b). (H and E X 400).
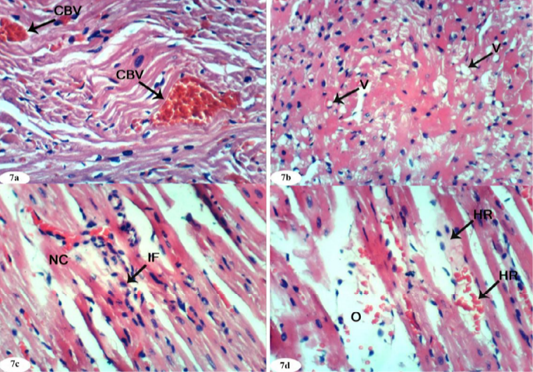
Figure 7: Photomicrographs of heart sections of doxorubicin-injected group showing congestion (CBV) of myocardial blood vessels (Photomicrograph 7a), vacuolization (V) of sarcoplasm of cardiac myocytes (Photomicrograph 7b), focal necrosis (NC) of cardiac myocytes, inflammatory cells’ infiltration (Photomicrograph 7c), intermuscular oedema (O) and haemorrhage (HR) (Photomicrograph 7d). (H and E X 400).
The histological changes in heart of normal control, doxorubicin-injected rats and doxorubicin-injected rats treated with rutin, quercetin and their combination were depicted in Figures 6a to 10b. Normal control group given the equivalent volumes of vehicles showed normal heart architecture (Figure 6a and 6b) with normal cardiac myocytes.
On the other hand, the heart of doxorubicin-injected rats exhibited marked changes like congestion of myocardial blood vessels (Figure 7a), vacuolation of sarcoplasm of cardiac myocytes (Figure 7b), focal necrosis of cardiac myocytes, inflammatory cells’ infiltration (Figure 7c) and intermuscular oedema and haemorrahage (Figure 7d).
Heart sections of doxorubicin-injected rats treated with rutin showed vacuolation of sarcoplasm of cardiac myocytes (Figure 8a) and no histopathological changes (Figure 8b). The treatment of doxorubicin-administered rats with quercetin produced no histopathological changes (Figure 9). Also, the treatment of doxorubicin-injected rats with a combination of rutin and quercetin showed no histopathological changes (Figure 10a and 10b).
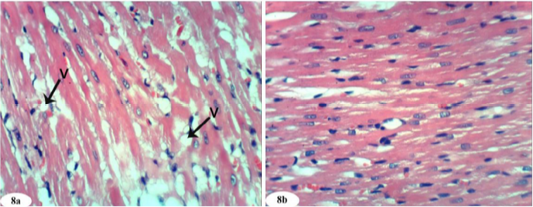
Figure 8: Photomicrographs of heart sections of doxorubicin-injected group treated with rutin showing vacuolization (V) of sarcoplasm of cardiac myocytes (Photomicrograph 8a) and improvement of the tissue in some animals (photomicrograph 8b). (H and E X 400).
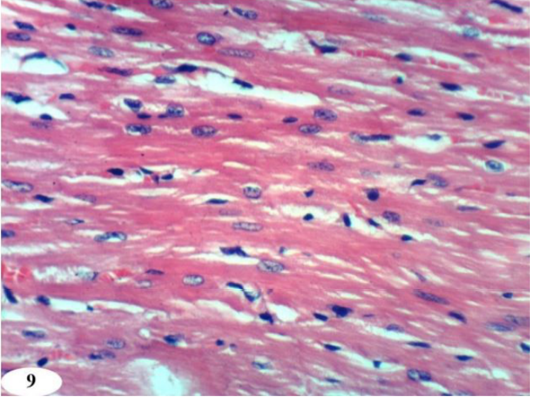
Figure 9: Photomicrographs of heart sections of doxorubicin-injected group treated with quercetin showing an improvement of the kidney. (H and E X 400).
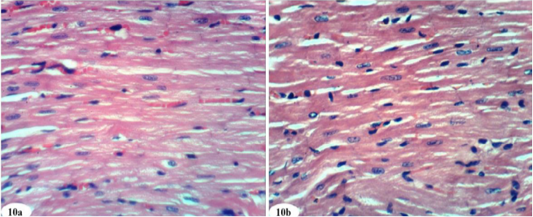
Figure 10: Photomicrographs of heart sections of doxorubicin-injected group treated with mixture of rutin and quercetin showing an improvement of the kidney. (Photomicrographs 10a and 10b). (H and E X 400).
