Advances in Animal and Veterinary Sciences
Research Article
Biometric Indicators, Testicular Parameters and Semen Characteristics in Peripubertal and Postpubertal Pleven Blackhead Rams
Stanimir Angelov Yotov*, Ivan Rosenov Fasulkov
Department of Obstetrics, Reproduction and Reproductive Disorders, Faculty of Veterinary Medicine, Trakia University, Student Campus, 6000 Stara Zagora, Bulgaria.
Abstract | The aim of this study was to investigate basic biometric indicators, testicular parameters, semen characteristics and testicular parenchymal echogenicity in peripubertal and postpubertal Pleven Blackhead rams. The animals were separated in two groups: group I (peripubertal rams, aged 5 - 8 months; n=8) and group II (postpubertal rams, aged 14 - 22 months; n=8). The biometric indicators age, body weight and scrotal circumference were determined by the routine methods. Semen was collected by electroejaculation and evaluated for volume of ejaculate, concentration of the spermatozoa and mass motility. Transversal diameters of the testes were measured by the electronic calipers of the ultrasound scanner. Testicular parenchymal echogenicity and pixel heterogeneity were assessed by measuring numerical pixel values (NPVs) and standard deviation of NPVs using Image ProPlus 7.0 analytical software. The results were statistically processed by t-test and correlation analysis. The current study indicated significantly (P < 0.05) higher mean values of scrotal circumference (30.7 ± 4.4 cm vs. 18.8 ± 5.9 cm), total transversal diameter (5.1 ± 0.5 cm vs. 2.9 ± 0.94 cm) and testicular parenchyma echogenicity (110.4 ± 14.2 vs. 74.8 ± 32.5) in postpubertal than peripubertal rams, but no effect of these parameters on concentration of the spermatozoa and mass motility was found. The age and the body weight correlated positively (P < 0.05) to scrotal circumference and total transversal diameter. Testicular parenchymal echogenicity showed a strong positive relationship (R = 0.92; P < 0.05) with pixel heterogeneity. In conclusion, the period between peripubertal and postpubertal age in rams is associated with significant alterations of scrotal circumference, transversal diameter of the testes and testicular parenchymal echogenicity with no effect on concentration of the spermatozoa and their mass motility. Computer-assisted quantitative analysis of the testicular echotexture may be introduced in a system for early assessment of the sexual maturity in rams.
Keywords | Ram, Semen, Testis, Biometry, Echotexture
Received | September 11, 2019; Accepted | February 17, 2020; Published | February 20, 2020
*Correspondence | Stanimir Angelov Yotov, Department of Obstetrics, Reproduction and Reproductive Disorders, Faculty of Veterinary Medicine, Trakia University, Student Campus, 6000 Stara Zagora, Bulgaria; Email: [email protected]
Citation | Yotov SA, Fasulkov IR (2020). Biometric indicators, testicular parameters and semen characteristics in peripubertal and postpubertal pleven blackhead rams. Adv. Anim. Vet. Sci. 8(2): 217-222.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.217.222
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Yotov et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
iNTRODUCTION
The early selection of males with high reproductive capacity is very important for effective reproductive performance of the small ruminant flocks (Gouletsou and Fthenakis, 2010; Allaoui et al., 2014; Saaed and Zaid, 2018). In this aspect, breeding soundness evaluation (BSE) is crucial for reproductive success in sheep production systems and maximising the productive longevity of the rams (Ridler et al., 2012; Menegassi et al., 2014). Many clinical methods have been used for evaluation of reproductive abilities of the rams before their inclusion in breeding programs but with variable success (Gouletsou and Fthenakis, 2010; Mozo et al., 2015; Camela et al., 2019). The ultrasonographic examination of scrotal content is a useful technique for diagnosis of male genital health and an excellent complementary test in evaluation of the reproductive ability of rams (Gouletsou, 2017; Elbaz et al., 2019). A quantitative computer-assisted analysis of ultrasonograms from reproductive organs can facilitate their interpretation and increase the diagnostic value of the ultrasound method (Pierson and Adams, 1995; Giffin et al., 2009). The numerical pixel values (NPVs) and standard deviation of numerical pixel values (sdNPVs) are criteria for evaluation of testicular echotexture or echogenicity and pixel heterogeneity, respectively (Chandolia et al., 1997; Giffin et al., 2014). The echogenicity of testicular parenchyma was shown as an indicator for semen production and quality that has been used for determination of testicular maturation in male animals (Ahmadi et al., 2013; Moxon et al., 2015; Tomlinson et al., 2017; Saaed and Zaid, 2018).