DISCUSSION
Doxorubicin, a quinone-containing anthracycline antibiotic, is an important agent against a wide spectrum of human neoplasms. However, its toxicity limits usage in cancer chemotherapy (Lv et al., 2013; Xin et al., 2015; Ahmed et al., 2019a). In addition, resistance to chemotherapeutic agents is one of the major disadvantages of long-term anticancer treatment. Repeated doxorubicin administration leads to drug-resistant cancer cells and increased cytotoxicity (Ahmed et al., 2019a). It has been shown that free radicals are involved in doxorubicin-induced toxicities (Ferreira et al., 2017). It has been revealed that doxorubicin caused severe injury in some organs like liver, heart and kidneys (Gokcimen et al., 2007). The chemical structure of doxorubicin causes the generation of free radicals and the induction of oxidative stress that result in cellular injury (Saad et al., 2001; Karaman et al., 2006). Doxorubicin tends to accumulate in mitochondria, causing changes in their structure and function. However, the main cause of doxorubicin side effects observed in human is an extremely high level of oxidative stress within the cell, eventually causing apoptotic cell death (Taskin and Dursun, 2015; Yapislar et al., 2016). The increased production of mitochondrial ROS and reactive nitrogen species (RNS) induced by doxorubicin leads to an excessive oxidative stress and is strongly linked to cell damage involving reduced protein synthesis and redox modifications of macromolecules (proteins, lipids, and DNA) such as nitrotyrosine formation, protein carbonylation, and LPO which increase in doxorubicin-exposed cardiac muscle and include cellular membrane damage (Cappetta et al., 2017).
Doxorubicin may undergo a one-electron reduction through a metabolic activation by nicotinamide adenine dinucleotide phosphate (NADPH)-cytochrome P-450 reductase. This reduction leads to the formation of the free radical semiquinone, which in turn can produce a variety of active ROS/RNS, including hydrogen peroxide (H2O2), hydroxyl radical (•OH) and peroxynitrite (ONOO) (Takemura and Fujiwara, 2007; Ahmed, 2013). These species can attack the cell membrane, damage several macromolecular cellular components, cause protein and LPO and consequently lead to apoptosis or death (Shiromwar and Chidrawar, 2011; Ayla et al., 2011). Doxorubicin cytotoxicity and genotoxicity may be mediated by free radicals derived from this drug and its capability to induce apoptosis through a wide variety of mechanisms including production of ROS, alkylation of cellular macromolecules, DNA intercalation and cross-linking, LPO, cell membrane damage, ceramide production and p53 induction in various tissues (Quiles et al., 2002; Lorenzo et al., 2002). Studies have proposed that oxidative stress, inflammation, and apoptosis are the main players that control doxorubicin pathogenesis (Liu et al., 2016).
The former approach of modulating the drug dosage was put forward, aiming to diminish the significant effects of oxidative stress, which is the major cause of cardiotoxicity, but subsequent studies revealed that minimizing or eliminating ROS did not solve the detrimental effects (Chio and Tuveson, 2017).
The present study revealed the effect of intraperitoneal injection of doxorubicin at dose level of 2 mg/kg b. w. 2 days/week, for 5 weeks on kidney and heart. Rutin and quercetin and their combination were given at dose level of 50 mg/kg b. w. were orally co-administered every other day to rats injected with doxorubicin to assess whether rutin or quercetin can prevent the side effects of doxorubicin on kidney and heart or not. The current results are in accordance with Injac et al. (2008), Abou Seif (2012), El-Moselhy and ElSheikh (2014) and Jacevic et al. (2018) who stated that doxorubicin-induced nephrotoxicity may be due to the finding that doxorubicin administration increased capillary permeability and glomerular atrophy. This attribution was supported by the histological findings of the present study that indicated the presence of atrophied glomeruli in association with marked inflammatory leucocytes’ infiltration, necrosis and vacuolation of endothelial lining glomerular tuft.
As regards the heart, the present study showed that doxorubicin-induced a cardiotoxicity manifested biochemically by a significant increase in serum CK-MB and LDH activities. These results are in agreement with Sahu et al. (2016), Francis and Nayak (2017) and Kim et al. (2017) and also are consistent with the previous study of Yagmurca et al. (2004) who are supporting doxorubicin-inducing cardiotoxicity in normal rats. It could be suggested that increased cardiac enzymes activities in serum might be due to an increase in their release and leakage from cytoplasm of cardiomyocytes to the plasma, following doxorubicin-induced damage of membranes. The elevation of the enzymes related to heart function in the present investigation is concomitant with histological deteriorations which include marked necrosis of cardiomyocytes and inflammatory leukocytic infiltration in heart tissue. In addition the heart sections showed congestion of myocardial blood vessels, vacuolization of sarcoplasm of cardiac myocytes, intermuscular oedema and haemorrhage.
The previous deleterious biochemical and histological alterations in the present study of doxorubicin-administered rats are associated with a marked elevation of kidney and heart LPO and a significant decrease of non-enzymatic antioxidant (GSH) content and enzymatic antioxidants including GPx, SOD, and GST enzyme activities. These results are in agreement with many other authors including Abd El-Aziz et al. (2001), Kalender et al. (2005), Yagmurca et al. (2007), Hozayen et al. (2014) and QuanJun et al. (2017) who stated that one of the most prevailing hypotheses of cellular damage from doxorubicin administration is the ability of the drug to produce ROS and suppress antioxidant defense system. They also revealed that the increased LPO play a critical role in in kidney and heart injuries. In the same line, Yeh et al. (2009), Elberry et al. (2010) and Kim et al. (2017) reported that rats injected with doxorubicin showed an increase in the level of kidney and heart LPO and depressed antioxidants such as GSH content and GPx activity. Furthermore, Khan et al. (2005) reported that doxorubicin might cause excessive consumption, reduced production or chemical deactivation of the antioxidant enzymes. The putative role of oxidative stress in the induction of doxorubicin nephritis may be supported by the protective effect of the activation of SOD on the development of doxorubicin nephropathy (Okasora et al., 1992).
Doxorubicin-induced renal toxicity due to oxidative stress was elucidated by Yilmaz et al. (2006) and Mohan et al. (2010). According to these publications, doxorubicin-induced severe nephrotic syndrome with massive albuminuria, proteinuria, hyperlipidemia, hypo-albuminemia and hypo-proteinemia associated with a marked suppression in the kidney antioxidant defense and activation of oxidative stress manifested by the increase in kidney LPO and a decrease of kidney GSH content and GPx, GST, SOD activities. The changes reflect many functional alterations such as a drop in glomerular filtration rate, glomerular capillary damage and tubular toxicity as well as approved doxorubicin-induced pathogenesis of nephropathy through involvement of free radicals (Soliman et al., 2014; Badary et al., 2000).
That is in agreement with Ganeshpurkar and Saluja (2017) who stated that the increased toxicity of doxorubicin in liver and heart may be due to its ability to deplete the GST. The doxorubicin treatment has actually reduced the activity of GST in a dose dependent manner. They declared also that doxorubicin treatment has been earlier reported to increase the LPO in rat and mice. This evidence was reported also by Papasani et al. (2014), Jagetia and Venkatesh (2017), Jagetia and Venkatesh (2015) and Kwatra et al. (2016).
In the present study, the development of oxidative cardiac injury due to multiple doses of doxorubicin was confirmed by the myocardial cell damage, the significant increase in LPO as well as the significant decrease in GSH, GPx, GST, and SOD activities in the heart tissue. These changes in the antioxidant parameters are in accordance with the reports Saad et al. (2001) and Paweorn et al. (2015).
Histopathological examination in this study of heart and kidney sections of doxorubicin-administered animals supported the obtained biochemical results. The significant increase in serum creatinine and urea levels in doxorubicin-administered rats was consistent with the doxorubicin-induced deleterious changes in the kidney which exhibited glomerular atrophy and vacuolization of renal glomeruli reflecting the nephrotoxic effects of doxorubicin (Hahn et al., 2004). In the heart, myocardial necrosis, vascular congestion, as well inflammatory cells infiltration. Similar observations have also been revealed in earlier studies on doxorubicin-induced cardiotoxicity (Saad et al., 2001). The myocardial necrosis and damage resulted in an increased leakage of cytoplasmic enzymes CK-MB and LDH into plasma.