Most researches related to ultrasound evaluation of the ram reproductive system provide only qualitative information from ultrasound pictures analysis and the results for the correlations between the different findings are controversial. Because of that, the investigations on ultrasound determination of the testicular parameters and quantitative computer-assisted analysis of the testicular echotexture according to age and breed of the rams are still in progress.
The aim of this study was to investigate basic biometric indicators, testicular parameters, semen characteristics and testicular parenchymal echogenicity in peripubertal and postpubertal Pleven Blackhead rams.
MATERIAL AND METHODS
The investigation was carried out with sixteen clinically healthy rams (Pleven Blackhead breed) without a history of previous semen collection, separated in two groups according to their age and sexual development. Group I (peripubertal) included 8 rams with average age 6.5 ± 1.6 months (5- 8 months) and body weight 35±8.3 kg and group II (postpubertal): 8 rams, average age 18±3.6 months (14- 22 months) and body weight 63 ± 10.3 kg. All animals were housed in the same production system, fed according to standard requirements for each animal category with daily rations included concentrate, hay, straw, mineral vitamin supplements and drinking of water at libitum. The animals were reared in group pens at a small ruminant unit, located at N 42.25 and E 25.38. The experiment was performed at the beginning of the breeding season (June). The study was conducted in accordance with the recommendations of Animal Ethics Committee and regulations for human attitude and animal protection.
Initially, a physical examination and determination of body weight (BW) were performed. The rams were restrained in standing position and scrotal circumference (SC) was measured by pulling the testes down into the lower part of the scrotum and placing a measuring tape around the widest point. Semen was collected by electro-ejaculator kit for small ruminants (Minitübe, Germany), then all ejaculates were placed on a water bath at 35°C and submitted to assessment within 5 min after collection. Volume of ejaculates (VE) was measured in graduated collecting tubes. Mass motility (MM) was evaluated microscopically on the base of sperm wave motion (scale 0-5) by Motic Image Plus Digital System (Motic China Group Ltd, 2001-2004), including a microscope, objectives with different magnification, digital camera and relevant software. Concentration of the spermatozoa (CS) (x109/ml) was determined by a Photometer SpermaCue (Minitüb, Germany), calibrated for small ruminant semen.
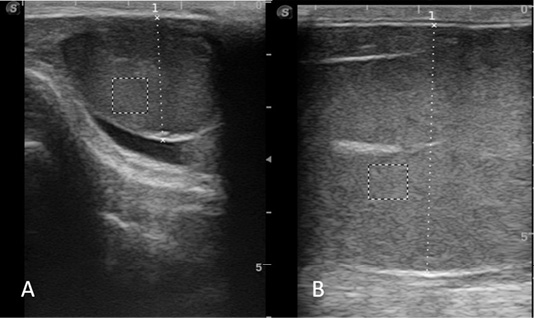
A trans-scrotal ultrasonography of both testes was performed with ultrasound scanner Sono Scape S2 Vet (Sono Scape Co. LTD, Shenzhen, China) and 7 MHz linear transrectal probe by the same operator. The values for focus, gain and brightness were set at the machine median settings and were kept constant during the study. Before examination, the testes were cleaned and dried by paper towels and ultrasound gel was applied as a coupling material between the scrotum and transducer. The ultrasound images were obtained in a longitudinal view plane and frozen when visualization of the mediastinum of the testes was clear and apparent (Figure 1A, B). The full visualization of the testes in some postpubertal animals was impossible because of insufficient length of the probe and only the transversal testicular diameter was discussed. The transversal diameters of left and right testes in each ram were measured by the electronic calipers of the ultrasound scanner and the mean value was read as total transversal diameter (TTD). All obtained images were transferred to a computer, followed by computer-assisted analysis of each ultrasound image in a gray-scale (0 pixels, absolute black - 250 pixels, absolute white) using Image ProPlus 7.0 analytical software (Media Cybernetics Inc., San Diego, CA, USA). Numerical pixel values (NPVs) and standard deviation of NPVs of three outlines of normal testicular tissue (width and height 50 x 50 mm) located in different place (below testicular mediastinum) were measured and their means were accepted as final values for each testis (Urt et al., 2018). A total testicular parenchymal echogenicity (TTPE) and total pixel heterogeneity (TPH) were given as mean values and standard deviations after their measurement in both testes of the same animal.
The results were processed by statistical program Statistica version 7.0 (Stat-Soft., 1984-2000 Inc., Tulsa, OK, USA). The parameters for each group were given as mean±standard deviation. Initially, the values were tested for normal distribution and homogeneity of variances by Kolmogorov-Smirnov and Lilliefors tests. In lack of normal distribution and homogeneity the values were transformed logarithmically. The mean values between both groups were compared by t - test for comparison of two means. The relationships between the basic parameters (age, BW, SC, VE, CS, MM, TTD, TTPE and TPH) were estimated by Pearson product moment analysis and calculation of Person’s coefficient of correlation (R). Statistical significance was considered at P level < 0.05.
RESULTS
The current study indicated significant differences (P < 0.05) between the values for age, body weight and scrotal circumference in the different groups (Table 1). Semen was successfully (without urine impurity) collected from four peripubertal animals only. In two rams the ejaculates were classified as inappropriate for evaluation. The mean volume of ejaculate was significantly greater (P < 0.05) in group II than in group I, while concentration and mass motility of the spermatozoa were comparable (P = 0.30 and P = 0.15). In both groups, no differences in transversal diameter, testicular parenchymal echogenicity and pixel heterogeneity between the left and the right testis were found. However, the mean values for left testis transversal diameter, right testis transversal diameter, and total transversal diameter were higher (P < 0.05) in postpubertal than peripubertal rams. Significantly (P < 0.05) increased means for left testis parenchymal echogenicity, right testis parenchymal echogenicity and total testicular parenchymal echogenicity were found in peripubertal rams. No differences in the mean values for pixel heterogeneity in both testes and total pixel heterogeneity between groups were detected (P > 0.05).
Data for relationships between the main parameters are presented in Table 2. Strong positive correlations were determined between the parameters age, body weight, scrotal circumference and total transversal diameter. Age of rams demonstrated strong (R ≥ 0.9; P < 0.01) correlations with body weight, scrotal circumference and total transversal diameter. The body weight and scrotal circumference correlations were similar to those recorded for age of animals (R ≥ 0.72; P < 0.05). Volume of ejaculate was positively related (R ≥ 0.77; P < 0.05) to concentration of the spermatozoa and total transversal diameter, while the concentration of the spermatozoa correlated with volume of ejaculate only (R = 0.80; P < 0.05). The testicular echogenicity correlated negatively with most parameters, but without statistical significance (P ≥ 0.06). The correlations between total pixel heterogeneity and age, body weight, scrotal circumference, volume of ejaculate, concentration of the spermatozoa, mass motility and total transversal diameter were also insignificant (P ≥ 0.08). However, the total testicular parenchymal echogenicity was positively associated to total pixel heterogeneity (R = 0.92; P < 0.05).
Table 1: Values of biometric indicators, semen characteristics and ultrasonographic parameters in the different groups (Mean±SD).
| Biometric indicators and semen characteristics | Group I (n=8) | Group II (n=8) |
| Age (months) |
6.5±1.6a |
18±3.6b |
| Body weight (kg) |
35±8.3a |
63±10.3b |
| Scrotal circumference (cm) |
18.8±5.9a |
30.7±4.4b |
| VE (ml) |
0.45±0.7a* |
1.12±0.32b |
|
CS (x 109) |
1.3±0.16a* |
1.7±0.72a |
| MM (0-5) |
3.5±0.71a* |
2.83±0.75a |
| Testicular diameters and echogenicity and pixel heterogeneity | ||
| LTTD (cm) |
2.9±1.1a |
5.0±0.8b |
| RTTD (cm) |
2.8±0.8a |
5.2±0.4b |
| TTD (cm) |
2.9±0.94a |
5.1±0.5b |
| LTPE (npv) |
101.4±19.9a |
67.0±26.1b |
| RTPE (npv) |
115.4±16a |
89.5±19.3b |
| TTPE (npv) |
110.4±14.2a |
74.8±32.5b |
| LTPH (sdnpv) |
12.1±1.0a |
11.5±3.7a |
| RTPH (sdnpv) |
12.7±0.7a |
11.9±4.0a |
| TPH (sdnpv) |
12.4±0.7a |
11.7±3.8a |
BW: body weight; SC: scrotal circumference; VE: volume of ejaculate; CS: concentration of the spermatozoa; MM: mass motility; LTTD: left testis transversal diameter; RTTD: right testis transversal diameter; TTD: total transversal diameter; LTPE: left testis parenchymal echogenicity; RTPE: right testis parenchymal echogenicity; TTPE: total testicular parenchymal echogenicity; LTPH: left testis pixel heterogeneity; RTPH: right testis pixel heterogeneity; TPH: total pixel heterogeneity; npv: numerical pixel values; sdnpv: standard deviation of numerical pixel values; For values with asterisk (*) n=6. Values with different superscripts in a row differ at P < 0.05
DISCUSSION
The determination of the reproductive capacity of pubertal animals or older rams before their introduction in breeding program is a prerequisite for effective reproductive management. The early selection of males during the first BSE allowed removal of unsound rams from the flock and purchase of new animals if necessary (Ridler et al., 2012). Ultrasound examination for interpreting uncertain clinical findings or for detecting early stages of pathological processes in ram genitalia is very important for the complete reproductive assessment (Gouletsou et al., 2003; Gouletsou and Fthenakis, 2010; Ahmadi et al., 2013; Saaed and Zaid, 2018).