Polyphenolic compounds are usually found in each edible and inedible plant, and that they are reported to possess multiple biological effects, as well as antioxidant activity. Flavonoids act as antioxidants by neutralizing oxidizing free radicals, including the superoxide and hydroxyl radicals. The redox properties of flavonoids also allow them to act as reducing agents and presence of flavonoids and phenolic compounds have been recognized as excellent scavengers of superoxide, hydroxyl ion and peroxyl radicals by inhibiting LPO. Orange contains phenols and flavonoids which can directly or indirectly reduce oxidative damage by preventing the excessive generation of free radicals (Abdel Moneim, 2014).
There are numerous studies showing that rutin decreases doxorubicin-induced cardiotoxicity. In this regard, Yang et al. (2017) stated that suppression of autophagy and apoptosis by administration of rutin could attenuate doxorubicin-induced cardiotoxicity.
In our investigation, the elevated creatinine and urea levels in doxorubicin-injected rats significantly decreased as a result of treatment with rutin and quercetin and that are in agreement with previous studies (Nazmi et al., 2016; Jambhulkar et al., 2014).
The rutin protective effect against nephrotoxicity can be attributed to its antioxidant and anti-inflammatory effect on ROS and some cytokines may be involved in the glomerular filtration rate damage (Cuzzocrea et al., 2002). This was accompanied by the improvement in serum creatinine, urea and uric acid levels and was associated with a decrease of LPO and an increase in GSH in the kidney. The attenuation of glutathione depleting effects of doxorubicin by quercetin in blood, heart and kidney tissues reveals the antioxidant effects produced by this flavonoid. The reduction (P< 0.01) in levels of MDA in kidney tissues of doxorubicin-injected rats pretreated with quercetin suggests the anti-lipid peroxidative property of test drug (Nazmi et al., 2016). Therefore, rutin and/or quercetin can protect the kidney from doxorubicin-induced injury via improvement in oxidant status.
In the present study, rutin and quercetin also reduced the elevated activities of serum enzymes related to the heart function including CK-MB and LDH in doxorubicin-injected rats. These findings are in agreement with Nazmi et al. (2016) who indicated that the pretreatment with quercetin resulted in significant reduction in the activities of AST and LDH toward near normal as compared with toxic control rats. This result could be due to protective effects of quercetin on the myocardium; thereby restricting the leakage of AST and LDH. These results were accompanied with decrease in heart LPO and elevation in GSH content as well as increase in GPx, GST and SOD activities. Thus, it can be suggested that the suppression of LPO and oxidative stress may be implicated in the improvements of the heart function and histological integrity as a result of treatment of doxorubicin-administered rats with rutin and quercetin.
Histopathological examination in this study of kidney and heart sections of doxorubicin-administered animals treated with rutin supports the above mentioned biochemical results. Kidney sections of doxorubicin-injected rats treated with rutin showed mild focal mononuclear inflammatory cells’ infiltration in some animals and showed no hisopathological changes in others. The treatment of doxorubicin-administered rats with quercetin produced mild congestion of renal intertubular blood vessels, vacuolation of endothelial lining glomerular tuft in some animals and showed no hisopathological changes in others. The treatment of doxorubicin-administered rats with a combination of rutin and quercetin exhibited more improvement of the kidney histological changes as compared with doxorubicin-administered control. It exhibited no histopathological changes. Heart sections of doxorubicin-administered rats treated with rutin showed vacuolation of sarcoplasm of cardiac myocytes in some animals and no histopathological changes in others. The treatment of doxorubicin-injected rats with quercetin or its combination with rutin produced no histopathological changes.
CONCLUSION
Both rutin and/or quercetin successfully prevented doxorubicin-induced kidney and heart injuries manifested by improvement in the function and histological integrity of kidney and heart. These preventive effects may be mediated via suppression of oxidative stress and inflammation as well as enhancement of the antioxidant defense system.
ACKNOWLEDGEMENT
The authors deeply thank Prof. Dr. Kawkab A. Ahmed, Professor of Histopathology, Pathology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt for help in examining and identifying lesions in the histological sections.
Authors Contribution
Heba Uallah R. Mahmoud performed the experimental work and participated in writing the draft of the manuscript; Osama M. Ahmed proposed the research plan, guided the experimental work and shared in writing and revising the manuscript. Hanaa I. Fahim revised the manuscript and also supervised the experimental work; Noha A. Ahmed participated in following up the experimental work and shared in writing and revising the manuscript; and Mohamed B. Ashour revised the manuscript and also supervised the experimental work.
Conflict of interest
The authors declare that there no conflict of interest.
REFERENCES