Table 2: Correlation coefficients between different findings in Pleven Blackhead rams.
| Age | BW | SC | VE | CS | MM | TTD | TTPE | TPH | |
| Age | - | ||||||||
| BW | 0.94** | - | |||||||
| SC | 0.90** | 0.93** | - | ||||||
| VE | 0.64 | 0.72* | 0.77* | - | |||||
| CS | 0.58 | 0.55 | 0.67 | 0.80* | - | ||||
| MM | - 0.35 | - 0.39 | - 0.19 | - 0.10 | - 0.26 | - | |||
| TTD | 0.91** | 0.95** | 0.96** | 0.86* | 0.69 | -0.26 | - | ||
| TTPE | - 0.12 | - 0.18 | - 0.06 | - 0.46 | - 0.15 | 0.03 | - 0.20 | - | |
| TPH | 0.14 | 0.08 | 0.20 | - 0.11 | 0.17 | - 0.02 | 0.12 | 0.92* | - |
BW: body weight; SC: scrotal circumference; VE: volume of ejaculate; CS: concentration of the spermatozoa; ММ: mass motility; TTD: total transversal diameter; TTPE: total testicular parenchymal echogenicity; TPH: total pixel heterogeneity. Level of significance at P < 0.05* and P < 0.01**
The mean values of scrotal circumference in both categories rams (18.8 ± 5.9 cm and 30.7 ± 4.4 cm) were close to values for young Synthetic Population Bulgarian Milk rams (23.7 ± 0.4 cm) and mature Kivircik animals (30.24 ± 0.91 cm), reported by Elmaz et al. (2007) and Metodiev et al. (2014), respectively. However, no correspondence was observed with the results (29.23 ± 3.32 cm and 33.23 ± 1.60 cm) in pubertal and sexually mature Santa Ines rams (Ribeiro et al., 2017) or the records (31.8 ± 0.4 cm and 33.3 ± 0.5 cm) for peri- and postpubertal Dorper rams (Camela et al., 2019). The different ram breed and age heterogeneity of the groups could be a reason for these discrepancies. According to Ridler et al. (2012) appropriate cut-off values for classifying an animal based on scrotal circumference are controversial, as they can vary due to age, season, breed and individual variation. А logical explanation for significantly (P < 0.05) larger scrotal circumference in the older rams was the higher age and body weight in these animals. It was confirmed by observed positive correlation between scrotal circumference and both indicators (R = 0.99 and R = 0.93; P<0.01). This result is in agreement with previous reports (Koyuncu et al., 2005; Allaoui et al., 2014) for strong positive relationship of SC with age and weight of the animals. The significant (P < 0.05) difference in the volume of ejaculate between the groups, with no effect of age and body weight on concentration of the spermatozoa and their mass motility indicate that, additional factors have stronger influence on the semen production and its characteristics. The lower volume of ejaculate in group I can be explained with incomplete development of reproductive organs in some peripubertal rams. An evidence for this suggestion was no collected semen from two younger animals in the first group by electroejaculation method. The positive correlation between VE and CS may be related to better hormonal stimulation of the sperm producing tissue and development of the accessory glands in sexually mature animals (Elmaz et al., 2007; Ribeiro et al., 2017).
Recently, ultrasound determination of testicular diameters and parenchymal echogenicity have been used for evaluation of the reproductive abilities in bulls and rams (Ribeiro et al., 2017; Tomlinson et al., 2017; Saaed and Zaid, 2018; Camela et al., 2019; Elbaz and Razek, 2019). The ultrasound measurement of different testicular diameters is commonly used for reproductive assessment of rams but the obtained results are rather various. The insignificant difference in the transversal diameters between right and left testis of the same animal in both groups was in line with the study of Ribeiro et al. (2017), as well as earlier data for a highly symmetrical manner of testicular development in rams (Chandolia et al., 1997). The significantly (P<0.05) higher transversal diameters of the testes in postpubertal rams and the positive correlations (R≥0.87; P<0.05) of TTD with age, BW and SC were comparable to the testicular sizes measured in immature and mature Awassi rams (Saaed and Zaid, 2018).
An ultrasonographic image is composed of pixels, and numerical pixel values and standard deviation of numerical pixel values are directly connected with tissues characteristics (Pirson and Adams, 1995; Chandolia et al., 1997). The determination of NPVs and standard deviation of NPVs by computer-assisted image analysis allows measurement of the tissues echotexture in a very objective way, imperceptible to the human eye (Singh et al., 2003; Giffin et al., 2009; Urt et al., 2018). Comparing mean echogenicity and pixel heterogeneity values in the different groups, no differences between the mean values for left and right testes were observed. This result was consistent with data reported by Camela et al. (2019). However, the means for LTPE, RTPE and TTPE in postpubertal Pleven Blackhead rams were lower than values (87.57 ± 12.06; 85.98 ± 12 and 86.78 ± 10.49) registered for the same findings in Santa Ines rams (Ribeiro et al., 2017). Regarding the intensity of the pixels of the predetermined regions and the testes position, Urt et al. (2018) observed a difference between left and right testes when testicular parenchyma with area of 400 mm2 was examined. Because of that, they recommended average intensity of the pixels of the left and right testes of the 1600 mm2 area (40 x 40 mm) for correct echotexture analysis. In our study, three testicular regions with area of 2500 mm2 (50 x 50 mm) were examined and average NPVs was accepted as ultimate value.
Mean numerical pixel values and pixel heterogeneity were significantly (P < 0.05) higher in peripubertal compared with postpubertal Pleven Blackhead rams. A testicular echogenicity varying highly with age, associated with an initial increase followed by a decline, corresponding to the mitotic and postmitotic phases of spermatogenesis in prepubescent ram lambs was reported by Giffin et al. (2014). Urt et al. (2018) related the differences in testicular parenchymal echogenicity to the stage of sexual maturity of the rams studied (end of puberty and beginning of sexual maturity) and the anatomical changes of the seminiferous tubules. Camela et al. (2019) accepted that the difference in testicular echotexture was due to changes of the testes’ histomorphology occurring at puberty and explaining the decline in both echotextural variables with seminiferous tubule area differentiation, manifesting in the presence of more mature germ cells in the majority of seminiferous tubules. According to Ahmadi et al. (2013) the mean pixel values of the testicular ultrasonogram correlated negatively with parenchymal protein content, while pixel heterogeneity correlated directly with extractable lipids.
The strong positive correlation (r=0.92; P<0.05) between testicular echogenicity and pixel heterogeneity supported the previous suggestions for use of both indicators in the assessment of the sexual maturity and the reproductive capacity in rams. In this regard, a pixel heterogeneity of testicular parenchyma obtained approximately 60 d prior to semen evaluation correlated inversely with percentage of the sperm cells with normal morphology and progressive motility, and directly with percentage of the sperm cells with abnormal tails and loose heads (Ahmadi et al., 2012). They concluded that scrotal ultrasonography combined with computer-assisted analyses of epididymal and testicular echotexture in rams was a valuable method for determining future semen parameters. We consider that additional investigations with a large number of animals can clarify some debatable issues attributed to quantitative computer-assisted analysis of the testicular ultrasonogram and use of the testicular parenchymal echogenicity as semen quality indicator.
CONCLUSION
The period between peripubertal and postpubertal age in rams is associated with significant alterations of scrotal circumference, transversal diameter of the testes and testicular parenchymal echogenicity with no effect on concentration of the spermatozoa and their mass motility. A computer-assisted quantitative analysis of the testicular echotexture can be introduced in a system for early assessment of the sexual maturity in rams. Further investigations are needed to determine reference valuess for each ultrasonographically measured testicular parameter depending on the age and the breed of the animals.
ACKNOWLEDGMENTS
The authors are very grateful to all people of Small ruminant unit of FVM for their help during the investigation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS CONTRIBUTION
SAY contributed to planning and performance of the investigations, statistical processing of the results, writing and revision of the manuscript. IRF contributed to performance of the investigations and preparation of the manuscript.
Conflict of interest
None to declare
REFERENCES